Abstract
Storage of energy as triglyceride in large adipose-specific lipid droplets is a fundamental need in all mammals. Efficient sequestration of fat in adipocytes also prevents fatty acid overload in skeletal muscle and liver, which can impair insulin signaling. Here we report that the Cide domain-containing protein Cidea, previously thought to be a mitochondrial protein, colocalizes around lipid droplets with perilipin, a regulator of lipolysis. Cidea-GFP greatly enhances lipid droplet size when ectopically expressed in preadipocytes or COS cells. These results explain previous findings showing that depletion of Cidea with RNAi markedly elevates lipolysis in human adipocytes. Like perilipin, Cidea and the related lipid droplet protein Cidec/FSP27 are controlled by peroxisome proliferator-activated receptor γ (PPARγ). Treatment of lean or obese mice with the PPARγ agonist rosiglitazone markedly up-regulates Cidea expression in white adipose tissue (WAT), increasing lipid deposition. Strikingly, in both omental and s.c. WAT from BMI-matched obese humans, expression of Cidea, Cidec/FSP27, and perilipin correlates positively with insulin sensitivity (HOMA-IR index). Thus, Cidea and other lipid droplet proteins define a novel, highly regulated pathway of triglyceride deposition in human WAT. The data support a model whereby failure of this pathway results in ectopic lipid accumulation, insulin resistance, and its associated comorbidities in humans.
Keywords: adipocyte, Cide, diabetes, fat droplet, fat metabolism
Type 2 diabetes mellitus and obesity are associated disorders and are increasing in incidence worldwide (1). It is now evident that adipose tissue plays a central role in regulating whole-body metabolism and glucose homeostasis in addition to its well known function to store and mobilize triglyceride (2). High concentrations of circulating fatty acids and triglyceride, observed in both obesity and lipodystrophy, are thought to cause muscle insulin resistance and decreased glucose tolerance. This hypothesis is supported by experiments showing that elevation of circulating fatty acids by infusion of lipid into humans impairs insulin sensitivity in skeletal muscle (3–5). Several mechanisms may account for the ability of abnormally high levels of fatty acids in muscle to inhibit insulin signaling, including the activation of protein kinases such as PKCθ, IKKβ, and JNK that are negative regulators of elements of the insulin signaling pathway, notably IRS proteins (6–8). Adipocytes can hypothetically protect muscle and liver from these deleterious effects of fatty acids by their large capacity to esterify them into triglyceride and to sequester large amounts of the triglyceride within lipid droplets. Evidence for this hypothesis is provided by the demonstrated reversal of insulin resistance by transplantation of adipose tissue into genetically manipulated “fatless” mice, which are devoid of adipocytes (9, 10).
The efficiency and capacity of adipocytes to esterify fatty acids into triglyceride and protect triglyceride stores within the cells is controlled within large lipid droplets surrounded by a phospholipid layer and lipid droplet proteins (11, 12). These proteins include the “PAT” domain-containing proteins perilipin TIP47 and ADRP, which are targeted to lipid droplets and regulate the size and biogenesis of these organelles (12). Recently we identified Cidec/FSP27 as a novel lipid droplet-associated protein in adipocytes and showed that Cidec/FSP27 negatively regulates lipolysis and promotes triglyceride accumulation (13, 14). Cidec/FSP27 is a CIDE-N domain-containing protein and belongs to CIDE family of proteins. The CIDE family of proteins has three members in mice (Cidea, Cideb, and FSP27) and humans (CIDEA, CIDEB, and CIDEC, similar to FSP27) (15). They have a common N-terminal CIDE-N domain and a C-terminal CIDE-C domain. A significant homology has been found between the CIDE-N domain of CIDE proteins and the regulatory domains of the apoptotic DNA fragmentation factors, DFF40 (caspase-activated nuclease) and DFF45 (DFF40 inhibitor) (16–19). Thus, it was surprising to find that Cidec/FSP27 functions as a lipid droplet-associated protein in mouse adipocytes to regulate fat deposition (13, 14).
The Cide domain-containing protein Cidea is also known to be highly expressed in adipose tissue. In mice it is largely restricted to brown adipose tissue (BAT) (18) whereas in humans Cidea is highly expressed in WAT (20, 21). In previous studies, Cidea was proposed to be a mitochondrial protein that negatively regulates the activity of the BAT uncoupling protein UCP1 (18, 19), consistent with its high expression in mouse BAT. However, based on the sequence similarity between Cidea and Cidec/FSP27 and on our findings revealing Cidec/FSP27 to be a lipid droplet-associated protein, a similar function for Cidea seemed likely. In the present studies we discovered that Cidea indeed localizes to lipid droplets and regulates triglyceride deposition in adipocytes as well as other cell types. Remarkably, we found that both Cidea and Cidec/FSP27 expression in WAT of obese human subjects also correlates positively with whole-body insulin sensitivity, as does the known lipid droplet protein perilipin. Taken together, these results are consistent with the hypothesis that Cide domain-containing proteins play an important role in sequestration of triglyceride within human adipocytes, promoting whole-body insulin sensitivity.
Results and Discussion
Identification of Cidea as a Lipid Droplet Protein.
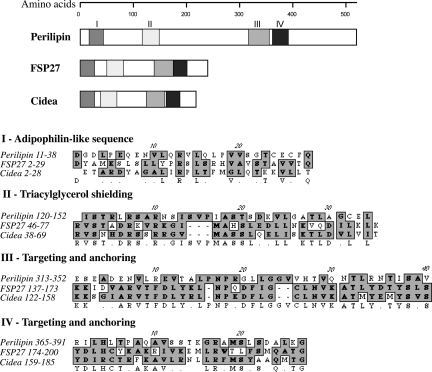
In considering that Cidea may share functions with the lipid droplet protein Cidec/FSP27, we noted that Cidea displays almost 61% sequence identity with Cidec/FSP27 [supporting information (SI) Figs. S1–S3]. We then compared amino acid sequences within the Cidea and Cidec/FSP27 proteins with those in the prototypic lipid droplet protein perilipin. This analysis of sequences within the Cidea and Cidec/FSP27 proteins revealed four regions of low but significant similarity to perilipin (Fig. 1). These regions included a short N-terminal sequence (I) with shared similarity to adipophilin, a segment (II) with similarity to a region of perilipin thought to shield lipid droplets from lipases, and two regions (III and IV) thought to function in the targeting and binding of perilipin to lipid droplets (22). Interestingly, there is no similarity between sequences in the PAT domain of perilipin, thought to be a signature structure for lipid droplet proteins, and sequences within the Cide proteins (data not shown).
Fig. 1.
Predicted structural motifs of mouse Cidea and FSP27 based on sequence homology with mouse perilipin. FSP27 (amino acids 2–29) and Cidea (amino acids 2–28) show sequence similarity of 32% and 22%, respectively, with a portion of the adipophilin-like sequence of perilipin (amino acids 11–38, I). In perilipin, amino acids 120–152 (II) represents a section of the triacyglycerol shielding region that plays a role in shielding stored triacylglycerol from cytosolic lipases. It has a sequence similarity of 40% with FSP27 (amino acids 46–77) and 51% with Cidea (amino acids 38–69). Similarly, lipid droplet targeting and anchoring regions of perilipin (amino acids 313–352, III; and amino acids 365–391, IV) have sequence similarities of 40% and 30% with respective sequences of FSP27 (amino acids 137–173 and 174–200) and 38% and 48% similarities with Cidea (amino acids 122–158 and 159–185).
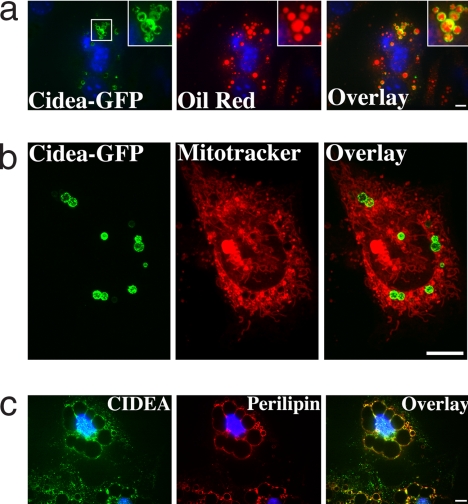
Fig. 2 confirms that the sequence similarities between the Cidea and perilipin reflect functional redundancies between these proteins. Ectopic expression of Cidea protein fused to GFP in 3T3-L1 adipocytes reveals its striking localization surrounding lipid droplets stained with oil red (Fig. 2a). The expressed Cidea-GFP protein localized around lipid droplets but not with mitochondria, as detected by MitoTracker dye (Fig. 2b; also see Fig. S4). These data are reminiscent of the first described endogenous lipid droplet-associated protein, perilipin (23, 24). Cidea is highly expressed in BAT but not WAT in the mouse. We therefore used human cultured adipocytes from WAT to assess its intracellular disposition (Fig. 2c). Endogenous Cidea protein was found concentrated around lipid droplets and colocalized with endogenous perilipin (Fig. 2c). Some punctate cytoplasmic staining was also observed in these experiments and requires further analysis. A previous report suggested that Cidea partially colocalizes with mitochondria in COS cells (18) and with mitochondrial UCP1 protein in BAT, but careful examination of expressed Cidea-GFP in COS cells in the present study showed little or no colocalization with mitochondria (Figs. S5 and S6). These studies cannot exclude the possibility that small amounts of Cidea may be associated with mitochondria but are not detected by these methods.
Fig. 2.
Cidea localizes at the surface of lipid droplets and colocalizes with perilipin in adipocytes. (a) Cidea-GFP expression in day-4 3T3-L1 adipocytes (Left). (Center) Staining of lipid droplets with oil red. The overlay of the confocal images (Right) clearly demonstrates the association of Cidea with lipid droplets. (Scale bar: 10 μm.) (b) Confocal microscopic image of a 3T3-L1 adipocyte (day 4) expressing Cidea-GFP. (Left) Expression of Cidea after 48 h of transfecting Cidea-GFP cDNA. (Center) Mitochondria stained with MitoTracker. (Scale bar: 10 μm.) The individual Z-sections are displayed in Fig. S4. (c) Immunofluorescence confocal microscopy displaying expression of endogenous CIDEA in human adipocytes (Left) using monoclonal anti-CIDEA (Novus Biologicals) antibody and anti-mouse Alexa Fluor 488 (Molecular Probes) secondary antibody. For perilipin staining (Center), guinea pig anti-perilipin primary antibody was stained with Texas red-labeled rabbit polyclonal to guinea pig IgG (Abcam). (Scale bar: 10 μm.)
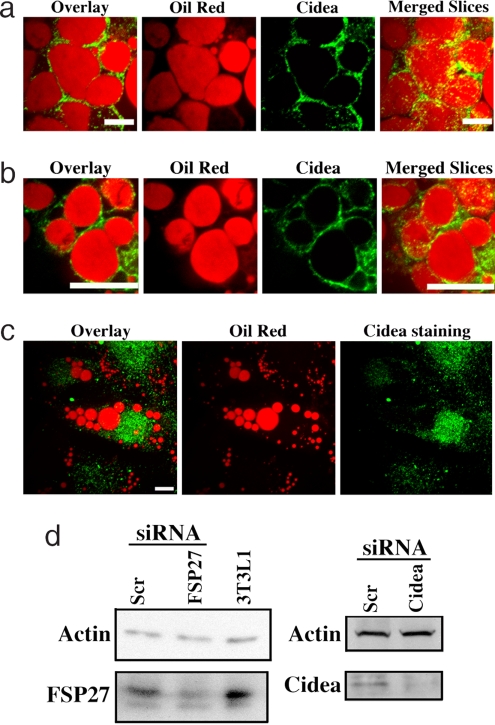
To further verify that Cidea is primarily localized around lipid droplets, we adopted an RNAi-based analysis in cultured brown adipocytes. These cells have low expression of FSP27 protein as compared with 3T3-L1 adipocytes (Fig. 3d). Immunofluorescence using Cidea monoclonal antibody showed Cidea localization around lipid droplets in these cells transfected with scrambled siRNA (Fig. 3a). Some droplets did not show Cidea staining, and some cytosolic punctuate staining was observed, which requires further analysis. To eliminate cross-reactivity of the antibody with FSP27 in these studies, we depleted FSP27 by 90% using RNAi. This had no effect on Cidea antibody staining around lipid droplets (Fig. 3 b and d). Importantly, depletion of Cidea itself with siRNA in these cells abolished the antibody staining around the lipid droplets, while some cytosolic punctuates were observed. This punctuate staining was partially due to nonspecific binding of the secondary antibody alone.
Fig. 3.
FSP27 depletion has no effect on Cidea localization at the surface of lipid droplets in adipocytes. Immunofluorescence confocal microscopy displaying localization of endogenous Cidea and staining of lipid droplets with oil red in cultured brown adipocytes transfected with scrambled siRNA (a), transfected with FSP27 siRNA (b), and transfected with Cidea siRNA (c). In a and b the left three panels show a single optical plane of 4 μm each and the far right panels display the merged Z-sections. (Scale bar: 10 μm.) (d) Western blots showing the expression of FSP27 and Cidea in cultured brown adipocytes transfected with FSP27 or Cidea siRNA. (Left) Protein lysate from 3T3-L1 adipocytes was loaded as a positive control for FSP27. Actin was labeled as a loading control.
Varying Expression Levels of Cidea Modulate Lipid Droplet Size and Basal Lipolysis.
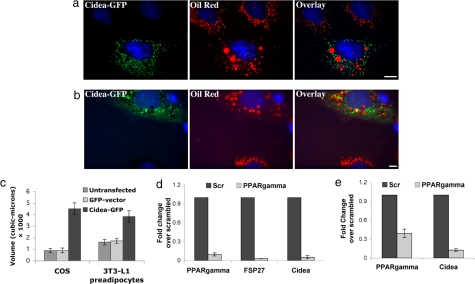
Cidea and Cidec/FSP27 mRNA dramatically increased by >3-fold and 50-fold, respectively (Table S1), as mouse adipocytes acquire lipid storage capacity during differentiation. FSP27-GFP expression even in nonadipose cells leads to lipid accumulation (16). We therefore tested the effect of increasing Cidea-GFP levels in 3T3-L1 preadipocytes on lipid accumulation in lipid droplets. These experiments revealed that preadipocytes expressing Cidea-GFP (Fig. 4a and Fig. S7) display lipid droplets of increased size as compared with neighboring untransfected cells. A similar result was obtained when Cidea-GFP (Fig. 4b and Fig. S7) expression in COS cells was examined—greatly increased lipid droplets. These results indicate that Cidea associates with lipid droplets and functions to strongly promote triglyceride accumulation even in cell types that do not normally store large amounts of neutral lipid. Morphometric analysis of oil red-stained lipid droplets in cells transfected with Cidea-GFP showed significantly higher volume than in untransfected cells or cells transfected with GFP vector alone (Fig. 4c and Figs. S7 and S8). These data reveal that Cidea (Fig. 4) and Cidec/FSP27 (13) function similarly in facilitating lipid droplet enlargement. Furthermore, in these nonadipose cells we observed that most of the Cidea was not directly associated with lipid droplets. This could be due to a requirement for other differentiation-dependent proteins to facilitate Cidea localization to lipid droplets.
Fig. 4.
Cidea-GFP expression in COS cells or 3T3-L1 preadipocytes enhances lipid droplet size. (a) COS cells were transfected with Cidea-GFP and cultured for 24 h before fixing and staining with oil red. Eight hours after transfection a 400 μM oleic acid/BSA mixture (from Sigma–Aldrich) was added to the medium. (b) 3T3-L1 preadipocytes were transfected with Cidea-GFP and cultured for 24 h before fixing and staining with oil red. Eight hours after transfection the 400 μM oleic acid/BSA mixture was added to the medium. (c) Morphometric analysis of lipid droplets in COS cells and 3T3-L1 preadipocytes that were transfected with Cidea-GFP or GFP vector alone or untransfected under the same conditions as in a and b. Student's t test comparison between untransfected and Cidea-GFP or comparison between GFP vector alone and Cidea-GFP in COS cells, P < 0.0001; in 3T3-L1 preadipocytes, P < 0.001. (d) Quantitative real-time analysis performed by using RNA isolated from 3T3-L1 adipocytes. The effect of siRNA-mediated PPARγ knockdown on Cidec/FSP27 and Cidea was measured (P < 0.0001). (e) Quantitative real-time analysis performed by using RNA isolated from differentiated brown adipocytes after PPARγ knockdown (P < 0.0001).
Perilipin has little or no effect on the triacyglycerol synthetic pathway but inhibits triglyceride hydrolysis in adipocytes (25, 26). As shown in Table S2, previous studies have also shown that depleting Cidea in human preadipocytes increases lipolysis. Nordstrom et al. (20) have shown that CIDEA mRNA expression is 50% lower in s.c. WAT of obese human subjects compared with lean subjects, and this correlates with a 2-fold increase in basal lipolysis. Moreover, 2–4 years after bariatric surgery and weight reduction, obese subjects displayed a doubling of CIDEA mRNA expression and a 40% reduced lipolytic rate in s.c. WAT (Table S2). Furthermore, TNF-α treatment of differentiated primary human fat cells has been shown to increase lipolysis in proportion to the reduced CIDEA mRNA levels (Table S2). Increased lipolysis was also observed in BAT from Cidea-null mice (18). In our own recent studies, increased glycerol release in response to RNAi-mediated depletion of Cidec/FSP27 in 3T3-L1 adipocytes was observed (13). These previously published studies, in combination with the data presented here, indicate that the physical localization of Cidea and Cidec/FSP27 with lipid droplets is associated with shielding triglycerides from hydrolysis by lipases.
Peroxisome proliferator-activated receptor γ (PPARγ) is a major regulator of adipogenesis, and its expression during the differentiation program in adipose tissue is followed by accumulation of triglycerides within adipocytes (27–29). We therefore studied the effect of PPARγ depletion by RNAi on Cidea and Cidec/FSP27 expression in adipocytes to determine whether these proteins are under the control of this transcription factor. As shown in Fig. 4d, ≈90% depletion of PPARγ mRNA in 3T3-L1 adipocytes leads to >90% depletion of Cidea and Cidec/FSP27 mRNA expression. Similar results of PPARγ depletion on Cidea mRNA were obtained in mouse brown adipocytes (Fig. 4e). These results suggest that Cidea and Cidec/FSP27 expression in adipocytes is mediated directly or indirectly by PPARγ. Furthermore, Cidea is undetectable in mouse liver but is highly expressed under conditions where PPARγ expression is increased, consistent with Cidea being a target gene for PPARγ (30, 31). A recent study has indeed revealed that the mouse Cidea gene promoter has a putative peroxisome proliferator response element where the transcription factors PPARα and PPARγ could bind and control Cidea gene transcription in the liver (31).
Cidea and Cidec/FSP27 mRNA Levels Are Up-Regulated Under Physiological Conditions That Enhance Triacylglycerol Deposition in Mice.
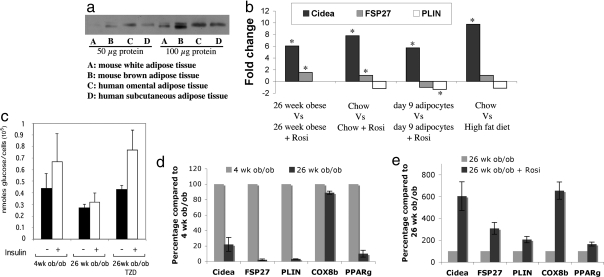
A puzzling aspect of Cide domain-containing protein biology relates to the differential expression of the Cide proteins in mouse adipose tissues versus human adipose tissue. Whereas Cidec/FSP27 is highly expressed in both mouse BAT and WAT as well as human WAT, Cidea is highly expressed in mouse BAT and human WAT, but not mouse WAT (18, 20, 21). This expression profile for endogenous Cidea is confirmed in our immunoblot studies using anti-Cidea antibody, as shown in Fig. 5a, where low Cidea expression is observed in mouse WAT compared with BAT or compared with human omental or s.c. WAT. The appearance of two bands for Cidea in mouse adipose tissues requires further analysis. We reasoned that if Cidea plays a major role in fat storage in mouse WAT, then its expression must be highly up-regulated under conditions of increased triglyceride deposition. We therefore determined Cidea mRNA levels under various physiological conditions, as shown in Fig. 5 b–e. Dramatic 6- to 10-fold increases in Cidea expression were observed in adipocytes under four different conditions known to be associated with increased triglyceride stores—treatment of ob/ob obese mice with rosiglitazone for 2 weeks, treatment of normal wild-type mice with rosiglitazone for 2 weeks, treatment of fully differentiated 3T3-L1 adipocytes with rosiglitazone for 24 h, and feeding of wild-type mice with a high-fat diet (Fig. 5b).
Fig. 5.
Rosiglitazone treatment of cultured adipocytes or intact mice markedly increases Cidea expression. (a) Western blot analysis of Cidea expression in mouse and human adipose tissues. (b) Fold change of Cidea, FSP27, and perilipin mRNA in 3T3-L1 adipocytes and primary adipocytes isolated from mice after rosiglitazone treatment based on expression data using MG-U74 Affymetrix GeneChips. Total RNA was isolated from 3T3-L1 adipocytes treated with or without 1 μM rosiglitazone for 24 h or primary fat cells of mice treated with or without 5 mg/kg rosiglitazone each day for 2 weeks. *, P < 0.05. (c) The graph represents amount of d-[U-14C]-glucose taken up and converted to triglycerides by primary adipocytes from 4-week-old ob/ob mice, 26-week-old ob/ob mice, and 26-week-old ob/ob mice treated with 5 mg/kg rosiglitazone each day for 2 weeks. Glucose conversion to triglyceride glycerol in adipocytes plus or minus insulin was calculated to nanomoles per 105 cells. The data represent the mean ± SEM of three experiments for each age group and condition. (d) Quantitative real-time analysis performed by using RNA isolated from adipose tissue (epididymal fat pads) of 4-week-old ob/ob and 26-week-old ob/ob mice. The 36B4 gene was used as a reference gene for quantitative analysis (P < 0.05). (e) Quantitative real-time analysis performed by using RNA isolated from adipose tissue (epididymal fat pads) of 26-week-old ob/ob mice and 26-week-old ob/ob mice treated with 5 mg/kg rosiglitazone each day for 2 weeks. The 36B4 gene was used as a reference gene for quantitative analysis (P < 0.05). All procedures in Fig. 5 were carried out according to the guidelines of the University of Massachusetts Medical School Institutional Animal Care and Use Committee.
We confirmed that the levels of Cidea and Cidec/FSP27 expression in mouse adipose tissue correlate with conditions of increased triglyceride deposition by measuring the rates of 14C-glucose conversion to triglyceride glycerol in adipose tissue from 4-week-old lean ob/ob mice compared with 26-week-old obese ob/ob mice or compared with the latter mice treated with rosiglitazone for 14 days. As shown in Fig. 5c, net triglyceride synthesis was decreased in 26-week-old obese ob/ob mice compared with 4-week-old lean ob/ob mice or with obese (26-week-old ob/ob) mice treated with rosiglitazone. Similarly, Cidea and Cidec/FSP27 expression was decreased during the progression of obesity from 4 weeks to 26 weeks and restored by rosiglitazone treatment of the obese mice (Fig. 5 d and e). These data are consistent with the concept that Cidea and Cidec/FSP27 expression is under the control of PPARγ, which also plummets during the progression of obesity in the ob/ob mouse model, but is activated by rosiglitazone. We confirmed that Cidea and Cidec/FSP27 expression depends on PPARγ levels in 3T3-L1 adipocytes by siRNA-mediated silencing of PPARγ, which markedly reduced mRNA levels of both proteins in these cells (Fig. 4 d and e). Interestingly, obesity in the ob/ob mice also caused dramatic decreases in the expression of other lipid droplet proteins in WAT, but, of the known lipid droplet proteins, Cidea was most responsive to the effects of rosiglitazone treatment (Fig. 5 c and d; also see Table S1).
Cidea and Cidec/FSP27 Expression Correlates Positively with Insulin Sensitivity in Obese Human Subjects Matched for BMI.
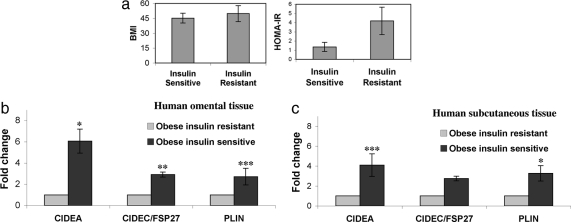
It is thought that triglyceride storage in adipocytes plays an important role in sequestering triglycerides and fatty acids away from the circulation and peripheral tissues, thus enhancing insulin sensitivity in liver and muscle (10, 32). If Cidea and Cidec/FSP27 function to decrease lipolysis and enhance triglyceride deposition in human WAT, it may play a role in enhancing whole-body insulin sensitivity. Nordstrom et al. (20) indeed reported negative correlations between Cidea expression in omental adipose tissue and both basal lipolysis and apparent insulin sensitivity (HOMA index) in a cohort of 186 lean and obese patients (also see Table S2). To further refine an analysis of the relationship between Cidea expression and insulin sensitivity, we assessed Cidea mRNA levels in s.c. and omental adipose samples from obese human subjects with similar high BMI values but different levels of insulin sensitivity (Fig. 6a).
Fig. 6.
Cidea mRNA levels are higher in WAT of obese, insulin-sensitive subjects as compared with obese, insulin-resistant subjects matched for BMI. (a) BMI and HOMA-IR comparisons of insulin-sensitive (n = 13) and insulin-resistant (n = 7) obese human subjects. Please note that BMI does not predict the degree of insulin resistance in this cohort of obese patients. HOMA-IR of 2.3 was used as a cut point to categorize the obese patients as insulin-sensitive (HOMA-IR ≤ 2.3) or insulin-resistant. (b) Real-time PCR analysis depicting a fold change in mRNA levels of various genes in omental adipose tissue of obese, insulin-sensitive individuals as compared with obese, insulin-resistant subjects. *, P < 0.0001; **, P < 0.001; ***, P < 0.05. (c) Real-time PCR analysis depicting a fold change in mRNA levels of various genes in s.c. adipose tissue of obese, insulin-sensitive individuals as compared with obese, insulin-resistant subjects. *, P < 0.0001; ***, P < 0.05. Fresh human omental and s.c. tissues were obtained under informed consent from patients undergoing gastric bypass surgery (University of Massachusetts Medical School Institution Review Boards docket number H-11033). Tissues were frozen after procurement and stored at −80°C for subsequent RNA and protein extractions.
In preliminary studies (33) it was observed that, in a population of 138 such obese subjects with high BMI, approximately half of the nondiabetic subjects exhibited apparent high insulin sensitivity (low HOMA-IR index) and approximately half exhibited the expected insulin resistance (high HOMA-IR index). Furthermore, insulin resistance measured by HOMA-IR index was not correlated with BMI values in this cohort of very obese patients. We thus segregated adipose samples from such obese patients based on high or low HOMA-IR index values, reflecting relative insulin sensitivity or insulin resistance (Fig. 6a). RT-PCR analysis revealed a highly significant 6-fold increase in Cidea expression in both omental and s.c. adipose tissue in the group of patients with low HOMA index (high insulin sensitivity) (Fig. 6 a and b), and this was also seen in omental adipose tissue from full genome microarray data (Table S3). Perilipin was also elevated significantly in omental and s.c. adipose tissue from the low HOMA group, although less dramatically, whereas Cidec/FSP27 was elevated significantly in omental adipose tissue from this group. Taken together, these data show a strong negative correlation between expression levels of the lipid droplet proteins Cidea/Cidec/perilipin and an index of insulin resistance (HOMA) in obese patients matched for BMI.
A previous study also showed that expression of Cidea in adipose tissue correlated inversely with whole-body insulin resistance in lean versus obese subjects (20) (Table S2). Here we extended these findings by showing that Cidea expression levels in human adipose tissue correlate with insulin sensitivity even when subjects are matched for BMI (Fig. 6). The data we present in this paper also provide a hypothetical mechanistic rationale for these results: Cidea enhances storage of triglyceride in lipid droplets of adipose tissues, decreasing fatty acid levels in the circulation, thereby protecting muscle and liver from high fatty acid levels that impair insulin sensitivity. Thus, up-regulation of Cidea, Cidec/FSP27, and perilipin expression by rosiglitazone may be associated with fat-sequestration and insulin-sensitizing effects of the drug in humans. Indeed, a recent study has reported an increased perilipin expression in s.c. fat of rosiglitazone-treated fatty rats (34), consistent with this hypothesis and our data in mice (Fig. 5). Failure to efficiently sequester lipid into the lipid storage droplets of adipocytes has been emphasized as a major contributor to the pathogenesis of insulin resistance (35). The exact extent to which the capacity for triglyceride deposition in adipose tissue contributes to insulin sensitivity in obese patients will be important to rigorously determine in future studies.
Our results emphasize that Cidea, previously known to be a BAT protein in mice, is highly abundant in human WAT (20) (Fig. 5a). The marked effect of Cidea to promote especially large lipid droplets when expressed in cells (Fig. 4) thus indicates a particularly important role for this protein in fat storage in human WAT. The potential failure to optimally store fat in adipose tissue in obesity may be exacerbated by the recruitment of macrophages into adipose tissue (36, 37) and the release of cytokines such as TNFα (38), known to down-regulate PPARγ and therefore decrease triglyceride synthesis. It is thus noteworthy that high Cidea expression also appears to down-regulate TNFα expression in human adipose tissue, which may be related to its effect to decrease lipolysis (20, 39). It has also been reported that a V115F polymorphism in human Cidea is associated with obesity in two Swedish samples of 981 women and 582 men (40). Thus, it is likely that Cidea plays a central role in controlling metabolic flux in human adipose tissues through its regulation of fat storage in lipid droplets.
Methods
siRNA.
siRNA was purchased from Dharmacon. Individual siRNA sequences include the following: scrambled, 5′-CAGUCGCGUUUGCGACUGG-3′; FSP27, 5′-CAACUAAGAAGAUCGAUGUUU-3′; perilipin, 5′-GCAGAACACUCUCCGGAACUU-3′; Cidea, 5′-GGACACCGGGUAGUAAGUA3′.
Cell Culture and siRNA Transfection in 3T3-L1 Adipocytes.
3T3-L1 fibroblasts were cultured, and adipocytes were transfected with siRNA duplexes as described previously (41–43).
Confocal Microscopy.
Images were taken on a Zeiss Axiophot microscope equipped with a Hamamatsu digital camera and processed by using Metamorph imaging software, version 6.1 (Universal Imaging).
Statistical Analysis.
Quantitative data are represented as mean ± SEM. For statistical analysis the differences between groups were examined with Student's paired t test, and P < 0.05 was considered statistically significant.
Materials and the remaining methods on cells, transfection, constructs, RNAi, RNA isolation, RT-PCR, oil red staining, and immunofluorescence can be found in SI Materials and Methods.
Supplementary Material
Acknowledgments.
We gratefully acknowledge Dr. Stephen J. Doxsey and Paul Furcinitti for use of their confocal microscopy facilities. This work was supported by National Institutes of Health Grants DK30898 and DK60837 and the Genomics and Bioinformatics Core Facilities of the University of Massachusetts Diabetes and Endocrinology Center (which is supported by National Institutes of Health Grant DK32520). FSP27 antibodies were a kind gift from Dr. Masato Kasuga and Dr. Naonobu Nishino from Kobe University Graduate School of Medicine, Kobe, Japan.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0802063105/DCSupplemental.
References
- 1.Smyth S, Heron A. Diabetes and obesity: The twin epidemics. Nat Med. 2006;12:75–80. doi: 10.1038/nm0106-75. [DOI] [PubMed] [Google Scholar]
- 2.Rajala MW, Scherer PE. Minireview: The adipocyte—at the crossroads of energy homeostasis, inflammation, and atherosclerosis. Endocrinology. 2003;144:3765–3773. doi: 10.1210/en.2003-0580. [DOI] [PubMed] [Google Scholar]
- 3.Ferrannini E, Barrett EJ, Bevilacqua S, DeFronzo RA. Effect of fatty acids on glucose production and utilization in man. J Clin Invest. 1983;72:1737–1747. doi: 10.1172/JCI111133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boden G, Chen X, Ruiz J, White JV, Rossetti L. Mechanisms of fatty acid-induced inhibition of glucose uptake. J Clin Invest. 1994;93:2438–2446. doi: 10.1172/JCI117252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dresner A, et al. Effects of free fatty acids on glucose transport and IRS-1-associated phosphatidylinositol 3-kinase activity. J Clin Invest. 1999;103:253–259. doi: 10.1172/JCI5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Itani SI, Ruderman NB, Schmieder F, Boden G. Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and IkappaB-alpha. Diabetes. 2002;51:2005–2011. doi: 10.2337/diabetes.51.7.2005. [DOI] [PubMed] [Google Scholar]
- 7.Nguyen MT, et al. JNK and tumor necrosis factor-alpha mediate free fatty acid-induced insulin resistance in 3T3–L1 adipocytes. J Biol Chem. 2005;280:35361–35371. doi: 10.1074/jbc.M504611200. [DOI] [PubMed] [Google Scholar]
- 8.Yu C, et al. Mechanism by which fatty acids inhibit insulin activation of insulin receptor substrate-1 (IRS-1)-associated phosphatidylinositol 3-kinase activity in muscle. J Biol Chem. 2002;277:50230–50236. doi: 10.1074/jbc.M200958200. [DOI] [PubMed] [Google Scholar]
- 9.Gavrilova O, et al. Surgical implantation of adipose tissue reverses diabetes in lipoatrophic mice. J Clin Invest. 2000;105:271–278. doi: 10.1172/JCI7901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lewis GF, Carpentier A, Adeli K, Giacca A. Disordered fat storage and mobilization in the pathogenesis of insulin resistance and type 2 diabetes. Endocr Rev. 2002;23:201–229. doi: 10.1210/edrv.23.2.0461. [DOI] [PubMed] [Google Scholar]
- 11.Londos C, Sztalryd C, Tansey JT, Kimmel AR. Role of PAT proteins in lipid metabolism. Biochimie. 2005;87:45–49. doi: 10.1016/j.biochi.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 12.Wolins NE, Brasaemle DL, Bickel PE. A proposed model of fat packaging by exchangeable lipid droplet proteins. FEBS Lett. 2006;580:5484–5491. doi: 10.1016/j.febslet.2006.08.040. [DOI] [PubMed] [Google Scholar]
- 13.Puri V, et al. Fat-specific protein 27, a novel lipid droplet protein that enhances triglyceride storage. J Biol Chem. 2007;282:34213–34218. doi: 10.1074/jbc.M707404200. [DOI] [PubMed] [Google Scholar]
- 14.Puri V, Virbasius JV, Guilherme A, Czech MP. RNAi screens reveal novel metabolic regulators: RIP140, MAP4k4 and the lipid droplet associated fat specific protein (FSP) 27. Acta Physiol (Oxford) 2008;192:103–115. doi: 10.1111/j.1748-1716.2007.01786.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liang L, Zhao M, Xu Z, Yokoyama KK, Li T. Molecular cloning and characterization of CIDE-3, a novel member of the cell-death-inducing DNA-fragmentation-factor (DFF45)-like effector family. Biochem J. 2003;370:195–203. doi: 10.1042/BJ20020656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Danesch U, Hoeck W, Ringold GM. Cloning and transcriptional regulation of a novel adipocyte-specific gene, FSP27. CAAT-enhancer-binding protein (C/EBP) and C/EBP-like proteins interact with sequences required for differentiation-dependent expression. J Biol Chem. 1992;267:7185–7193. [PubMed] [Google Scholar]
- 17.Inohara N, Koseki T, Chen S, Wu X, Nunez G. CIDE, a novel family of cell death activators with homology to the 45 kDa subunit of the DNA fragmentation factor. EMBO J. 1998;17:2526–2533. doi: 10.1093/emboj/17.9.2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhou Z, et al. Cidea-deficient mice have lean phenotype and are resistant to obesity. Nat Genet. 2003;35:49–56. doi: 10.1038/ng1225. [DOI] [PubMed] [Google Scholar]
- 19.Chen Z, Guo K, Toh SY, Zhou Z, Li P. Mitochondria localization and dimerization are required for CIDE-B to induce apoptosis. J Biol Chem. 2000;275:22619–22622. doi: 10.1074/jbc.C000207200. [DOI] [PubMed] [Google Scholar]
- 20.Nordstrom EA, et al. A human-specific role of cell death-inducing DFFA (DNA fragmentation factor-alpha)-like effector A (CIDEA) in adipocyte lipolysis and obesity. Diabetes. 2005;54:1726–1734. doi: 10.2337/diabetes.54.6.1726. [DOI] [PubMed] [Google Scholar]
- 21.Su AI, et al. Large-scale analysis of the human and mouse transcriptomes. Proc Natl Acad Sci USA. 2002;99:4465–4470. doi: 10.1073/pnas.012025199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Subramanian V, Garcia A, Sekowski A, Brasaemle DL. Hydrophobic sequences target and anchor perilipin A to lipid droplets. J Lipid Res. 2004;45:1983–1991. doi: 10.1194/jlr.M400291-JLR200. [DOI] [PubMed] [Google Scholar]
- 23.Blanchette-Mackie EJ, et al. Perilipin is located on the surface layer of intracellular lipid droplets in adipocytes. J Lipid Res. 1995;36:1211–1226. [PubMed] [Google Scholar]
- 24.Greenberg AS, et al. Perilipin, a major hormonally regulated adipocyte-specific phosphoprotein associated with the periphery of lipid storage droplets. J Biol Chem. 1991;266:11341–11346. [PubMed] [Google Scholar]
- 25.Brasaemle DL. Thematic review series: Adipocyte biology. The perilipin family of structural lipid droplet proteins: stabilization of lipid droplets and control of lipolysis. J Lipid Res. 2007;48:2547–2559. doi: 10.1194/jlr.R700014-JLR200. [DOI] [PubMed] [Google Scholar]
- 26.Ducharme NA, Bickel PE. Minireview: Lipid droplets in lipogenesis and lipolysis. Endocrinology. 2008;149:942–949. doi: 10.1210/en.2007-1713. [DOI] [PubMed] [Google Scholar]
- 27.Lazar MA. Becoming fat. Genes Dev. 2002;16:1–5. doi: 10.1101/gad.964002. [DOI] [PubMed] [Google Scholar]
- 28.Rosen ED, Walkey CJ, Puigserver P, Spiegelman BM. Transcriptional regulation of adipogenesis. Genes Dev. 2000;14:1293–1307. [PubMed] [Google Scholar]
- 29.Tontonoz P, Hu E, Spiegelman BM. Regulation of adipocyte gene expression and differentiation by peroxisome proliferator activated receptor gamma. Curr Opin Genet Dev. 1995;5:571–576. doi: 10.1016/0959-437x(95)80025-5. [DOI] [PubMed] [Google Scholar]
- 30.Cherkaoui-Malki M, et al. Identification of novel peroxisome proliferator-activated receptor alpha (PPARalpha) target genes in mouse liver using cDNA microarray analysis. Gene Expression. 2001;9:291–304. doi: 10.3727/000000001783992533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Viswakarma N, et al. Transcriptional regulation of Cidea, mitochondrial cell death-inducing DNA fragmentation factor alpha-like effector A, in mouse liver by peroxisome proliferator-activated receptor alpha and gamma. J Biol Chem. 2007;282:18613–18624. doi: 10.1074/jbc.M701983200. [DOI] [PubMed] [Google Scholar]
- 32.Boden G, Shulman GI. Free fatty acids in obesity and type 2 diabetes: Defining their role in the development of insulin resistance and beta-cell dysfunction. Eur J Clin Invest. 2002;32(Suppl 3):14–23. doi: 10.1046/j.1365-2362.32.s3.3.x. [DOI] [PubMed] [Google Scholar]
- 33.Perugini RA, et al. Metabolic characterization of nondiabetic severely obese patients undergoing Roux-en-Y gastric bypass: Preoperative classification predicts the effects of gastric bypass on insulin-glucose homeostasis. J Gastrointest Surg. 2007;11:1083–1090. doi: 10.1007/s11605-007-0158-3. [DOI] [PubMed] [Google Scholar]
- 34.Kim HJ, et al. Depot-specific regulation of perilipin by rosiglitazone in a diabetic animal model. Metabolism. 2007;56:676–685. doi: 10.1016/j.metabol.2006.12.017. [DOI] [PubMed] [Google Scholar]
- 35.Unger RH. Minireview: Weapons of lean body mass destruction: The role of ectopic lipids in the metabolic syndrome. Endocrinology. 2003;144:5159–5165. doi: 10.1210/en.2003-0870. [DOI] [PubMed] [Google Scholar]
- 36.Weisberg SP, et al. Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu H, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hotamisligil GS, Arner P, Caro JF, Atkinson RL, Spiegelman BM. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest. 1995;95:2409–2415. doi: 10.1172/JCI117936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ryden M, et al. Targets for TNF-alpha-induced lipolysis in human adipocytes. Biochem Biophys Res Commun. 2004;318:168–175. doi: 10.1016/j.bbrc.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 40.Dahlman I, et al. The CIDEA gene V115F polymorphism is associated with obesity in Swedish subjects. Diabetes. 2005;54:3032–3034. doi: 10.2337/diabetes.54.10.3032. [DOI] [PubMed] [Google Scholar]
- 41.Jiang ZY, et al. Insulin signaling through Akt/protein kinase B analyzed by small interfering RNA-mediated gene silencing. Proc Natl Acad Sci USA. 2003;100:7569–7574. doi: 10.1073/pnas.1332633100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Powelka AM, et al. Suppression of oxidative metabolism and mitochondrial biogenesis by the transcriptional corepressor RIP140 in mouse adipocytes. J Clin Invest. 2006;116:125–136. doi: 10.1172/JCI26040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Puri V, et al. RNAi-based gene silencing in primary mouse and human adipose tissues. J Lipid Res. 2007;48:465–471. doi: 10.1194/jlr.D600033-JLR200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.