Abstract
Adaptation of photosynthesis in marine environment has been examined in two strains of the green, picoeukaryote Ostreococcus: OTH95, a surface/high-light strain, and RCC809, a deep-sea/low-light strain. Differences between the two strains include changes in the light-harvesting capacity, which is lower in OTH95, and in the photoprotection capacity, which is enhanced in OTH95. Furthermore, RCC809 has a reduced maximum rate of O2 evolution, which is limited by its decreased photosystem I (PSI) level, a possible adaptation to Fe limitation in the open oceans. This decrease is, however, accompanied by a substantial rerouting of the electron flow to establish an H2O-to-H2O cycle, involving PSII and a potential plastid plastoquinol terminal oxidase. This pathway bypasses electron transfer through the cytochrome b6f complex and allows the pumping of “extra” protons into the thylakoid lumen. By promoting the generation of a large ΔpH, it facilitates ATP synthesis and nonphotochemical quenching when RCC809 cells are exposed to excess excitation energy. We propose that the diversion of electrons to oxygen downstream of PSII, but before PSI, reflects a common and compulsory strategy in marine phytoplankton to bypass the constraints imposed by light and/or nutrient limitation and allow successful colonization of the open-ocean marine environment.
Keywords: marine environment, PTOX, water cycle, eletron flow, photoprotection
Picoplankton is defined as a unicellular organism with a cell size of 0.2–3 μm (1). Studies relating to the ecology of this group have greatly expanded over the last decade and suggest that these tiny organisms play major roles in biogeochemical cycling in the oceans, especially in oligotrophic areas (1). Picoplankton communities contain both prokaryotic and eukaryotic organisms that can be either heterotrophic or autotrophic. Although picoeukaryotes are a minor component of picoplankton communities with respect to cell number, they are major contributors to the primary productivity in coastal areas (1, 2). Within picoeukaryotes, the smallest known organism belongs to the genus Ostreococcus (Prasinophyceae, Mamiellales). This genus has a cosmopolitan distribution, and several strains have been isolated or detected in samples of diverse geographical origins (3). The genome of two of these strains has been sequenced recently (4, 5). Ostreococcus tauri, OTH95 was the first characterized strain, isolated in 1995 from a coastal Mediterranean lagoon in France (6, 7). It is a naked, non-flagellated cell with a single mitochondrion and chloroplast. The chloroplast has simple structural features relative to that of plants; it is reduced in size, with only three layers of stacked thylakoid membranes (7). Furthermore, whereas genes encoding LHCI and prasinophyte-specific, chlorophyll-binding proteins (LHCPs) are present in the O. tauri OTH95 genome, the LHCII genes are absent (8, 9).
Since the description of this first strain, several morphologically indistinguishable Ostreococcus isolates originating from surface or deep waters have been established in culture. By analogy with the prokaryotic cyanobacterium Prochlorococcus (10, 11), these strains have been defined as low- or high-light strains (12). Although the low- and high-light Ostreococcus strains exhibit differences in their growth characteristics under various light regimes, it is not clear whether these differences reflect long-term adaptations (speciation) or transient acclimation processes. Furthermore, there is little information concerning the photosynthetic properties of Ostreococcus; these properties would strongly influence growth characteristics and the ecologic niche in which these organisms could thrive.
Here, we show that photosynthesis in Ostreococcus has been largely and constitutively shaped by the environment, as evidenced by the comparison between the surface/high-light strain O. tauri OTH95 and the deep/low-light oceanic strain Ostreococcus RCC809. In the former, photosynthesis is very similar to that observed in plants and freshwater algae. Conversely, RCC809 is prone to overreduction of the photosynthetic chain because of an increased light absorption and diminished electron flow capacity as a consequence of reduced cellular PSI content. This leads to increased photosensitivity, which is actively counterbalanced by a singular photoprotection mechanism that involves bypassing the PSI limitation by establishing a H2O-to-H2O cycle: A substantial fraction of PSII-generated electrons are rerouted to oxygen, thanks to the activity of a plastoquinol terminal oxidase-like enzyme operating upstream of the cytochrome b6 f complex.
Results
The Low-Light/Deep-Sea RCC809 Strain of Ostreococcus Exhibits a Reduced Ability to Acclimate to High-Light Intensities.
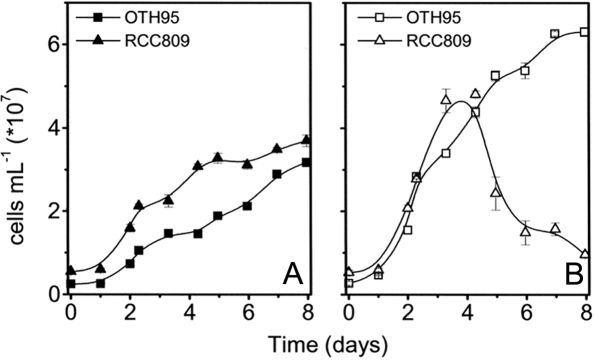
The surface/high-light strain O. tauri OTH95 was the first strain described in the genus (6, 7), and its genome was recently sequenced (4). The Ostreococcus deep/low-light strain RCC809 was isolated at 105 m of depth from the tropical zone of the Atlantic ocean. Although the two strains show similar morphologic features, they belong to different clades according to their ribosomal RNA sequences. Moreover, they show different growth capacities under high irradiance (12). Both Ostreococcus strains can sustain growth for 4 d under light intensities of 10–100 μE m−2 s−1 (12) (Fig. 1). However, we observed a strong photosensitivity in the deep sea RCC809 strain (Fig. 1B). Although this strain is still viable after 4 d at 100 μE m−2 s−1, the cells could not be rejuvenated after 8–10 d at 100 μE m−2 s−1 (data not shown), suggesting that sustained exposure to this light intensity is above the acclimation threshold of RCC809 (see ref. 13 for further discussion). In contrast, the OTH95 surface strain grew well at 100 μE m−2 s−1 (Fig. 1B).
Fig. 1.
Growth kinetics of OTH95 and RCC809 Ostreococcus strains at different light intensities. The two strains OTH95 (squares) and RCC809 (triangles) were grown at low light [10 μE·m−2·s−1, blue filter (A)] or moderate/high light [100 μE·m−2·s−1 (B)]. Error bars indicate standard error of the mean of three independent measurements.
We compared the functional properties of each strain after growth under conditions close to those prevailing in their natural environment: RCC809 cells were grown at 10 μE m−2 s−1 of blue light, whereas 100 μE m−2 s−1 of white light was used for OTH95. These light environments supported near-optimal growth rates for each strain. The strains were also switched to the opposite growth light to distinguish between acclimation and adaptation processes that have prevailed for their selection in their natural environment. Cultures were maintained for at least 3 d under the chosen light conditions before photosynthesis was measured.
PSII Absorption Capacity Is Enhanced in RCC809.
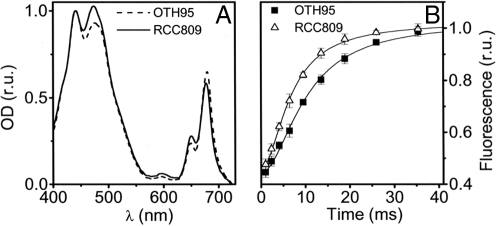
Changes in the PSII absorption capacity are typically observed during acclimation of plants and algae to changing light conditions (14, 15). Although the absorption spectra of RCC809 and OTH95 were similar, we observed a larger contribution of chlorophyll-b (Chl-b) in the overall absorption in the low-light/deep-sea strain (Fig. 2A), suggesting that the relative amount of light-harvesting complexes (i.e., the ones containing Chl-b) was larger in the low light-adapted than in the high light-adapted strain. The difference in pigment content was confirmed by the HPLC analysis of the pigments extracted from whole cells. RCC809 was found to have a ≈50% higher Chl-b/a ratio than OTH95 (Table 1). Furthermore, the difference in the PSII antenna size was confirmed by measuring the rate of the fluorescence rise under limiting light excitation in the presence of the PSII inhibitor 3-(3,4-dichlorophenyl)-1,1-dimethylurea (DCMU). In RCC809, the PSII antenna size was increased by a factor of ≈1.5 relative to OTH95 under all growth light conditions [Fig. 2B and supporting information (SI) Fig. S1]. This suggests that, at least under the conditions explored here, the different PSII antenna content in the two strains does not reflect a reversible acclimation process but, rather, a constitutive adaptation to their natural light environments.
Fig. 2.
Comparative absorption and fluorescence characteristics of OTH95 and RCC809 strains. (A) Absorption spectrum of intact OTH95 (dashed line) and RCC809 (solid line) cells. Curves were normalized to absorption at 440 nm. (B) Fluorescence transients of OTH95 (squares) and RCC809 (triangles) in the presence of 40 μM DCMU. Curves were normalized to the same value of variable fluorescence to allow a better comparison. Error bars indicate standard error of the mean of three independent measurements.
Table 1.
Photosynthetic parameters of the Ostreococcus strains OTH95 and RCC809
| Parameter | OTH95 | RCC809 |
|---|---|---|
| Chl-b/Chl-a | 0.65 ± 0.05 | 1.01 ± 0.08 |
| PSI | 1.41 ± 0.15 | 0.42 ± 0.2 |
| PSII | 1 | 1.08 ± 0.22 |
| PSI/PSII | 1.41 ± 0.15 | 0.39 ± 0.08 |
| NPQ10μE | 1.53 ± 0.47 | 0.09 ± 0.07 |
| NPQ100μE | 1.65 ± 0.35 | 0.75 ± 0.16 |
| ((Ax)/(Ax + Vx))10μE | 0.01 ± 0.001 | 0.01 ± 0.003 |
| ((Ax)/(Ax + Vx))100μE | 0.04 ± 0.01 | 0.210 ± 0.026 |
Chl-b/a ratios were measured by HPLC pigment analysis of light-adapted cells. The high cellular Chl-b/a value is attributable to an elevated cellular content of LHCP, which has a very high Chl-b/a ratio (≈1.3) (8, 12). NPQ induced by ΔpH was calculated as the nigericin-sensitive fraction of steady-state fluorescence quenching (Fm − Fm′/Fm′), which was induced by exposure of cells grown at 10 or 100 μE·m−2·s−1 of saturating illumination (46). PSI/PSII ratios were estimated from PSI and PSII charge separation capacity. Values were normalized to Chl concentrations, and the value for PSII centers in OTH95 (100 μE·m−2·s−1) was arbitrary set to 1. Ax/(Ax + Vx) is the deepoxidation state of xanthophylls. Standard errors are relative to at least three replicate experiments.
Reduced Electron Flow from PSII to PSI in RCC809 Is Compensated for by an Increased Electron Flow to Oxygen.
In addition to changes in the size of the light-harvesting apparatus, photosynthetic organisms modify the stoichiometry of their reaction centers in response to light and nutrient levels (14–18). We tested this possibility by quantifying the fraction of active PSI and PSII centers in the two Ostreococcus strains. This was done through monitoring the electrochromic shift signal (ECS), a technique previously used to evaluate the PSI/PSII ratio in freshwater green algae (19). The ECS is triggered by the light-induced electric field that develops across the thylakoid membrane upon charge separation within the reaction centers of the two photosystems. The field modifies the spectrum of pigment-containing complexes because of the Stark effect (20). When illuminated, both Ostreococcus strains display an identical ECS signal characterized by more symmetric and sharper peaks than those observed in plants (Fig. S2 A and B). These features likely reflect the specific carotenoid composition of the antenna complex LHCP of Mamiellales (8) and the different protein environment of the chromophores (21).
As a prerequisite to the determination of PSI/PSII ratios, we established that the amplitude of the ECS signal was a linear function of the number of light-induced charge separations (Fig. S3). We then assessed the reaction center stoichiometry from the amplitude of the signal measured in cells that were dark-adapted for at least 4 hours before being exposed to a single turnover flash of saturating intensity. Under these conditions, the amplitude of the fast ECS (100 μs) is proportional to the photochemical activity of both PSI and PSII and does not depend on either the relative absorption cross-section of the PSs (because the flash intensity is saturating) or on the rate of the intersystem electron flow (because the absorption changes are measured before intersystem electron transfer occurs) (20). The relative contribution of PSII was calculated from the difference between the signal measured in the absence and presence of the PSII inhibitors DCMU and hydroxylamine (19). Conversely, the PSI contribution was estimated as the amplitude of the signal that was insensitive to these inhibitors. The PSI/PSII stoichiometry was ≈1.4 in OTH95 and ≈0.4 in RCC809. This difference reflects a marked decrease in the PSI signal in RCC809 relative to OTH95 (Table 1), compared on a Chl basis. These stoichiometries were preserved in each strain independent of the light intensity at which the cells were grown (Fig. S2 C and D), suggesting that the differences in reaction center stoichiometry between the two strains reflect an adaptation to the natural environments in which the strains grow.
To determine the consequences of the decreased PSI/PSII stoichiometry observed in RCC809 on its overall photosynthetic capacity, we measured the light saturation curves of oxygen evolution for both strains. Maximum oxygen evolution (Pmax) was attained at lower light intensities for RCC809 than for OTH95. Thus, RCC809 is capable of maintaining efficient photosynthesis at limiting light, despite the reduced amount of PSI. Conversely, Pmax was twice as large for OTH95 (215 ± 17 μmol O2·h−1·mg Chl−1) relative to RCC809 (104 ± 6 μmol O2·h−1·mg Chl−1) once the values were normalized to the Chl concentration (Fig. 3A and Fig. S4). This suggests that the light-saturated rate of electron flow from H2O to CO2 was limited by the decreased PSI content in RCC809. The reduced photosynthetic activity in RCC809 was accompanied by a decrease in the quantum yield of linear electron flow as measured by the fluorescence parameter ΦPSII (22) (Fig. 3B). However, the relative decrease in ΦPSII in RCC809 was much smaller than the overall drop in the rate of oxygen evolution. Such a difference was unexpected because, in principle, both parameters should equally reflect the efficiency of electron flow. However, this result would be predicted if there were an “alternative” electron flow downstream of PSII and, in particular, a sustained electron transfer from PSII to molecular oxygen. Several alternative electron sinks exist in the chloroplast that use O2 as the terminal electron acceptor. Utilization of oxygen as a Hill acceptor in photosynthesis was first described by Mehler in 1951 (23). Later, it was established that oxygen reduction can take place either downstream of PSI [leading to the so-called “Mehler reaction” (24)] or between PSII and PSI. The latter reaction is driven by the plastoquinol terminal oxidase (PTOX) (25), an enzyme that catalyzes electron flow from reduced plastoquinol to O2 during the so-called chlororespiratory process (reviewed in ref. 26). Either the Mehler reaction or the PTOX activity could promote an H2O-to-H2O cycle that would lead to a decrease in the net oxygen evolution yield without affecting the quantum yield of PSII as evaluated from the fluorescence parameter ΦPSII.
Fig. 3.
Light saturation curves of oxygen evolution, ΦPSII and the effect of pgal in OTH95 (squares) and RCC809 (triangles) cells. (A) Oxygen evolution was monitored by using a Clark electrode under increasing light intensity, adjusted every 2 min. Photosynthetic activity was calculated as “net photosynthesis” (i.e., photosynthesis after correction for respiration) at any given light intensity. (B) ΦPSII, calculated as (Fm′ − Fs)/Fm′ (22). (C) Effect of pgal on both oxygen evolution and ΦPSII. Values are expressed as a percentage of change with respect to the untreated sample. Error bars indicate standard error of the mean of three independent measurements.
Genes encoding PTOX homologs are present both in OTH95 (CAL55767 and CAL58090) and in RCC809 (H.M., unpublished work). Thus, we tested the possible involvement of PTOX (or a PTOX-related activity) in diverting PSII-generated electrons to molecular oxygen. This was done by measuring the effect of propylgallate (pgal, a known inhibitor of PTOX) (27) on both oxygen evolution and ΦPSII in the two strains. Even at a relatively low concentration (100 μM), this inhibitor strongly decreased the ΦPSII value of RCC809, consistent with the occurrence of electron flow to molecular oxygen catalyzed by PTOX (Fig. 3C). As expected, pgal only slightly increased the yield of oxygen evolution in this strain: Indeed, an H2O-to-H2O cycle will stimulate oxygen emission by PSII, but this rise is strictly compensated by O2 consumption by PTOX. Thus, in the presence of pgal and on a Chl basis, as expected, both oxygen evolution and ΦPSII are reduced by half in RCC809 compared with OTH95.
There was no major effect of pgal on ΦPSII or oxygen evolution in OTH95, suggesting (i) that the H2O-to-CO2 electron flow is the main pathway for PSII-driven electron flow in this strain and (ii) that pgal addition does not result in a specific inhibition of photosynthesis in Ostreococcus, at least under the conditions used in our studies.
A ΔμH+ Is Generated in a Cytochrome b6f/PSI-Independent Manner in the RCC809.
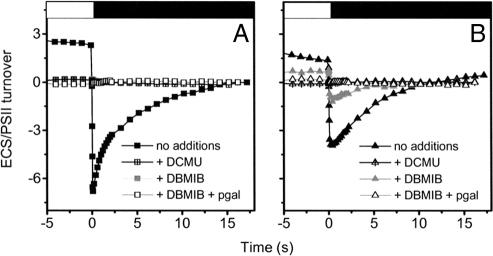
As a consequence of the H2O-to-H2O cycle in RCC809—mediated by PSII and PTOX or a PTOX-like enzyme—one expects at least a fraction of the light-generated electrochemical proton gradient to be established independently of electron flow downstream the plastoquinone pool (i.e., of PSI activity). Indeed, the combined oxidation of water by PSII and of plastoquinol by the oxidase results in a vectorial transfer of H+ from the stroma to the luminal space (26). This possibility was evaluated by measuring the inversion of the ECS at the end of continuous illumination (28). Inversion of the membrane potential upon switching the light off is assumed to stem from fast H+ flux through the CF0/F1 ATP synthase complex. Owing to the low dielectric constant of the thylakoid membranes (29), the high H+ buffering capacity of the lumen (30), and the slow rate of charge redistribution along the membranes (28), the relaxation of the electric component (ΔΨ) of the ΔμH+ becomes faster than the complete dissipation of the ΔpH. This leads to a transient excess of positive charges in the stroma, which inverts the membrane potential. Thus, the amplitude of the inverted membrane potential can be taken as an indication of the size of the light-induced ΔpH (31). Although this parameter provides only a qualitative estimation of the ΔpH in vivo, the values obtained support the notion that RCC809 maintains a lower ΔpH than OTH95 under our experimental conditions (Fig. 4 and Fig. S5). In addition, although inhibition of PSII activity with DCMU completely abolished the generation of a ΔpH in both strains, a fraction of this proton gradient was maintained in RCC809 cells (but not in OTH95) when electron flow was blocked by addition of the cytochrome b6f inhibitor dibromothymoquinone (DBMIB) (Scheme 1). Further addition of pgal completely suppressed residual electrochemical proton gradient generation, consistent with this extra ΔpH being linked to the activity of a PTOX-like oxidase that mediates an H2O-to-H2O cycle.
Fig. 4.
Estimation of the electrochemical proton gradient generated upon steady-state illumination in OTH95 (A) and RCC809 (B) cells. Membrane potential is evaluated by absorption changes at 505–525 nm on untreated cells (black symbols), with 40 μM DCMU (crossed symbols), 5 μM DBMIB (gray symbols), and 5 μM DBMIB and 100 μM pgal (open symbols). Samples were illuminated for 2 min with saturating light (white box). The light was then switched off (black box), and the membrane potential decay was followed until full relaxation. The amplitude of the membrane potential was normalized to 1 PSII membrane charge separation, corresponding to the contribution of PSII to the signal generated by a saturating laser flash (see Figs. S2 and S3).
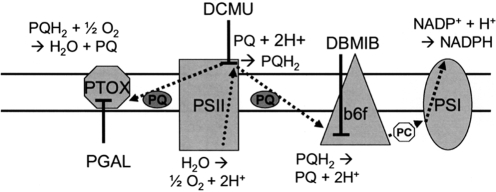
Scheme 1.
Photosynthetic electron flow. The site of coupling between electron and proton transfer, as well as the sites of inhibition by DCMU and DBMIB, are shown. Activity of PTOX or a PTOX-like oxidase (inhibited by pgal) may allow diversion of electrons from the plastoquinone pool (PQH2) to oxygen and may stimulate proton deposition into the lumen by a DCMU-sensitive, DBMIB-insensitive reaction. See text for further details.
The fact that an unusual mode of ΔpH generation takes place in RCC809 through a PSII/PTOX-like, oxidase-coupled electron flow probably accounts for the different photoprotective responses observed in the two strains. Whereas nonphotochemical dissipation of absorbed energy (NPQ) (32) could readily occur in OTH95 in high light, independent of the growth conditions (Table 1), NPQ could be observed in RCC809 only upon exposure of the cells to 100 μE·m−2·s−1 for several days. This NPQ was paralleled by the accumulation (Table 1) of antheraxanthin [i.e., the only product of violaxanthin deepoxidation in Prasinophytes (33)], as expected if the extra PTOX-dependent ΔpH generated at this light may sustain a greater activity of violaxanthin deepoxidase (32) in RCC809.
Discussion
Despite their identical morphology, the two Ostreococcus strains characterized in this work—OTH95 and RCC809 (12)—display distinct photosynthetic traits that have been shaped by their contrasting growth environments. The OTH95 strain was isolated in the Thau coastal lagoon in the western Mediterranean; i.e., a rather shallow environment rich in nutrients and often subjected to strong illumination (especially in the summertime). In contrast, RCC809 is an oceanic strain, isolated in the deep sea (≈100-m depth), where it will not encounter light stress but is likely subjected to nutrient limitation (e.g., Fe). The most striking feature observed in our analysis is the H2O-to-H2O cycle generated by PSII in RCC809 but not in OTH95. We characterized this process, specific to RCC809, as a PTOX-mediated electron sink based on two independent observations: (i) the inhibition of the quantum yield of PSII-driven electron flow (ΦPSII) by pgal without any effect on the oxygen evolution capacity, and (ii) the generation of a ΔpH in the light under conditions in which electron flow is blocked at the level of the cytochrome b6f complex (by DBMIB). Generation of this extra ΔpH is inhibited by blocking electron flow at the level of PSII by DCMU or by adding pgal at a concentration that inhibits ΦPSII.
Alternative electron flow to oxygen is prominent in RCC809; ≈50% of the electrons generated from water oxidation can be routed to the reduction of molecular oxygen, at the cost of CO2 fixation. No such sustained activity of a water-to-water cycle has been observed in vascular plants or freshwater green algae (26, 34). However, an important rerouting of photosynthetic electrons to molecular oxygen has been shown in marine prokaryotes under laboratory conditions (35) or in situ, where the organisms may be experiencing nutrient limitation (36). It is thus conceivable that this phenomenon represents a typical response of organisms adapted to life in the nutrient-poor oligotrophic oceans.
What, then, is the rationale for such an adaptation mechanism? Previous work has emphasized that algae and plants respond to environmental changes by modulating the size of their light harvesting complexes as well as the stoichiometry of their reaction centers (13–16, 37–41). However, whereas the changes in the antenna size of RCC809 are consistent with this organism being adapted to a low-light environment, the low PSI/PSII ratio in this strain is typical of high-light-acclimated plants (14). It is thus tempting to speculate that the situation observed in RCC809 reflects a constitutive adaptation to a sustained nutrient limitation (e.g., Fe) experienced by this strain. Owing to its relatively high Fe content, PSI is strongly decreased in freshwater (17) or marine (18) algae that are exposed to an Fe-depleted environment. Independent of the origin of the large decrease in the PSI content observed in RCC809, the unusually high activity of the PTOX-like oxidase may be essential for this strain to survive, even at moderate light intensities. The concomitant increase in the light harvesting capacity of PSII and the decrease in PSI cellular content would rapidly promote overreduction of the plastoquinone pool, eventually leading to sustained photodamage, should this strain be subjected to higher light. However, this potentially lethal situation is actively counterbalanced in RCC809 by the oxidase-mediated H2O-to-H2O cycle. By acting as an efficient electron sink, this process allows reoxidation of the electron transport components and the opening of PSII traps. Consistent with this hypothesis, RCC809 is capable of maintaining a partially oxidized electron transport chain even at light intensities where electron flow to CO2 is limited by PSI performances (Fig. 3).
Furthermore, by bypassing the bottleneck of electron transfer through PSI, this process has a major advantage compared with electron flow to other alternative sinks [i.e., the Mehler reaction or the transfer of reducing equivalents to the mitochondria via the malate shunt (42)], which require activity of both photosystems. The H2O-to-H2O cycle also provides other benefits to the cell: (i) by allowing the development of a ΔpH-mediated NPQ response, it enhances photoprotection. Previous work has demonstrated that the ΔpH activates the VDE enzyme (responsible for antheraxanthin and zeaxanthin synthesis), leading to an enhanced thermal dissipation capacity (reviewed in ref. 32). Thus, the extra ΔpH generated in RCC809 may be responsible for the accumulation of antheraxanthin that is observed after several days of exposure to 100 μE·m−2·s−1. This antheraxanthin promotes the onset of reversible fluorescence quenching, which may prolong viability of RCC809 during extended periods in high light. Moreover, (ii) the extra ΔpH can fuel ATP synthesis for housekeeping purposes, even in the presence of reduced PSI performances. It is known from previous work in plants and algae that electron diversion at the PSI acceptor side (via the Mehler reaction, the malate shunt, or cyclic flow around PSI) may increase the amount of ATP synthesized per electron transferred in PSII. This likely allows establishing the correct ATP/NADPH ratio required for CO2 assimilation by the Calvin–Benson cycle (see ref. 43 for a discussion). In analogy to the cyclic electron flow observed around PSI, activation of a H2O-to-H2O cycle around PSII—under nutrient starvation and/or any other condition leading a significant drop in the PSI levels—may increase the ATP synthesized per electron transferred in PSII and help sustaining cell survival. Furthermore, although this process was observed under controlled laboratory conditions, a comparison with previous observations in diatoms and cyanobacteria (18, 35) suggests that electron diversion downstream of PSII may be widespread among the prokaryotic and eukaryotic components of phytoplankton. Thus, we propose that rerouting electrons to a plastoquinol oxidase represents a common and compulsory strategy to allow successful adaptation of photosynthetic taxa to the oligotrophic ocean environment.
Materials and Methods
Growth Conditions.
Ostreococcus OTH95 (O. tauri) (7) and RCC809 strains were obtained from the Observatoire Océanologique de Banyuls-sur-Mer, France, and grown in K medium (44). They were grown either in white light at an intensity of 100 μE·m−2·s−1 or in blue light at an intensity of 10 μE·m−2·s−1 (filter 183 Moonlight Blue Filter; Leefilters) in a 12-h light/12-h dark regime at 18–20°C with mild agitation. This temperature represents a compromise between the range experienced by OTH95 (20–25°C) and RCC809 (15–20°C) in their natural environments (see http://bulletin.mercator-ocean.fr). Flow cytometric analyses were performed with a FACScan flow cytometer (Becton Dickinson) equipped with an air-cooled argon laser providing 15 mW at 488 nm.
Fluorescence and Oxygen Measurements.
Fluorescence emission was measured by using a home-built instrument, as described previously (45). ΦPSII, the quantum yield of PSII (22), was calculated as (Fm′ − Fs)/Fm′, where Fm′ is the maximum fluorescence emission level induced by a pulse of saturating light (≈5,000 μE·m−2·s−1), and Fs is the steady-state level of fluorescence emission. NPQ was calculated as (Fm − Fm′)/Fm′ (46), where Fm is the maximum fluorescence emission level in the dark measured with the saturating pulse of light. The ΔpH-sensitive NPQ extent was evaluated as the fraction of fluorescence quenching that was selectively suppressed by addition of the H+/K+ exchanger nigericin (10 μM). Oxygen evolution was measured with a Clark electrode (Hansatech).
Spectroscopic Measurements.
In vivo absorption was measured with a home-built spectrophotometer, based on a detecting diode array (AVS-USB 200; Ocean Optics). Kinetics measurements were performed by using a home-built xenon lamp-based spectrophotometer (19). Detection flashes were provided by a xenon flashlamp (3-μs duration at half-height) with light filtered through a monochromator (HL; Jobin Yvon). Actinic light was provided either by a dye laser or by a continuous green LED source. PSI and PSII charge separation capacity was calculated from changes in the amplitude of the fast phase of the ECS signal (at 505–525 nm) upon excitation with a saturating laser flash in the presence or absence of the PSII inhibitors DCMU (20 μM) and hydroxylamine (1 mM). The latter compound was added to destroy the manganese cluster responsible for oxygen evolution and to prevent recombination between the donor and acceptor side of PSII, which would preclude correct estimation of the PSI/PSII ratio.
Pigment Analysis.
Pigments were extracted from whole cells in methanol, and debris was removed by centrifugation at 10,000 × g for 15 min. A total of 15 μl of pigment extract was subjected to reverse-phase HPLC analysis using commercial equipment (Waters). A Nova Pak C18, 60A column (150-mm length, 4-μm pore size) was used for separation. The Chl-a and Chl-b concentrations were estimated according to Lichtenthaler (47).
Note Added in Proof.
While this manuscript was being processed for publication, a study (48) of the acclimation properties of the two Ostreococcus strains was published. Although the two studies are consistent for the light intensity we used, Six et al. observed contrasting acclimation behaviors when Ostreococcus was exposed for several months to much higher light intensity.
Supplementary Material
Acknowledgments.
We thank Fabrice Franck (Liège) and Bernard Genty (Cadarache) for valuable suggestions. M. Radoux is also thanked for technical assistance. This research was supported by Belgian “Fonds National de la Recherche Scientifique” (FNRS) Grant F.4735.06 (to P.C.) and by the Centre National de la Recherche Scientifique. H.M. and E.D. were supported by the “Marine Genomics Europe” European Network of Excellence (2004–2008) (GOCE-CT-2004-505403). S.B. was supported by National Science Foundation Grant OCE-0450874 (to A.R.G.) P.C. is a research associate of the Belgian FNRS.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0802762105/DCSupplemental.
References
- 1.Li WKW. Primary productivity of prochlorophytes, cyanobacteria, and eukaryotic ultraphytoplankton: Measurements from cytometric sorting. Limnol Oceanogr. 1994;39:169–175. [Google Scholar]
- 2.Worden AZ, Nolan JK, Palenik B. Assessing the dynamic and ecology of marine picoplankton: The importance of eukaryotic component. Limnol Oceanogr. 2004;49:168–179. [Google Scholar]
- 3.Zhu F, Not F, Massana R, Marie D, Vaulot D. Mapping of the picoeukaryotes in marine systems with quantitative PCR of the 18S rRNA gene. FEMS Microbiol Ecol. 2005;52:79–92. doi: 10.1016/j.femsec.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 4.Derelle E, et al. Genome analysis of the smallest free-living eukaryote Ostreococcus tauri unveils many unique features. Proc Natl Acad Sci USA. 2006;103:11647–11652. doi: 10.1073/pnas.0604795103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palenik B, et al. The tiny eukaryote Ostreococcus provides genomic insights into the paradox of plankton speciation. Proc Natl Acad Sci USA. 2007;104:7705–7710. doi: 10.1073/pnas.0611046104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Courties C, et al. Smallest eukaryotic organism. Nature. 1994;370:255. [Google Scholar]
- 7.Chrétiennot-Dinet MJ, et al. A new marine picoeukaryote: Ostreococcus tauri gen et sp. Nov. (Chlorophyta, Prasinophyceae) Phycologia. 1995;4:285–292. [Google Scholar]
- 8.Six C, Worden AZ, Rodriguez F, Moreau H, Partensky F. New insights into the nature and phylogeny of prasinophyte antenna proteins: Ostreococcus tauri, a case study. Mol Biol Evol. 2005;22:2217–2230. doi: 10.1093/molbev/msi220. [DOI] [PubMed] [Google Scholar]
- 9.Derelle E, et al. DNA libraries for sequencing the genome of Ostreococcus tauri (chlorophyta, prasinophyceae): The smallest free-living eukaryotic cell. J Phycol. 2002;38:1150–1156. [Google Scholar]
- 10.Moore LR, Rocap G, Chisholm SW. Physiology and molecular phylogeny of coexisting Prochlorococcus ecotypes. Nature. 1998;393:464–467. doi: 10.1038/30965. [DOI] [PubMed] [Google Scholar]
- 11.Rocap G, Distel DL, Waterbury JB, Chisholm SW. Resolution of Prochlorococcus and Synechococcus ecotypes by using 16S–23S ribosomal DNA internal transcribed spacer sequences. Appl Environ Microbiol. 2002;68:1180–1191. doi: 10.1128/AEM.68.3.1180-1191.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rodriguez F, et al. Ecotype diversity in the marine picoeukaryote Ostreococcus (Chlorophyta, Prasinophyceae) Environ Microbiol. 2005;7:853–859. doi: 10.1111/j.1462-2920.2005.00758.x. [DOI] [PubMed] [Google Scholar]
- 13.Walters RG. Towards an understanding of photosynthetic acclimation. J Exp Bot. 2005;56:425–447. doi: 10.1093/jxb/eri060. [DOI] [PubMed] [Google Scholar]
- 14.Anderson JM, Chow WS, Park YI. The grand design of photosynthesis: Acclimation of the photosynthetic apparatus to environmental clues. Photosynth Res. 1995;46:129–139. doi: 10.1007/BF00020423. [DOI] [PubMed] [Google Scholar]
- 15.Falkowski PG, Owens TG. Light-shade adaptation: Two strategies in marine phytoplankton. Plant Physiol. 1980;66:592–595. doi: 10.1104/pp.66.4.592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bailey S, Walters RG, Jansson S, Horton P. Acclimation of Arabidopsis thaliana to the environment: the existence of separate low light and high light responses. Planta. 2001;231:794–801. doi: 10.1007/s004250100556. [DOI] [PubMed] [Google Scholar]
- 17.Moseley JL, et al. Adaptation to Fe-deficiency requires remodeling of the photosynthetic apparatus. EMBO J. 2002;21:6709–6720. doi: 10.1093/emboj/cdf666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Strzepek RF, Harrison PJ. Photosynthetic architecture differs in coastal and oceanic diatoms. Nature. 2004;431:689–692. doi: 10.1038/nature02954. [DOI] [PubMed] [Google Scholar]
- 19.Joliot P, Delosme R. Flash-induced 519 nm absorption change in green algae. Biochim Biophys Acta. 1974;357:267–284. doi: 10.1016/0005-2728(74)90066-8. [DOI] [PubMed] [Google Scholar]
- 20.Witt HT. Energy conversion in the functional membrane of photosynthesis. Analysis by light pulse and electric pulse methods. The central role of the electric field. Biochim Biophys Acta. 1979;505:355–427. doi: 10.1016/0304-4173(79)90008-9. [DOI] [PubMed] [Google Scholar]
- 21.Goss R, Wilhelm C, Garab G. Organization of the pigment molecules in the chlorophyll a/b/c containing alga Mantoniella squamata (Prasinophyceae) studied by means of absorption, circular and linear dichroism spectroscopy. Biochim Biophys Acta. 2000;1457:190–199. doi: 10.1016/s0005-2728(00)00101-8. [DOI] [PubMed] [Google Scholar]
- 22.Genty B, Harbinson J, Briantais JM, Baker NR. The relationship between non-photochemical quenching of chlorophyll fluorescence and the rate of photosystem 2 photochemistry in leaves. Photosynth Res. 1990;25:249–257. doi: 10.1007/BF00033166. [DOI] [PubMed] [Google Scholar]
- 23.Mehler AH. Studies on reactions of illuminated chloroplasts. I. Mechanism of the reduction of oxygen and other Hill reagents. Arch Biochem Biophys. 1951;33:65–77. doi: 10.1016/0003-9861(51)90082-3. [DOI] [PubMed] [Google Scholar]
- 24.Ort DR, Baker NR. A photoprotective role for O(2) as an alternative electron sink in photosynthesis? Curr Opin Plant Biol. 2002;5:193–198. doi: 10.1016/s1369-5266(02)00259-5. [DOI] [PubMed] [Google Scholar]
- 25.Streb P, et al. Evidence for alternative electron sinks to photosynthetic carbon assimilation in the high mountain plant species Ranunculus glacialis. Plant Cell Environ. 2005;28:1123–1135. [Google Scholar]
- 26.Peltier G, Cournac L. Chlororespiration. Annu Rev Plant Biol. 2002;53:523–550. doi: 10.1146/annurev.arplant.53.100301.135242. [DOI] [PubMed] [Google Scholar]
- 27.Josse EM, Alcaraz JP, Labouré AM, Kuntz M. In vitro characterization of a plastid terminal oxidase (PTOX) Eur J Biochem. 2003;270:3787–3794. doi: 10.1046/j.1432-1033.2003.03766.x. [DOI] [PubMed] [Google Scholar]
- 28.Cruz JA, Sacksteder CA, Kanazawa A, Kramer DM. Contribution of electric field (Δψ) to steady-state transthylakoid proton motive force (pmf) in vitro and in vivo. Control of pmf parsing into Δψ and ΔpH by ionic strength. Biochemistry. 2001;40:1226–1237. doi: 10.1021/bi0018741. [DOI] [PubMed] [Google Scholar]
- 29.Vredenberg WJ. Electrostatic interactions and gradients between chloroplast compartments and cytoplasm. In: Barber L, editor. The Intact Chloroplast. Amsterdam: Elsevier/North Holland Biomedical; 1976. pp. 53–87. [Google Scholar]
- 30.Junge W, McLaughlin S. The role of fixed and mobile buffers in the kinetics of proton movement. Biochim Biophys Acta. 1987;890:1–5. doi: 10.1016/0005-2728(87)90061-2. [DOI] [PubMed] [Google Scholar]
- 31.Kramer DM, Cruz JA, Kanazawa A. Balancing the central roles of the thylakoid proton gradient. Trends Plants Sci. 2003;8:27–32. doi: 10.1016/s1360-1385(02)00010-9. [DOI] [PubMed] [Google Scholar]
- 32.Horton P, Ruban AV, Walters RG. Regulation of light harvesting in green plants. Annu Rev Plant Physiol Plant Mol Biol. 1996;47:655–684. doi: 10.1146/annurev.arplant.47.1.655. [DOI] [PubMed] [Google Scholar]
- 33.Goss R, Böhme K, Wilhelm C. The xanthophyll cycle of Mantonielle squamata converts violaxanthin into antheraxanthin but not zeaxanthin : Consequences for the mechanism of enhanced non-photochemical energy dissipation. Planta. 1998;205:613–621. [Google Scholar]
- 34.Cournac L, et al. Electron flow between photosystem II and oxygen in chloroplasts of photosystem I-deficient algae is mediated by a quinol oxidase involved in chlororespiration. J Biol Chem. 2000;275:17256–17262. doi: 10.1074/jbc.M908732199. [DOI] [PubMed] [Google Scholar]
- 35.Bailey S, et al. Alternative photosynthetic electron flow to oxygen in marine Synechococcus. Biochim Biophys Acta. 2008;1777:269–276. doi: 10.1016/j.bbabio.2008.01.002. [DOI] [PubMed] [Google Scholar]
- 36.Mackey KRM, Paytan A, Grossman AR, Bailey S. A photosynthetic strategy for coping in a high-light, low-nutrient environment. Limnol Oceanogr. 2008;53:900–913. [Google Scholar]
- 37.Demmig-Adams B. Survey of thermal energy dissipation and pigment composition in sun and shade leaves. Plant Cell Physiol. 1998;39:474–482. [Google Scholar]
- 38.Berry J, Bjorkman O. Photosynthetic response and adaptation to temperature in higher-plants. Annu Rev Plant Physiol Plant Mol Biol. 1980;31:491–543. [Google Scholar]
- 39.LaRoche J, Mortain-Bertrand A, Falkowski P. Light intensity induced changes in cab mRNA and light harvesting complex II apoprotein levels in the unicellular chlorophyte Dunaliella tertiolectica. Plant Physiol. 1991;97:147–153. doi: 10.1104/pp.97.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Durnford DG, Falkowski PG. Chloroplast redox regulation of nuclear gene transcription during photoacclimation. Photosynth Res. 1997;53:229–241. [Google Scholar]
- 41.Im CS, Grossman AR. Identification and regulation of high light-induced genes in Chlamydomonas reinhardtii. Plant J. 2001;30:301–313. doi: 10.1046/j.1365-313x.2001.01287.x. [DOI] [PubMed] [Google Scholar]
- 42.Scheibe R, Backhausen JE, Emmerlich V, Holtgrefe S. Strategies to maintain redox homeostasis during photosynthesis under changing conditions. J Exp Bot. 2005;56:1481–1489. doi: 10.1093/jxb/eri181. [DOI] [PubMed] [Google Scholar]
- 43.Allen J. Photosynthesis of ATP-electrons, proton pumps, rotors, and poise. Cell. 2002;110:273–276. doi: 10.1016/s0092-8674(02)00870-x. [DOI] [PubMed] [Google Scholar]
- 44.Keller MD, Selvin RC, Claus W, Guillard RRL. Media for the culture of oceanic ultraphytoplankton. J Phycol. 1987;23:633–638. [Google Scholar]
- 45.Rappaport F, Beal D, Joliot A, Joliot P. On the advantages of using green light to study fluorescence yield changes in leaves. Biochim Biophys Acta. 2007;1767:56–65. doi: 10.1016/j.bbabio.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 46.Bilger W, Björkman Ö. Role of the xanthophyll cycle in photoprotection elucidated by measurements of light-induced absorbance changes, fluorescence and photosynthesis in leaves of Hedera canariensis. Photosynth Res. 1990;25:173–186. doi: 10.1007/BF00033159. [DOI] [PubMed] [Google Scholar]
- 47.Lichtenthaler HK. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987;148:350–382. [Google Scholar]
- 48.Six C, et al. Contrasting photoacclimation costs in ecotypes of the marine eukaryotic picoplankter Ostreococcus. Limnol Oceanogr. 2008;53:255–265. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.