Abstract
It has been hypothesized that insulin resistance is mediated by a deficiency of mitochondria in skeletal muscle. In keeping with this hypothesis, high-fat diets that cause insulin resistance have been reported to result in a decrease in muscle mitochondria. In contrast, we found that feeding rats high-fat diets that cause muscle insulin resistance results in a concomitant gradual increase in muscle mitochondria. This adaptation appears to be mediated by activation of peroxisome proliferator-activated receptor (PPAR)δ by fatty acids, which results in a gradual, posttranscriptionally regulated increase in PPAR γ coactivator 1α (PGC-1α) protein expression. Similarly, overexpression of PPARδ results in a large increase in PGC-1α protein in the absence of any increase in PGC-1α mRNA. We interpret our findings as evidence that raising free fatty acids results in an increase in mitochondria by activating PPARδ, which mediates a posttranscriptional increase in PGC-1α. Our findings argue against the concept that insulin resistance is mediated by a deficiency of muscle mitochondria.
Keywords: mitochondrial biogenesis, mitochondrial dysfunction, PPARδ, skeletal muscle, PGC-1α
It has been hypothesized that insulin resistance in patients with impaired or diabetic glucose tolerance is mediated by a deficiency of mitochondria in skeletal muscle (1, 2). The mechanism by which a decrease in mitochondria is proposed to cause insulin resistance is accumulation of intramyocellular lipids caused by a decrease in the capacity to oxidize fat (2). This hypothesis is based on the finding that type 2 diabetics and insulin-resistant individuals with impaired glucose tolerance have ≈30% less mitochondria in their muscles than insulin-sensitive control subjects (3–7). In support of this concept, recent studies have reported that raising serum free fatty acids (FFA) by a high-fat diet in humans (8), or by feeding mice or rats high-fat diets (8–10), results in decreases in skeletal muscle peroxisome proliferator-activated receptor γ coactivator-1α (PGC-1α) mRNA (8–10) and the mRNA levels of various mitochondrial constituents (8). In contrast, a number of earlier studies provided evidence that high-fat diets induce increases in mitochondrial marker enzymes (11–14), and Turner et al. (15) recently reported that a high-fat diet resulted in increases in mitochondrial biogenesis and fatty acid oxidative capacity in skeletal muscle of mice.
We have found that raising serum FFA in rats by feeding them a high-fat diet and giving them daily heparin injections results in an increase in muscle mitochondria (16). The initial purpose of the present study was to determine whether the more modest increase in FFA induced by a high-fat diet also results in increased mitochondrial biogenesis with an increase in the capacity of muscle to oxidize fat. We found that a high-fat diet does induce an increase in muscle mitochondria. This finding made it possible to evaluate whether a high-fat diet causes muscle insulin resistance despite increases in mitochondria and fat oxidative capacity.
Overexpression of peroxisome proliferator-activated receptor (PPAR)δ in muscles of transgenic mice, or activation of PPARδ in skeletal muscle in vivo by using a specific PPARδ activator, has been shown to result in increases in skeletal muscle mitochondria (17, 18). In these studies, an increase in muscle mitochondria occurred in the absence of an increase in PGC-1α mRNA (17, 18). This finding led to the conclusion that the increase in mitochondria is mediated directly by PPARδ (17–19). We have shown that raising serum FFA, which are endogenous ligands of PPARδ (20), results in activation of PPARδ, as evidenced by a large increase in PPARδ binding to the PPAR response element in the carnitine palmitoyl transferase 1 (CPT-1) promoter (16). Muscle CPT-1 expression is regulated by PPARδ in skeletal muscle (21). This finding, viewed in the context of the previous studies of PPARδ overexpression and activation, makes it probable that activation of PPARδ is the first step in the pathway by which raising FFA leads to an increase in mitochondria. However, it did not seem possible that PPARδ could directly induce mitochondrial biogenesis, because it regulates expression of a limited number of mitochondrial proteins. It is well established that the biogenesis of normal, functional mitochondria requires the activation of a number of transcription factors in addition to PPARδ (22–24). The coordinated activation of these transcription factors and, thus, the regulation of mitochondrial biogenesis, is mediated by the transcription coactivator PGC-1 (22, 24). In this context, another aim of this study was to test the hypothesis that raising FFA and overexpressing PPARδ both result in an increase in PGC-1α protein expression by a posttranscriptional mechanism.
Results
Body Weights, Intraabdominal Fat, and Serum Free Fatty Acids.
Feeding rats the high-fat diets for 4–5 wk was not sufficiently long to result in a significantly greater increase in body weight than occurred in chow-fed controls. However, the omental, epididymal, and retroperitoneal fat depots were significantly greater in the high-fat diet-fed groups than in the chow-fed group (7.7 ± 0.5 g vs. 12.2 ± 1.1 g, P < 0.01). The high-fat diet-fed rats had higher serum FFA levels than the chow-fed animals (756 ± 68 μM vs. 249 ± 24 μM, P < 0.01).
High-Fat Diets Induce an Increase in Skeletal Muscle Mitochondria.
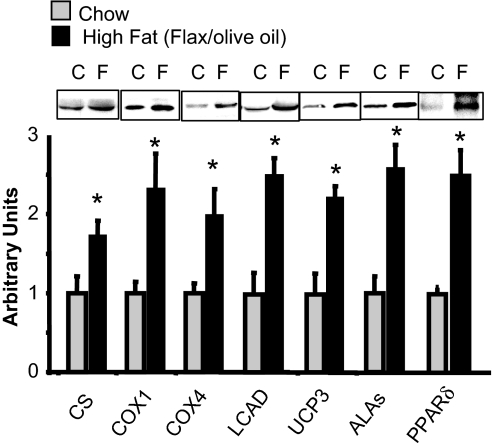
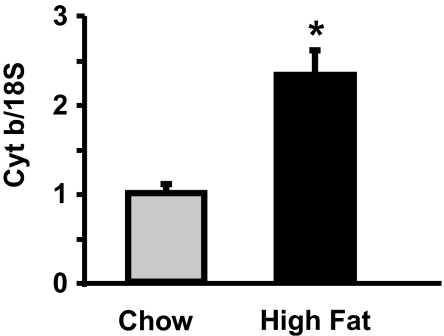
In our previous study of the effect of raising FFA on mitochondrial biogenesis in skeletal muscle, we fed rats a high-fat diet and gave them daily injections of heparin to raise serum FFA to high levels (16). The rationale for this approach was to try to mimic the effects of exercise on FFA levels. One purpose of the present study was to determine whether the more modest increase in FFA that results from feeding a high-fat diet also induces an increase in muscle mitochondria. As shown in Fig. 1, there were significant increases in a range of mitochondrial proteins in epitrochlearis muscles of rats fed the flaxseed/olive oil diet for 5 wk. There was also a significant increase in the capacity of skeletal muscle to oxidize palmitate [10.2 ± 1.8 (chow) vs. 17.8 ± 2.5 (high fat) nmol/min per g, P < 0.02], providing evidence for an increase in functional mitochondria. Further evidence for a high-fat diet-induced increase in muscle mitochondria is provided by the finding of an increase in mitochondrial DNA copy number, as evidenced by an increase in the ratio of cytochrome b to 18S DNA (Fig. 2).
Fig. 1.
A high-fat diet induces an increase in skeletal muscle mitochondria. Western blot analysis of mitochondrial proteins from triceps muscles of rats fed the flax seed oil/olive oil diet. Values are mean ± SE for 6–10 muscles. *, P < 0.05, high fat versus chow. C, control; F, high fat; COX1, cytochrome oxidase subunit 1; COX4, cytochrome oxidase subunit 4; ALAs, aminolevulinate synthase.
Fig. 2.
High-fat diet induces an increase in mitochondrial DNA copy number. Mitochondrial DNA copy number was evaluated in triceps muscles by determining the ratio of cytochrome b DNA to 18S DNA. Values are means ± SE for four rats per group. *, P < 0.05.
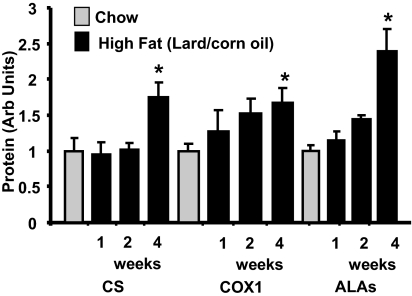
Because previous studies in which high-fat diets were reported to result in decreases in PGC-1α and/or muscle mitochondria used either lard- or milk-based diets (8–10), we also examined the effect of a diet containing 32% lard and 18% corn oil. As shown in [supporting information (SI) Fig. S1], feeding rats the lard/corn oil diet for 4 wk resulted in increases in a range of mitochondrial proteins in muscle.
The Flax Seed/Olive Oil Diet Induces Insulin Resistance of Muscle Glucose Transport.
The lard/corn oil diet and other high-fat diets induce insulin resistance of muscle glucose transport in rats within a few weeks and, depending on type of fat and genetic background, diabetes in rats (25–28). The flax seed/olive oil diet also results in decreases in insulin responsiveness of glucose transport in the slow-twitch red soleus muscle [2-deoxyglucose (2DG) transport: chow, 1.6 ± 0.2 (basal) and 5.9 ± 0.6 (insulin) μmol·ml−1·30 min−1 versus high fat, 1.4 ± 0.1 (basal) and 3.4 ± 0.3 (insulin) μmol·ml−1·30 min−1, P < 0.05] and the fast-twitch white epitrochlearis muscle [2DG transport: chow, 0.52 ± 0.07 (basal) and 2.5 ± 0.2 (insulin) μmol·ml−1·30 min−1 versus high fat, 0.53 ± 0.05 (basal) and 1.8 ± 0.14 (insulin) μmol·ml−1·30 min, P < 0.05]. Because the high-fat diet-induced increases in mitochondria and in the capacity to oxidize fat occurred during the period in which insulin resistance developed, the muscle insulin resistance is clearly not due to mitochondrial deficiency.
Time Course of the Increase in Muscle Mitochondria.
In contrast to induction of increases in mitochondrial biogenesis by other stimuli such as exercise (29–31), cold (32), or increases in cytosolic calcium (33), which occur rapidly (hours), the high-fat diet-induced increase in mitochondria occurs slowly (weeks). As shown in Fig. 3, no increases in expression of mitochondrial proteins were observed after 1 wk, and only small increases were present after 2 wk. Exceptions were long-chain acyl CoA dehydrogenase (LCAD) and uncoupling protein 3 (UCP3), which had increased 50–60% by 2 wk (data not shown). Transcription of LCAD and UCP3 is regulated by PPARδ (34, 35). A significant adaptive response of mitochondrial marker proteins had occurred by 4 wk (Fig. 3). The mRNA content of a range of mitochondrial markers was not different after 1 wk of fat feeding (data not shown), however, increases began to be evident by 2 wk and further increases were evident after 4 wk (Fig. S2 A and B).
Fig. 3.
A high-fat diet induces a gradual increase in muscle mitochondria. Time course of the increase in mitochondrial enzyme proteins in response to the high-fat, lard/corn oil diet. Values are means ± SE for four to eight muscles per group. *, P < 0.05 versus other groups. Values are means ± SE for four muscles per group. COX1, cytochrome oxidase subunit 1; ALAs, aminolevulinate synthase.
PGC-1α Response to the High-Fat Diets.
FFA are natural ligands of the PPARs (20). Both overexpression and activation of PPARδ result in increased mitochondrial biogenesis in the absence of an increase in PGC-1α mRNA in skeletal muscle in vivo (17–19). In these studies, PGC-1α protein was not measured, and it was concluded that overexpression or activation of PPARδ directly mediates mitochondrial biogenesis.
It did not seem possible to us that PPARδ could directly mediate mitochondrial biogenesis, because it regulates expression of only a subset of mitochondrial proteins. Mitochondrial biogenesis requires the coordinated expression of genes encoded in both the nuclear and mitochondrial genomes, and this process requires activation and/or increased expression of a number of transcription factors in addition to PPARδ (22–24). These include the nuclear respiratory factors 1 and 2 (NRF-1 and NRF-2), mitochondrial transcription factor A (TFAM), the myocyte specific enhancer factors (MEFs), and the estrogen related receptors (ERRs) (22–24). The coordinated increase in transcription of genes encoding mitochondrial proteins is mediated by the transcription coactivator PGC-1α, which activates the NRFs, ERRs, PPARs, and MEFs and, thus, plays the key role in mediating adaptive mitochondrial biogenesis (22–24).
In this context, we hypothesized that the increase in muscle mitochondria induced by activation or overexpression of PPARδ is mediated by induction of an increase in PGC-1α protein expression by a posttranscriptional mechanism. We have previously reported that raising FFA by means of a high-fat diet plus daily heparin injections for 4 wk does not result in an increase in PGC-1α mRNA in muscle (16). The high-fat diets also did not cause an increase in PGC-1α mRNA (Fig. S3). In fact, we observed a significant decrease in PGC-1α mRNA after 1 wk of a high-fat diet, with a return to the control level after 2 wk (Fig. S3). Other investigators have also reported that high-fat diets (8–10) bring about a decrease in PGC-1α mRNA.
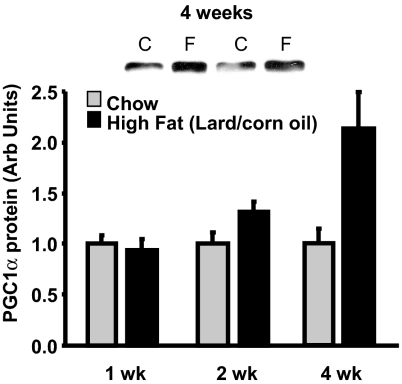
As shown in Fig. 4, there was a 2.5-fold increase in PGC-1α protein in muscle of rats fed the high-fat diets for 4 wk. In contrast to the induction of PGC-1α by other stimuli, such as exercise (29–31), cold (32), and calcium (33), which occurs rapidly (hours) and is mediated by increased transcription, the high-fat diet-induced increase in PGC-1α protein expression occurs slowly (weeks) and in the absence of an increase in PGC-1α mRNA. We used an anti-PGC-1α antibody obtained from Calbiochem in the Western blots shown in Fig. 4. To further confirm the finding of an increase in PGC-1α protein, we also used three other anti-PGC-1α antibodies directed at different amino acid sequences of the PGC-1α protein. The results obtained with all four antibodies show an increase in PGC-1α protein expression in muscles of the high-fat diet-fed animals (Fig. S4).
Fig. 4.
A high-fat diet induces a gradual increase in PGC-1α protein. Time course of the increase in PGC-1α protein in epitrochlearis muscles of rats fed the lard/corn oil diet. C, control; F, high fat. Values are means ± SE for four to eight muscles per group.
Overexpression of PPARδ in Skeletal Muscle Results in an Increase in PGC-1α Protein.
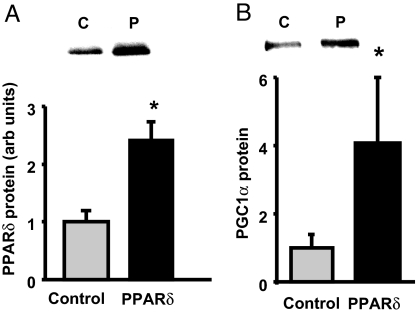
The finding that overexpression of PPARδ in skeletal muscle of transgenic mice does not result in an increase in PGC-1α mRNA led to the conclusion that the increase in mitochondria is mediated directly by PPARδ (17–19). In light of our finding that raising fatty acids, which activate PPARδ, induces a posttranscriptionally mediated increase in PGC-1α protein, it seemed important to determine whether overexpression of PPARδ also results in an increase in PGC-1α protein. Three weeks after electric pulse-mediated transfer of a full-length PPARδ gene into tibialis anterior muscle, PPARδ protein was increased ≈2-fold above control level (Fig. 5A). This increase in PPARδ protein resulted in a ≈4-fold increase in PGC-1α protein expression (Fig. 5B). As in previous studies (17, 18), overexpression of PPARδ did not result in an increase in PGC-1α mRNA (Fig. S5).
Fig. 5.
Overexpression of PPARδ in muscle results in an increase in PGC-1α protein. (A) PPARδ protein in tibialis anterior muscles in which PPARδ (P) was overexpressed by electric pulse field gene transfer. *, P < 0.05. (B) PGC-1α protein expression is increased in muscles overexpressing PPARδ. *, P < 0.05. Values are means ± SE for four muscles per group. C, control.
Discussion
The major findings of the first phase of this study are that feeding rats high-fat diets results in an increase in mitochondria and in the capacity of muscle to oxidize fat concomitant with development of muscle insulin resistance. It has been hypothesized that skeletal muscle insulin resistance is mediated by a mitochondrial deficiency that limits fat oxidation and results in accumulation of intramyocellular lipids (2). This concept is based on the finding that muscles of insulin-resistant individuals generally contain ≈30% less mitochondria than those of insulin-sensitive control subjects (3–7). This phenomenon has also been referred to as mitochondrial dysfunction (1, 2), although a detailed evaluation has provided evidence that the remaining mitochondria function normally (36). The present findings seem incompatible with the concept that muscle insulin resistance is mediated by mitochondrial deficiency.
Our findings that high-fat diets induce increases in PGC-1α protein, a range of mitochondrial proteins, and fat oxidative capacity in skeletal muscle of rats differ markedly from those of a recent study, in which feeding mice a high-fat diet for 3 wk was reported to decrease muscle mRNA levels of PGC-1α and mitochondrial respiratory chain constituents by ≈90% and PGC-1α and cytochrome c protein levels by ≈40% (8). However, a number of earlier studies provided evidence that high-fat diets result in an increase in mitochondrial enzymes in muscle (11–14), and we have shown that large increases in FFA induce an increase in mitochondrial biogenesis (16). Furthermore, Turner et al. (15) recently reported that, as in the present study, feeding rats a high-fat diet resulted in increases in mitochondrial enzyme activities, mitochondrial respiratory chain subunit protein levels, and fat oxidation capacity in skeletal muscle. Further evidence that fatty acids stimulate mitochondrial biogenesis comes from the finding that lowering serum FFA by giving acipimox to rats resulted in a ≈30% decrease in mRNA content of mitochondrial proteins (37) and that overexpression of lipoprotein lipase in muscle results in a massive increase in mitochondria (38).
It is surprising that the mitochondrial deficiency causes insulin resistance concept has been so widely accepted, because it seems untenable in the context of what is known regarding the capacity of skeletal muscle for oxidative metabolism. The mechanism by which a 30% decrease in muscle mitochondria has been proposed to cause muscle insulin resistance is an impairment in the ability to oxidize fat, resulting in accumulation of intramyocellular lipids (1, 2). Actually, the rate of substrate oxidation in resting muscle is not determined/limited by mitochondrial oxidative capacity but by the rate of ATP breakdown/ADP formation, which is regulated by the cells' need for energy (39). The energy/substrate requirement of resting muscle cells is determined by “housekeeping” functions, such as maintenance of transmembrane potential by the Na+/K+ ATPase, protein synthesis, etc., and is very low relative to the maximal capacity of muscle for substrate oxidation. Increasing the supply of FFA or glucose to resting muscle can change the relative proportions of these substrates that are oxidized but does not result in an increase in substrate oxidation above that required to supply the energy needed for ATP repletion, regardless of its content of mitochondria.
Oxygen utilization by limb skeletal muscles when individuals are exercising at a work rate that elicits their maximal capacity to use oxygen (VO2max) during running or cycling is in the range of 200 ml·kg muscle−1·min−1 for sedentary and 400 ml·kg muscle−1·min−1 for well trained individuals (40). Overweight, middle-aged patients with insulin resistance are generally in poor physical condition and have a low VO2max, in the range of 22–26 ml of O2·kg body wt−1·min·1 (7). If one assumes that such an individual has an extremely low muscle capacity to use oxygen of 100 ml·kg−1·min−1 that is only 50% as great as that of normal sedentary individuals, this still represents a 33-fold increase above resting muscle oxygen uptake, which is ≈3 ml·kg muscle−1·min−1. In light of the enormous difference in the capacity of muscle to oxidize substrate and the rate of substrate oxidation required to regenerate ATP in resting muscle, a 30% decrease in muscle mitochondria is irrelevant in terms of the ability of resting muscle to oxidize fat.
On the other hand, in pathological states in which mitochondrial number and/or function are so severely reduced as to limit the rate of substrate oxidation in resting muscle, both basal- and insulin-stimulated glucose transport are increased (41, 42) despite massive accumulation of intramyocellular lipid (41). Thus, mitochondrial deficiency and dysfunction sufficiently severe to limit fat oxidation increases, rather than decreases, insulin action. Similarly, severe hypoxia, which can be thought of as the ultimate degree of mitochondrial dysfunction, markedly increases basal- and insulin-stimulated glucose transport (43, 44). These effects of mitochondrial deficiency/dysfunction are mediated by activation of AMP-dependent protein kinase (AMPK) (41, 42, 44), which stimulates glucose transport and induces an increase in expression of the GLUT4 glucose transporter (45, 46).
If increases in FFA do not mediate a decrease in mitochondria, what is responsible for the lower than usual amount of mitochondria in skeletal muscle of insulin-resistant patients? Nair and coworkers (6) have hypothesized that, rather than causing insulin resistance, a reduction in mitochondrial content is mediated by insulin resistance. Although our findings shed no light on this question, we think it is likely that exercise deficiency is the cause of the mitochondrial deficiency. The amount of mitochondria in muscle can vary over a considerable range, increasing with endurance exercise training and decreasing with physical inactivity (47). The insulin resistance syndrome and type 2 diabetes are, to a large extent, mediated by exercise deficiency in genetically predisposed individuals who do not balance reduced energy expenditure with reduced energy intake, resulting in obesity.
The central finding of the second phase of this study is that a high-fat diet induces an increase in muscle mitochondria by increasing the expression of PGC-1α protein via a posttranscriptional mechanism. We have shown that raising FFA levels activates PPARδ in skeletal muscle in vivo, as evidenced by increased binding to the PPAR response element in the carnitine palmitoyl transferase 1 promoter (16). This finding is not surprising because fatty acids are endogenous ligands responsible for activating the PPARs (20). In the studies on mouse skeletal muscle in vivo, in which PPARδ was overexpressed or activated by using a PPARδ ligand, mitochondrial biogenesis increased in the absence of an increase in PGC-1α mRNA (17, 18). Similarly, raising serum FFA (16) and feeding rats high-fat diets resulted in increased biogenesis of mitochondria in skeletal muscle despite no increase in PGC-1α mRNA. Because it did not seem possible that PPARδ could directly mediate mitochondrial biogenesis, we hypothesized that increases in PPARδ expression and/or activity bring about an increase in PGC-1α protein expression by a posttranscriptional mechanism. This hypothesis proved correct, because we found that both the high-fat diets and the increased expression of PPARδ induce increases in skeletal muscle PGC-1α protein expression.
It seems likely that the reason that the increase in PGC-1α protein was missed in previous studies is that it has become common practice to just measure mRNA levels and to refer to increases in gene transcription as increased gene expression. This approach ignores the fact that genes are expressed as proteins and that gene expression can be regulated at a number of steps during translation as well as posttranslationally. As an example of posttranslational regulation, it has been shown that overexpression of calpastatin, the endogenous inhibitor of the protease calpain, results in large increases in expression of GLUT4, CAMKII, and AMPK proteins despite a decrease or no change in their mRNA levels (48, 49).
The regulation of PGC-1α expression by PPARδ differs markedly between skeletal muscle in vivo and myotubes in culture. In contrast to the finding in mouse and rat skeletal muscle that PPARδ overexpression or activation does not result in an increase in PGC-1α mRNA, a number of studies have shown that PPARδ induces an increase in PGC-1α gene transcription, with a rapid increase in PGC-1α mRNA, in myotubes (19, 50, 51). This remarkable difference is currently unexplained, but it does show that skeletal muscle and myotubes have important differences that make it inappropriate to refer to myotubes as “skeletal muscle” and shows that findings on myotubes cannot always be directly extrapolated to skeletal muscle.
In conclusion, our results show that a high-fat diet that raises FFA results in a gradual increase in mitochondria in rat skeletal muscle, with an increase in the capacity for fat oxidation, concomitant with development of muscle insulin resistance. This finding argues against the concept that muscle insulin resistance is mediated by a deficiency of muscle mitochondria. We interpret our findings as evidence that the increase in muscle mitochondria induced by raising FFA levels is mediated by activation of PPARδ, which brings about a gradual increase in PGC-1α expression by a posttranscriptional mechanism.
Materials and Methods
Animal Care.
This work was approved by the Animal Studies Committee of Washington University School of Medicine. Male Wistar rats weighing ≈50 g were housed 2 or 3 per cage with a 12:12 light/dark cycle. Animals were given ad libitum access to one of three diets, a standard chow diet (PicoLab Rodent Diet 20, 5053) or one of two high-fat diets. The standard chow diet (chow) consisted of 23.5% calories from protein, 11.9% calories from fat, and 64.5% calories from carbohydrate. The high-fat flax seed/olive oil diet consisted of 20% calories from protein (243 g/kg casein), 50% calories from fat (187.5 g/kg flax seed oil and 93.7 g/kg olive oil), and 30% of calories from carbohydrate (210.8 g/kg corn starch, 105.4 g/kg sucrose, and 59.6 g/kg bran). The high-fat lard/corn oil diet consisted of the following: 23% calories from protein (294.5 g/kg casein), 50% calories from fat (180g/kg lard and 100g/kg corn oil), and 27% calories from carbohydrate (347.4 g/kg sucrose). Both of the high-fat diets included 22 g of vitamin mix (Teklad Premier #40077), 51 g of mineral mix (Teklad Premier #170915), 5 g of methionine (Teklad Premier #10850), and 1.3 g of choline chloride per kg of diet.
Measurement of Glucose Transport Activity.
Rats fed the high-fat flax/olive oil diet were used to measure the skeletal muscle insulin responsiveness. On the evening before the experiment, animals had food removed at 1800 hours. Rats were anesthetized with 50 mg/kg sodium pentobarbital and epitrochlearis muscles and soleus strips were dissected out for in vitro incubation. Maximum insulin stimulated (2 milliunits/ml) glucose transport activity was measured by using the glucose analog 2-deoxy-d-[3H]glucose (2DG), and [U-14C]mannitol (0.3 μCi/ml) was used to calculate extracellular space as described previously (52).
Measurement of Palmitate Oxidation.
The capacity of muscle to oxidize fatty acids was determined by measuring the rate of [14C]CO2 production from [14C] palmitate as described previously (53–55).
Western Blotting.
Muscles were homogenized in ice-cold buffer containing the following: 250 mM sucrose, 10 mM HEPES/1 mM EDTA (pH 7.4), 1 mM each of Pefabloc (Roche), EDTA, and NaF, 1 μg/ml each of aprotinin, leupeptin, and pepstatin, 0.1 mM bpV(phen), and 2 mg/ml β-glycerophosphate. Homogenates were subjected to three freeze/thaw cycles and centrifuged for 10 min. at 700 × g. Protein concentration was determined by using the Lowry Method (56). Aliquots were solubilized in Laemmli buffer and subjected to SDS/PAGE. The following antibodies were used for immunoblotting: δ aminolevulinate synthase (Alpha Diagnostic), cytochrome oxidase subunit 1, cytochrome oxidase subunit IV (Molecular Probes), citrate synthase (CS) (Alpha Diagnostic), PGC-1α [516557 (Calbiochem), sc-13067 (Santa Cruz), AB3242 (Upstate), and a gift from Dan Kelly (Washington University Medical School)], UCP3 (Chemicon), PPARδ (Affinity Bioreagents), or LCAD (a gift from Dan Kelly). After incubation with the appropriate secondary antibody, bands were visualized by ECL and quantified by densitometry.
Semiquantitative RT-PCR.
PGC-1α mRNA was measured by semiquantitative RT-PCR as described previously (57). The sense and antisense primers for PGC1α were 5′-GTGCAGCCAAGACTCTGTATGG-3′ (forward) and 5′-GTCCAGGTCATTCACATCAAGTTC-3′ (reverse). Muscle RNA isolated from a subset of animals was subjected to a RT2 Profiler Custom PCR Array (SuperArray Bioscience) designed to simultaneously measure eight mitochondria-related transcripts as well as two housekeeping transcripts [β-actin and ribosomal protein L10A (RPL10)], according to the manufacturer's protocol (SuperArray Bioscience). The data were normalized to the housekeeping genes by the ΔΔCt method as described in the RT2 Profiler protocol.
Muscle Mitochondrial DNA Content.
Muscle mitochondrial DNA content was determined as described previously (16). Briefly, DNA isolation was performed by using organic extraction (58). PCR was performed by using an 18S primer for a nuclear marker (catalog no. 1718; Ambion) and primers for cytochrome b (forward, ACAAAATCCCATTCCATCCA; reverse, GTTGGGAATGGAGCGTAGAA). PCR was performed with Promega master mix containing Taq polymerase, dNTPs, MgCl2, and PCR buffers.
Plasmid DNA Constructs.
To add myc-tag to the N terminus of PPARδ, PPARδ cDNA (Origene) was PCR cloned with the forward primer, 5′-CACCATGGAACAAAAACTCATCTCAGAAGAGGATCTGGAGCAGCCACAGGAGGAAGC-3′, and reverse primer, 5′-TTAGTACATGTCCTTGTAGATCTCC-3′. The PCR fragment was inserted into pCR2.1Topo (Invitrogen). For the construction of the pCAG/myc-ppard, EcorI fragment of pCR2.1/myc-ppard was inserted into EcorI site of pCAGEN (59). The plasmid DNAs were transformed and amplified by using DH-5α bacteria and purified with a plasmid maxi kit (Qiagen). To generate adenovirus-bearing myc-PPARδ cDNA, the PCR fragment was subcloned into the pENTER/D-Topo vector and cDNA inserts were transferred to a pAdCMV/V5-DEST vector by using LR clonase (Invitrogen). Adenovirus was generated by following vendor's instructions.
PPARδ DNA Electroporation.
The overexpression of PPARδ in the tibialis anterior (TA) muscle was accomplished by using an electric pulse-mediated gene transfer technique (60). Rats were anesthetized with isoflurane gas. The tibialis anterior (TA) muscle was injected with 100 μg of plasmid DNA containing the PARδ-myc or empty vector in 0.1 ml of saline by using a 28-gauge needle at a rate of 0.04 ml/min. After the injection, an electric field was applied to the TA muscle by using a S88 square-pulse stimulator (Grass) with a 533 model two-needle array (BTX). The electric field application consisted of eight pulses of 100-ms duration (at a frequency of 1 Hz and amplitude of 100 volts), which were applied perpendicular to the long axis of the muscle. Muscles were harvested 3 wk after electroporation.
Immunoprecipitation of myc PPARδ.
Three weeks after myc PPARδ gene transfer, tibialis anterior muscles were homogenized in ice-cold buffer. Homogenates were rotated at 4°C for 1 h then centrifuged at 13,000 × g for 15 min. Supernatant fraction was collected and protein content was measured. Homogenate samples containing 1 mg of protein were incubated overnight with anti-mycTag antibody (catalog no. 2276; Cell Signaling Technology). The next morning, 20 μl of protein A/G magnetic beads (Bioclone Inc.) were added, and samples were incubated for 3 h. After four 1-ml washes, samples were diluted in Laemmli buffer, boiled for 5 min, and subjected to SDS/PAGE.
Statistical Analysis.
Results are presented as means ± standard error. Comparisons between control and treatment were made by using an unpaired Student t test.
Supplementary Material
Acknowledgments.
C.R.H. was supported by an American Diabetes Association Mentor-Based Postdoctoral Fellowship and Individual National Research Service Award DK076410; D.C.W. was supported by an American Diabetes Association Mentor-Based Postdoctoral Fellowship and Individual National Research Service Award DK070425. This work was supported by National Institutes of Health Grants AG000425 and DK18986.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
See Commentary on page 7627.
This article contains supporting information online at www.pnas.org/cgi/content/full/0802057105/DCSupplemental.
References
- 1.Morino K, Petersen KF, Shulman GI. Molecular mechanisms of insulin resistance in humans and their potential links with mitochondrial dysfunction. Diabetes. 2006;55:S9–S15. doi: 10.2337/db06-S002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lowell BB, Shulman GI. Mitochondrial dysfunction and type 2 diabetes. Science. 2005;307:384–387. doi: 10.1126/science.1104343. [DOI] [PubMed] [Google Scholar]
- 3.Patti M-E, et al. Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: Potential role of PGC1 and NRF1. Proc Natl Acad Sci USA. 2003;100:104–109. doi: 10.1073/pnas.1032913100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Petersen KF, et al. Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. N Eng J Med. 2004;350:664–671. doi: 10.1056/NEJMoa031314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kelley DE, He J, Menshikova EV, Ritov VB. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes. 2002;51:2944–2950. doi: 10.2337/diabetes.51.10.2944. [DOI] [PubMed] [Google Scholar]
- 6.Asmann YW, et al. Skeletal muscle mitochondrial functions, mitochondrial DNA copy numbers, and gene transcript profiles in type 2 diabetic and nondiabetic subjects at equal levels of low or high insulin and euglycemia. Diabetes. 2006;55:3309–3319. doi: 10.2337/db05-1230. [DOI] [PubMed] [Google Scholar]
- 7.Mootha VK, et al. PGC-1α-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nat Genet. 2003;34:267–273. doi: 10.1038/ng1180. [DOI] [PubMed] [Google Scholar]
- 8.Sparks LM, et al. A high-fat diet coordinately downregulates genes required for mitochondrial oxidative phosphorylation in skeletal muscle. Diabetes. 2005;54:1926–1933. doi: 10.2337/diabetes.54.7.1926. [DOI] [PubMed] [Google Scholar]
- 9.Crunkhorn S, et al. PGC-1 expression is reduced in obesity: potential pathogenic role of saturated fatty acids and p38 MAP kinase activation. J Biol Chem. 2007;282:15439–15450. doi: 10.1074/jbc.M611214200. [DOI] [PubMed] [Google Scholar]
- 10.Koves TR, et al. Peroxisome proliferator-activated receptor-γ co-activator 1α-mediated metabolic remodeling of skeletal myocytes mimics exercise training and reverses lipid-induced mitochondrial inefficiency. J Biol Chem. 2005;280:33588–33598. doi: 10.1074/jbc.M507621200. [DOI] [PubMed] [Google Scholar]
- 11.Miller WC, Bryce GR, Conlee RK. Adaptations to a high-fat diet that increase exercise endurance in male rats. J Appl Physiol. 1984;56:78–83. doi: 10.1152/jappl.1984.56.1.78. [DOI] [PubMed] [Google Scholar]
- 12.Nemeth PM, et al. Metabolic response to a high-fat diet in neonatal and adult rat muscle. Am J Physiol. 1992;262:C282–C286. doi: 10.1152/ajpcell.1992.262.2.C282. [DOI] [PubMed] [Google Scholar]
- 13.McAinch AJ, et al. Dietary regulation of fat oxidative gene expression in different skeletal muscle fiber types. Obesity Res. 2003;11:1471–1479. doi: 10.1038/oby.2003.197. [DOI] [PubMed] [Google Scholar]
- 14.Simi B, Sempore B, Mayet M-H, Favier RJ. Additive effects of training and high-fat diet on energy metabolism during exercise. J Appl Physiol. 1991;71:197–203. doi: 10.1152/jappl.1991.71.1.197. [DOI] [PubMed] [Google Scholar]
- 15.Turner N, et al. Exess lipid availability increases mitochondrial fatty acid oxidative capacity in muscle. Evidence against a role for reduced fatty acid oxidation in lipid-induced insulin resistance in rodents. Diabetes. 2007;56:2085–2092. doi: 10.2337/db07-0093. [DOI] [PubMed] [Google Scholar]
- 16.Garcia-Roves PM, et al. Raising plasma fatty acid concentration induces increased biogenesis of mitochondria in skeletal muscle. Proc Natl Acad Sci USA. 2007;104:10709–10713. doi: 10.1073/pnas.0704024104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luquet S, et al. Peroxisome proliferator-activated receptor δ controls muscle development and oxydative capability. FASEB J. 2003;17:2299–2301. doi: 10.1096/fj.03-0269fje. [DOI] [PubMed] [Google Scholar]
- 18.Wang Y-X, et al. Regulation of muscle fiber type and running endurance by PPARδ. PLoS Biol. 2004;2:1532–1539. doi: 10.1371/journal.pbio.0020294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barish GD, Narkar VA, Evans RM. PPARδ: A dagger in the heart of the metabolic syndrome. J Clin Invest. 2006;116:590–597. doi: 10.1172/JCI27955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Forman BM, Chen J, Evans RM. Hypolipidemic drugs, polyunsaturated fatty acids, and eicosanoids are ligands for peroxisome proliferator-activated receptors α and δ. Proc Natl Acad Sci USA. 1997;94:4312–4317. doi: 10.1073/pnas.94.9.4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dressel U, et al. The peroxisome proliferator-activted receptor β/δ agonist, GW501516, regulates the expression of genes involved in lipid catabolism and energy uncoupling in skeletal muscle cells. Mol Endocrinol. 2003;17:2477–2493. doi: 10.1210/me.2003-0151. [DOI] [PubMed] [Google Scholar]
- 22.Kelly DP, Scarpulla RC. Transcriptional regulatory circuits controlling mitochondrial biogenesis and function. Genes Dev. 2004;18:357–368. doi: 10.1101/gad.1177604. [DOI] [PubMed] [Google Scholar]
- 23.Scarpulla RC. Nuclear control of respiratory gene expression in mammalian cells. J Cell Biochem. 2006;97:673–683. doi: 10.1002/jcb.20743. [DOI] [PubMed] [Google Scholar]
- 24.Lin J, Handschin C, Spiegelman BM. Metabolic control through the PGC-1 family of transcription coactivators. Cell Metab. 2005;1:361–370. doi: 10.1016/j.cmet.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 25.Storlien LH, et al. Fat-feeding causes widespread in vivo insulin resistance, decreased energy expenditure, and obesity in rats. Am J Physiol. 1986;251:E576–E583. doi: 10.1152/ajpendo.1986.251.5.E576. [DOI] [PubMed] [Google Scholar]
- 26.Han D-H, Hansen PA, Host HH, Holloszy JO. Insulin resistance of muscle glucose transport in rats fed a high-fat diet: A reevaluation. Diabetes. 1997;46:1761–1767. doi: 10.2337/diab.46.11.1761. [DOI] [PubMed] [Google Scholar]
- 27.Hansen PA, et al. A high-fat diet impairs stimulation of glucose transport in muscle. Functional evaluation of potential mechanisms. J Biol Chem. 1998;273:26157–26163. doi: 10.1074/jbc.273.40.26157. [DOI] [PubMed] [Google Scholar]
- 28.Rosholt MN, King PA, Horton ES. High-fat diet reduces glucose transporter responses to both insulin and exercise. Am J Physiol. 1994;266:R95–R101. doi: 10.1152/ajpregu.1994.266.1.R95. [DOI] [PubMed] [Google Scholar]
- 29.Pilegaard H, Saltin B, Neufer PD. Exercise induces transient transcriptional activation of the PGC-1α gene in human skeletal muscle. J Physiol. 2003;546:851–858. doi: 10.1113/jphysiol.2002.034850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Akimoto T, et al. Exercise stimulates Pgc-1α transcription in skeletal muscle through activation of the p38 MAPK pathway. J Biol Chem. 2005;280:19587–19593. doi: 10.1074/jbc.M408862200. [DOI] [PubMed] [Google Scholar]
- 31.Wright DC, et al. Exercise-induced mitochondrial biogenesis begins before the increase in muscle PGC-1α expression. J Biol Chem. 2007;282:194–199. doi: 10.1074/jbc.M606116200. [DOI] [PubMed] [Google Scholar]
- 32.Cao W, et al. p38 mitogen-activated protein kinase is the central regulator of cyclic AMP-dependent transcription of the brown fat uncoupling protein 1 gene. Mol Cell Biol. 2004;24:3057–3067. doi: 10.1128/MCB.24.7.3057-3067.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wright DC, et al. Calcium induces increases in peroxisome proliferator-activated receptor γ coactivator-1α and mitochondrial biogenesis by a pathway leading to p38 mitogen-activated protein kinase activation. J Biol Chem. 2007;282:18793–18799. doi: 10.1074/jbc.M611252200. [DOI] [PubMed] [Google Scholar]
- 34.Evans RM, Barish GD, Wang Y-X. PPARs and the complex journey to obesity. Nat Med. 2004;10:1–7. doi: 10.1038/nm1025. [DOI] [PubMed] [Google Scholar]
- 35.Wang Y-X, et al. Peroxisome-proliferator-activated receptor δ activates fat metabolism to prevent obesity. Cell. 2003;113:159–170. doi: 10.1016/s0092-8674(03)00269-1. [DOI] [PubMed] [Google Scholar]
- 36.Boushel R, et al. Patients with type 2 diabetes have normal mitochondrial function in skeletal muscle. Diabetologia. 2007;50:790–796. doi: 10.1007/s00125-007-0594-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bajaj M, et al. Paradoxical changes in muscle gene expression in insulin-resistant subjects after sustained reduction in plasma free fatty acid concentration. Diabetes. 2007;56:743–752. doi: 10.2337/db06-0840. [DOI] [PubMed] [Google Scholar]
- 38.Levak-Frank S, et al. Muscle-specific overexpression of lipoprotein lipase causes a severe myopathy characterized by proliferation of mitochondria and peroxisomes in transgenic mice. J Clin Invest. 1995;96:976–986. doi: 10.1172/JCI118145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McGilvery RW. Biochemistry: A Functional Approach. Philadelphia: Saunders; 1970. [Google Scholar]
- 40.Anderson P, Saltin B. Maximal perfusion of skeletal muscle in man. J Physiol (London) 1985;366:233–249. doi: 10.1113/jphysiol.1985.sp015794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Han D-H, et al. UCP-mediated energy depletion in skeletal muscle increases glucose transport despite lipid accumulation and mitochondrial dysfunction. Am J Physiol. 2004;286:E347–E353. doi: 10.1152/ajpendo.00434.2003. [DOI] [PubMed] [Google Scholar]
- 42.Wredenberg A, et al. Respiratory chain dysfunction in skeletal muscle does not cause insulin resistance. Biochem Biophys Res Commun. 2006;350:202–207. doi: 10.1016/j.bbrc.2006.09.029. [DOI] [PubMed] [Google Scholar]
- 43.Cartee GD, et al. Stimulation of glucose transport in skeletal muscle by hypoxia. J Appl Physiol. 1991;70:1593–1600. doi: 10.1152/jappl.1991.70.4.1593. [DOI] [PubMed] [Google Scholar]
- 44.Fisher JS, et al. Activation of AMP kinase enhances sensitivity of muscle glucose transport to insulin. Am J Physiol. 2002;282:E18–E23. doi: 10.1152/ajpendo.2002.282.1.E18. [DOI] [PubMed] [Google Scholar]
- 45.Winder WW, Hardie DG. AMP-activated protein kinase, a metabolic master switch: Possible roles in Type 2 diabetes. Am J Physiol. 1999;277:E1–E10. doi: 10.1152/ajpendo.1999.277.1.E1. [DOI] [PubMed] [Google Scholar]
- 46.Winder WW, Thomson DM. Cellular energy sensing and signaling by AMP-activated protein kinase. Cell Biochem Biophys. 2007;47:332–347. doi: 10.1007/s12013-007-0008-7. [DOI] [PubMed] [Google Scholar]
- 47.Holloszy JO, Coyle EF. Adaptations of skeletal muscle to endurance exercise and their metabolic consequences. J Appl Physiol. 1984;56:831–839. doi: 10.1152/jappl.1984.56.4.831. [DOI] [PubMed] [Google Scholar]
- 48.Otani K, et al. Calpain system regulates muscle mass and glucose transporter GLUT4 turnover. J Biol Chem. 2004;279:20915–20920. doi: 10.1074/jbc.M400213200. [DOI] [PubMed] [Google Scholar]
- 49.Otani K, Polonsky KS, Holloszy JO, Han D-H. Inhibition of calpain results in impaired contraction-stimulated GLUT4 translocation in skeletal muscle. Am J Physiol. 2006;291:E544–E548. doi: 10.1152/ajpendo.00510.2005. [DOI] [PubMed] [Google Scholar]
- 50.Dimopoulos N, Watson M, Green C, Hundal HS. The PPARδ agonist, GW501516, promotes fatty acid oxidation but has no direct effect on glucose utilisation or insulin sensitivity in rat L6 skeletal muscle cells. FEBS Lett. 2007;581:4743–4748. doi: 10.1016/j.febslet.2007.08.072. [DOI] [PubMed] [Google Scholar]
- 51.Hondares E, et al. PPARδ, but not PPARα, activates PGC-1α gene transcription in muscle. Biochem Biophys Res Commun. 2007;354:1021–1027. doi: 10.1016/j.bbrc.2007.01.092. [DOI] [PubMed] [Google Scholar]
- 52.Young DA, Uhl JJ, Cartee GD, Holloszy JO. Activation of glucose transport in muscle by prolonged exposure to insulin: Effects of glucose and insulin concentration. J Biol Chem. 1986;261:16049–16053. [PubMed] [Google Scholar]
- 53.Baar K, et al. Skeletal muscle overexpression of nuclear respiratory factor 1 increases glucose transport capacity. FASEB J. 2003;17:1666–1673. doi: 10.1096/fj.03-0049com. [DOI] [PubMed] [Google Scholar]
- 54.Molé PA, Oscai LB, Holloszy JO. Adaptation of muscle to exercise. Increase in levels of palmityl CoA synthetase, and in the capacity to oxidize fatty acids. J Clin Invest. 1971;50:2323–2330. doi: 10.1172/JCI106730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ojuka EO, et al. Intermittent increases in cytosolic Ca2+ stimulate mitochondrial biogenesis in muscle cells. Am J Physiol. 2002;283:E1040–E1045. doi: 10.1152/ajpendo.00242.2002. [DOI] [PubMed] [Google Scholar]
- 56.Lowry OH, Rosenbrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 57.Terada S, Wicke S, Holloszy JO, Han D-H. The PPARδ activator GW505516 has no acute effect on glucose transport in skeletal muscle. Am J Physiol. 2006;290:E607–E611. doi: 10.1152/ajpendo.00430.2005. [DOI] [PubMed] [Google Scholar]
- 58.Strauss WM. Preparation of genomic DNA from mammalian tissue. In: Ausubel FM, editor. Current Protocols in Molecular Biology. New York: Wiley; 1998. pp. 2.2.1–2.2.3. [DOI] [PubMed] [Google Scholar]
- 59.Matsuda T, Cepko CL. Electroporation and RNA interference in the rodent retina in vivo and in vitro. Proc Natl Acad Sci USA. 2004;101:16–22. doi: 10.1073/pnas.2235688100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Akimoto T, Sorg BS, Yan Z. Real-time imaging of peroxisome proliferator-activated receptor-gamma coactivator-1α promoter activity in skeletal muscles of living mice. Am J Physiol. 2004;287:C790–C796. doi: 10.1152/ajpcell.00425.2003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.