Abstract
The pivotal question in the debate on the ecological effects of climate change is whether species will be able to adapt fast enough to keep up with their changing environment. If we establish the maximal rate of adaptation, this will set an upper limit to the rate at which temperatures can increase without loss of biodiversity.
The rate of adaptation will primarily be set by the rate of microevolution since (i) phenotypic plasticity alone is not sufficient as reaction norms will no longer be adaptive and hence microevolution on the reaction norm is needed, (ii) learning will be favourable to the individual but cannot be passed on to the next generations, (iii) maternal effects may play a role but, as with other forms of phenotypic plasticity, the response of offspring to the maternal cues will no longer be adaptive in a changing environment, and (iv) adaptation via immigration of individuals with genotypes adapted to warmer environments also involves microevolution as these genotypes are better adapted in terms of temperature, but not in terms of, for instance, photoperiod.
Long-term studies on wild populations with individually known animals play an essential role in detecting and understanding the temporal trends in life-history traits, and to estimate the heritability of, and selection pressures on, life-history traits. However, additional measurements on other trophic levels and on the mechanisms underlying phenotypic plasticity are needed to predict the rate of microevolution, especially under changing conditions.
Using this knowledge on heritability of, and selection on, life-history traits, in combination with climate scenarios, we will be able to predict the rate of adaptation for different climate scenarios. The final step is to use ecoevolutionary dynamical models to make the link to population viability and from there to biodiversity loss for those scenarios where the rate of adaptation is insufficient.
Keywords: climate change, phenology, microevolution, phenotypic plasticity, intergovernmental panel on climate change, scenarios
1. Introduction
The world's climate is changing at an unprecedented rate and this change will continue over the following decades (IPCC 2007). There is ample evidence that climate change has ecological consequences (Walther et al. 2002; Parmesan & Yohe 2003; Root et al. 2003; Parmesan 2006). The two best recorded climate-change-induced shifts are changes in phenology, i.e. in timing of vegetation development (Menzel & Fabian 1999), in spawning date in frogs and toads (Beebee 1995), return date of migrant birds (Hüppop & Hüppop 2003) and butterflies (Sparks et al. 2005), egg hatching date in insects (Visser & Holleman 2001), laying dates in birds (Crick et al. 1997), etc. and in range shifts, in the distribution of butterflies (Parmesan et al. 1999), breeding range (Thomas & Lennon 1999) or overwintering range (Austin & Rehfisch 2005) of birds, etc. Less widespread documented consequences of climate change are shifts in body size (Millien et al. 2006; Yom-Tov et al. 2006) and in changes in the strength of competition between species (Bertness & Ewanchuk 2002; Jiang & Morin 2004).
The pivotal question in the debate on the ecological effects of climate change is whether these observed shifts are sufficiently large, i.e. whether species will be able to adapt fast enough to their changing world. Establishing the maximal rate of adaptation is also of crucial importance in the general debate on climate change. The rate of temperature increase up to 2100 is not determined yet as it strongly depends on socio-economic developments worldwide. The intergovernmental panel on climate change (IPCC) predicts climate change for six of such socio-economic scenarios (IPCC 2007). It is up to biologists to predict the ecological consequences for these different IPCC scenarios, for instance, in terms of reduced population viability or loss of biodiversity. This should then, in turn, be taken into account in the discussion on which IPCC scenario the world should aim for. As the magnitude of the ecological consequences will strongly depend on the rate of adaptation of species to their changing environment, assessing this rate of adaptation will set an upper limit to the rate at which temperatures can increase without loss of population viability or biodiversity.
In this paper, I will discuss the various mechanisms by which species can adapt to climate change and will argue that the rate of adaptation (see §2 for definitions) will be primarily set by the rate of microevolution, a rate that is estimated to be alarmingly low in vertebrates (Gienapp et al. 2008). I will mainly use examples from research on timing of reproduction in birds and focus mainly on the effect of climate change on temperature, rather than rainfall, etc., simply because temperature effects are the best studied. Furthermore, I will highlight the importance of long-term pedigreed population studies for this research.
2. Adapting to a warming world
In a warming world, species need to adapt; that is, populations need to shift their distribution of phenotypes such that the average fitness for the shifted phenotypic distribution is higher than that of the original distribution when compared within the current environment. The rate of adaptation is simply this change in the distribution of phenotypes per year. Adaptation can work via a change in the genetic composition of the population, when some genotypes increase while other genotypes, with a lower fitness, decline in frequency (microevolution). But adaptation can also work via different forms of phenotypic plasticity, and there has been ample debate on which of these two forms contribute most to the observed shifts in phenotype distribution in relation to climate change (Przybylo et al. 2000; Reale et al. 2003; Gienapp et al. 2008).
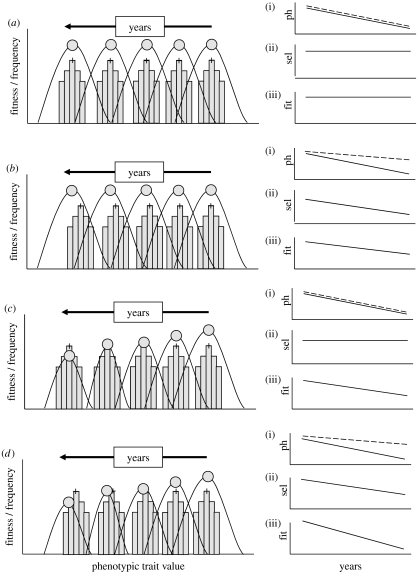
The effects of climate change on phenotype distributions are very apparent; it is less clear how we should interpret these shifts. Are these signs of a disruption in the ecology of species, or are these an indication that species are adapting to a changing world? This question cannot be answered without establishing whether the observed shifts are sufficiently strong. Intuitively, one would define a shift as sufficiently strong when the average fitness of a population does not decline. As shown in figure 1, this definition however does not hold. Even when the rate of adaptation matches the changes in the optimal phenotype values exactly, fitness may still decline simply owing to a decline in habitat quality (see also §4). A more accurate definition is, therefore, that the distribution of phenotypes of a species shifts at the appropriate rate when there is stabilizing selection throughout the period of the mean phenotype shift. If so, the rate of adaptation perfectly matches the rate of climate change and thus any decline in fitness is not because the distribution of phenotypes is lagging behind: the rate of adaptation is sufficient. A complication with this definition of a sufficient rate of adaptation is that in some populations for some traits there was already directional selection prior to climate change (Merilä et al. 2001). In such cases, the question is whether this directional selection is getting stronger.
Figure 1.
Over the years, due to climate change, the optimal trait value may shift as is indicated by the per year fitness curve, where the optimal trait value for that year is indicated by the dot at the highest fitness value. The actual distribution of trait values is indicated by the histogram, and the median trait value is indicated with a plus symbol. (a) There is no decline in fitness for individuals with the optimal trait value, and a perfect match between the shift in the optimal and the actual trait values. This is depicted in (a(i)) where the optimal trait value shift is indicated with a solid line and the actual trait value shift with a dashed line (ph, phenotypic trait value). In this scenario, there is no change in the selection on the trait value ((a(ii)) sd, selection differential) nor a decline in mean fitness ((a(iii)) fit, fitness). (b) There is again no decline in fitness for the optimal trait value, but the shift in the actual trait values is less than that for the optimal trait values. In this case, the lines in (b(i)) are no longer parallel, there will be increasing directional selection (b(ii)) and mean fitness will decline (b(iii)). The decline in fitness is due to an insufficient rate of adaptation. (c) There is a decline in fitness for the optimal trait values (due to, for instance, a decline in habitat quality), but a perfect match between the shift in the optimal and the actual trait values. Thus in (c(i)) the lines run parallel, and there is no increase in direction selection (c(ii)), but there is a decline in fitness (c(iii)). This decline is now not due to an insufficient rate of adaptation. (d) Finally, there is both a decline in fitness for the optimal trait values, and a shift in the actual trait values, which is less than that for the optimal trait values. Hence in (d(i)) the lines diverge, there is increasing direction selection (d(ii)) and a strong decline in fitness (d(iii)).
For great tits (Visser et al. 1998) and pied flycatchers (Both & Visser 2001), we have shown that there is increased directional selection on laying date (c.f. figure 1b(ii)), and thus that the rate of adaptation is insufficient in these populations. Interestingly, in UK great tits there is not such an increased directional selection (Cresswell & McCleery 2003), and thus the rate of adaptation is sufficient for that population (see Visser & Both (2005) for further discussion).
For the analyses on increased selection, the phenotypes of individuals need to be linked to their fitness, and for many species such data are not available. In these cases, we can compare the shifts in the observed and the optimal phenotype (c.f. figure 1 the (i) panels). We can use, as a first approximation, the shift in the phenology of a species' food compared to its own shift in phenology to investigate whether the rate of adaptation has been sufficient (Visser & Both 2005). In the majority of cases, the phenology of food shifts at a different rate leading to mistimed reproduction or growth (Stenseth & Mysterud 2002; Visser et al. 2004). This would indicate that in many species the shifts in adaptation are insufficient to match the changes in their environment.
What is constraining the rate of adaptation? Three types of responses to climate change have been described: dispersal to suitable habitats elsewhere, change in the phenotype distribution without a change in genotypes via phenotypic plasticity, and genetic change, i.e. microevolution (Holt 1990; Davis et al. 2005; Gienapp et al. 2008). It has often been suggested that the rate of adaptation can be quite high as phenotypic plasticity works almost instantaneously: if it becomes warmer, any trait that is phenotypically plastic with respect to temperature will shift. This can be via different forms of phenotypic plasticity: the response of an individual to environmental conditions within the same year (within the individual, within the same year), via learning (within the individual, across years) or via maternal effects (across individuals). In addition, the response in the form of dispersal to suitable habitats elsewhere, or, complementary, the immigration of novel genotypes into a population (an across population mechanism), can operate on relatively short time scales. Given these different mechanisms to adapt at relatively short time scales, microevolution does not seem essential to adapt to climate change. Below I explain why this is untrue and why adaptation to climate change, also via phenotypic plasticity or immigration of novel genotypes, will involve microevolution.
(a) Reaction norms
When the same genotype gives rise to different phenotypes in different environments this is termed phenotypic plasticity (Pigliucci 2001). Both traits that are expressed only once in a lifetime (non-labile traits, e.g. amphibian metamorphosis) and those that are expressed repeatedly within individual lifespan (labile traits, e.g. breeding time in iteroparous organisms) can be phenotypically plastic (Nussey et al. 2007). Among years, the same individual starts egg laying at different dates, hence laying date is a phenotypically plastic labile trait. The ultimate reason for this plasticity is that the optimal laying date varies from year to year, for instance, because the food abundance peaks at different times in years with different spring temperatures. If animals could measure these annual food peaks directly and produce offspring ‘instantaneously’ this plasticity would be perfect. But the proximate cues involved in the plasticity, i.e. the components of the environment that affect the phenotype, are often not the same variables that determine the optimal laying date. For instance, laying date is affected by temperatures much earlier in the season than when selection on laying date operates. Environmental variables are only useful as cues if they predict the future via correlations between environmental variables that serve as proximate cues and the environmental variables that are determining selection, i.e. the covariance between phenotype and fitness (van Noordwijk & Muller 1994; Visser et al. 2004).
Despite the fact that animals are phenotypically plastic in response to temperature, this is not sufficient to adapt to climate change. This seems a paradox but the key insight is that reaction norms will no longer be adaptive due to the disruption of the correlation between temporally spaced environmental variables by climate change (Visser et al. 2004). Hence, the cues no longer accurately predict future conditions: for instance, while in the past a certain temperature would correspond with a food peak for the offspring in 30 days, it would now correspond with a food peak in 20 days. This leads to mistimed reproduction as birds are, for instance, not sensitive enough to these cues, like temperature. As a consequence, microevolution of phenotypic plasticity is needed.
Environmental variables, like temperature or photoperiod, can be cues, i.e. they contain information about the future selective environment (Visser & Lambrechts 1999). The environment can, however, also select more directly on reproductive decisions. For instance, in timing of reproduction there is strong selection on a close match between the offsprings' needs and the abundance of food (Visser et al. 2006) but as egg production is costly, both in terms of energy (Stevenson & Bryant 2000) and fitness (Visser & Lessells 2001), selection may also operate via the cost of reproduction at the egg-laying stage (harsh conditions may ‘constrain’ early laying, cf. Perrins 1970). If so, animals may shift their phenology to a lesser extent than the shift in phenology of the peak date in food availability (cf. Jonzen et al. (2007) for a similar argument). However, this will not lead to directional selection for early laying per se as laying too early will lead to a lower fitness via increased mortality risks for the parents due to their increased effort in the egg-laying phase. In fact, it may lead to selection of life-history traits that determine these costs of reproduction associated with egg production, like egg size. As these life-history traits will also be heritable, climate change will lead to selection on multiple life-history traits simultaneously (see also Visser et al. 2003; Garant et al. in press), and again microevolution is needed, which will affect the reaction norm of timing of reproduction. To calculate optimal reaction norms, we need to integrate the selection on all phases of the reproductive cycle, which may perhaps be possible via annual routine models (McNamara & Houston 2008). Climate change can thus affect selection on reaction norms via, for instance, higher costs of reproduction associated with egg production, but I want to stress that also in that case this is caused by the disruption of the correlation between temporally spaced environmental variables: if the phenology of the environment at the time of egg production shifts as much as that at the time of chick rearing, the cost of reproduction associated with egg production remains the same (see Visser et al. (1998) for further discussion).
In their simplest form, reaction norms (the curve describing the relationship between a trait and the environmental variable) have a slope (i.e. the sensitivity of the trait to the environment) and an elevation (i.e. the trait value in the average environment, Pigliucci (2001)). Both can be under selection and it is useful to make a distinction between these. However, there is often a genetic covariance between slope and elevation, which may well constrain the response to selection on the reaction norm. This genetic covariance itself may also be under selection (Sgro & Hoffmann 2004) but this discussion falls outside the scope of this review.
Selection in any particular year will operate on the phenotype expressed in that specific annual environment, and hence direct selection on reaction norms (which is the phenotypic response over a range of environments) seems difficult. However, what matters for an individual is its lifetime reproductive success and hence it has to do well in all the years it reproduces, not just in 1 year. Moreover, what matters for genotypes is their fitness over a whole range of lifetimes, which will have to do well over a wide range of environmental conditions. This does enable direct selection on reaction norms, especially in long-lived species.
(i) Selection on the elevation of the reaction norm
As a measure of an individual's average phenotype, much attention has been focused on the elevation of the reaction norm and the potential to respond to selection. When these traits are heritable and under directional selection, the elevation of the reaction norm is expected to show an evolutionary change in value (Falconer & Mackay 1996). However, there are just two examples for vertebrates that have shown a response to climate change (see recent reviews Bradshaw & Holzapfel 2006; Gienapp et al. 2008). The genetic shift in phenology of the North American red squirrel (Tamiasciurus hudsonicus; Reale et al. 2003) has, however, been questioned (Postma 2006) while the blackcap (Sylvia atricapilla) for example (Berthold et al. 1992) may be a genetic response to environmental changes other than climate change. Gienapp et al. (2008) provide a set of conditions that need to be fulfilled in order to demonstrate climate change driven genetic change and conclude that studies that demonstrate such genetic change are ‘conspicuously scarce’.
One of the hypotheses that is most commonly put forward to explain a lack of a response to selection is that both phenotype and fitness values are correlated to a third, unmeasured factor, like quality (Price et al. 1988). However, both for the great tit (Parus major) population of the Hoge Veluwe (The Netherlands; Gienapp et al. 2006) and the collared flycatcher (Ficedula albicollis) population of Gotland (Sweden; Sheldon et al. 2003) there was no statistically significant difference between the selection differential on laying date phenotype or the breeding value for laying date, indicating that breeding time and fitness are causally linked.
Another reason why evolutionary stasis on wild populations is common (see Merilä et al. (2001) for a complete overview of explanations) might be that the assumption that the heritability of a trait is constant over time does not hold. The annual variation in environmental conditions may mean that both selection on a trait and the additive genetic variance of that trait vary from year to year (Wilson et al. 2006). In Soay sheep (Ovis aries), selection was weaker in good environments, and there was a negative correlation between the magnitude of selection and the magnitude of the genetic variance that year, which has implications for the predicted response to selection (Wilson et al. 2006). This has been studied for very few species, leaving open the possibility that in other species selection and genetic variance are positively correlated, speeding up the rate of microevolution.
The simplest reason for the lack of an observed genetic response to selection is that this response is very small and therefore difficult to detect. In one of the Dutch long-term great tit populations (the Hoge Veluwe), the heritability for laying date was found to be 0.17, and the predicted response was just 1.5 days over 30 years (Gienapp et al. 2006). Very long time series are needed to detect such low rates of response to selection, and given that climate change started to have an impact on natural systems ca 1980, it may take many more years before such time series are available.
(ii) Selection on plasticity of the reaction norm
While under some conditions there is selection on simply being earlier in all environments (selection on main trait value), under different conditions there may be selection on the degree of plasticity (the slope of the reaction norm, i.e. on the sensitivity of the trait phenotype for the environmental variable). This would be the case if a species is still well timed in cold years but at times its reproduction is too late in warm years. Indeed, for a Dutch great tit population, it has been shown that there is now selection on the strength of phenotypic plasticity (Nussey et al. 2005).
For microevolution in reaction norm slope there needs to be heritable variation in slope. For labile traits, we can often collect a number of trait values for the same individual. From that, it can be calculated whether there is variation among individuals in how they respond to their environment (I×E interactions, Nussey et al. 2007). Of the five examples of wild-vertebrate populations reviewed by Nussey et al. (2007), in four there is an I×E interaction. However, to get a response to selection genotypes need to differ in their response to the environment (G×E interactions). This has only been tested in two of these studies, in the collared flycatcher (Brommer et al. 2005) and the great tit (Nussey et al. 2005), and in a study on Soay sheep (Wilson et al. 2006), and in two of these three a G×E interaction was found. Clearly, we need many more studies to estimate the heritability on reaction norm slope in wild populations in general, and from there we estimate the rate of microevolution in this slope. In the great tit, for example (Nussey et al. 2005), no such response was detected.
In insects, the G×E interaction can be estimated by exposing relatives to different environments and measuring how their trait value depends on the environmental values (Nussey et al. 2007). In the timing of egg hatch of winter moths (which should be synchronized with bud burst of their host tree) there is a G×E interaction, and the reaction norm slope of egg hatch against temperature is predicted to change (van Asch et al. 2007). However, although there is a genetic response to selection in reaction norm elevation, no change in slope could be detected (van Asch et al. unpublished data). Obviously, many more studies are needed but it may be that a response to selection on reaction norm slopes is more difficult than on elevation (Wijngaarden & Brakefield 2001).
(b) Special cases of phenotypic plasticity
There are two special cases of phenotypic plasticity, which have been suggested as mechanisms for species to adapt to climate change without the need for (slow) genetic changes; learning and maternal effects. Both these mechanisms can be described as reaction norms; in case of learning with the animal's past experiences as the environmental axis and for maternal effect the component of the environment affected by the mother. As in the previous section, there can be selection on both the elevation and the slope of these reaction norms.
(i) Learning
Animals can adapt to climate change if they learn from their experiences. Learning can be seen as a form of phenotypic plasticity: but instead of the current environment affecting the phenotype it is the environment experienced during earlier reproductive events by the animal. When animals reproduce too late as a first-year breeder they may shift their timing when they get older, and be better synchronized with their food source. Learning of phenology has been experimentally shown in blue tits (Cyanistes caeruleus), which respond to the degree in which they were mistimed in one year in their laying date in the next year (Grieco et al. 2002). It is unclear in how many species such learning plays a role.
Learning of timing is expected to have evolved to deal with spatial, rather than temporal, variation in seasonality. As with the phenotypic plasticity described earlier, it is essential that the environmental variables used to shape the phenotype are correlated with the environmental variables that correlate with fitness but in this case it is the environment in one year predicting the environment in the following year. For this to work, if the location where a bird breeds is early in one year, it should be early again the next year (Visser et al. 2006). This type of learning is not simply a carry over effect from being mistimed in one year affecting laying dates in the next year as animals that lay too late in one year will lay earlier the next year (Gienapp & Visser 2006).
Climate change may lead to selection on learning as individuals who shift their phenotype strongly to their experiences during previous reproductive attempts are likely to have a higher fitness. But this is more complicated than it looks. If a warm year is followed by another warm year, as is often the case in the last decades, animals that take past experiences into account will have a higher fitness. But if a warm year is followed by a cold year this is not so, animals may easily shift their phenotype too much. This is because this type of learning has evolved to deal with spatial variation where there is this strong year to year consistency. When ‘using’ this leaning to adapt to temporal variation, a single cold year in a row of warm years will select against animals with a steep reaction norm slope of laying date versus past experiences. It is therefore not probable that very strong learning will be selected for, and the optimal reaction norm slope will depend on the year to year variation in temperature.
Given that selection on learning may be affected by climate change, it would be of interest to study the heritability of the degree of learning. For this, the effect of previous experiences needs to be separated from the effects of current conditions, which can be done by using first-time breeders as a reference (Nager & Van Noordwijk 1995; Grieco et al. 2002). Next, using pedigreed populations, the heritability of this learning effect can be estimated. Selection may also act on the ‘default trait value’ for first breeders but this is just the elevation on the reaction norm described in the previous section (although we should consider that animals also use environmental cues which provide information where they are ‘spatially’, i.e. at an early or late site).
Will learning play a substantial role in adaptation to climate change? An obvious drawback is that this learned experience cannot be passed on to the next generation. Especially in short-lived species, where a large part of the population are first-year breeders, this will not lead to sufficient adaptation at the population level as these first-year breeders will remain mistimed.
(ii) Maternal effects
Maternal effects are modifications of offspring phenotype caused by the environment provided by the mother during development (Mousseau & Fox 1998). These can be seen as a special form of phenotypic plasticity where the trait value depends on environmental variables that are under control of the mother. This opens the possibility of adaptation without genetic change (Kirkpatrick & Lande 1989) and maternal effects, therefore, are potentially important mechanisms when adapting to climate change. They can play a role via the environment as experienced by the offspring, such as photoperiod during the nestling period, and thus, where the maternal effect runs via the mother's timing, or maternal effects can run via direct effects provided by the mother, such as better care or yolk hormones.
An example of a maternal effect that runs via the mother's timing comes from blackcaps. In these migratory birds, the perceived photoperiod in the nestling phase affects their seasonal timing of moult and autumn migration (Coppack et al. 2001; Coppack & Pulido 2004): the longer the photoperiod experienced in the nestling phase, the earlier in life (i.e. at a younger age) the onset of migration. But this effect is not complete: for each later day a young bird is born, it starts migration half a day earlier in life, and thus in absolute calendar dates half a day later. Whether photoperiod during the nestling phase also affects the interval between fledging and first-time breeding is not known. In general, such an effect will be difficult to separate from genetic resemblance between mothers and daughters as early born offspring will have early reproducing parents. One way around this may be to compare the heritability in laying date for first- versus second-brood offspring (van Noordwijk 2006). These offspring are related to their mother in the same way but were raised under different photoperiodic environments. To my knowledge, not much is known about the effects of better care or maternal hormones on timing of reproduction of the offspring; although in great tits, there is an effect of female fledging mass on the clutch size she herself lays (Haywood & Perrins 1992).
Maternal effects have not evolved to cope with climate change but to cope with environmental variation that is predictable from the environment provided by the mother. Maternal effects may, under climate change, be constraining adaptation as has been suggested for the blackcap example. If laying date advances, offspring will be raised under shorter photoperiods, which in turn will lead to earlier autumn migration, perhaps too early as the growing season is prolonged under climate change (Coppack & Pulido 2004). This is a similar argument as presented earlier under the phenotypic plasticity section: if climate change leads to uncoupling of environmental variables, reaction norms, or here the maternal effects, are no longer adaptive.
The strength of maternal effects can be under selection (Kirkpatrick & Lande 1989), both at the maternal and the offspring level not only because the maternal effect themselves may have a genetic basis, i.e. determining the environment provided by the mother (maternal genes affecting the offspring phenotype), but also the response of the offspring reacting to the environment provided by the mother may be genetically determined. For the offspring, this is similar to selection on reaction norm slope and elevation. If, due to climate change, maternal effects are no longer adaptive, selection will occur and provided that the strength of the maternal effect is heritable, this will lead to microevolution in maternal effect strength. Thus, although maternal effects may play an important role in adapting to climate change, they themselves will be under selection and also here the rate of microevolution is essential for the speed at which species can adapt.
(c) Immigration
One of the responses to climate change is that individuals move away to more suitable habitats (Holt 1990). I will discuss the complementary case to ‘moving away’, i.e. the immigration of novel genotypes into the population as this fits in better with the question of how populations adapt to climate change.
Immigration of novel genotypes into a population can potentially affect the rate of adaptation to climate change but it is a very different mechanism from the ones as discussed above. It will just supply genetic variation on which selection can act. Given that climate change has led to range expansions from more southern to northern areas (Parmesan & Yohe 2003), it may well be that current immigrants into breeding populations originate from southern populations. These animals may have genotypes that are better adapted to warmer conditions and hence dispersal may lead to more genetic variation, and hence speed up adaptation rate (Garant et al. 2007). The prediction is that while in the past immigrants could be preventing local adaptation (Postma & van Noordwijk 2005), and hence there was selection against such immigrants, immigrants may now promote adaptation to the changed environment, and they will now be selected for.
This scenario is, however, implicitly based on the assumption that when southern genotypes move north, they will encounter the set of environmental conditions under which they have evolved in their original range. And this is not so, it is important to realize that the climate of The Netherlands from the 1980s will not be present anywhere on the planet in 2020. There will be more northern areas that have the same mean annual temperature, but they will have a different photoperiod and probably also different rainfall patterns. Furthermore, not all species within a food chain shift north at the same rate, and thus the southern immigrants will now depend on, for instance, trees with a northern genotype, and thus the food chain this immigrants are now a part of will respond differently to climate than in the original range of these migrants.
Immigrating genotypes from southern populations will thus be better adapted in terms of temperature, but not in terms of, for instance, photoperiod. Photoperiod plays a major role in seasonal timing in vertebrates (onset of gonadal development, Gwinner (1986)) and invertebrates (diapause, Bradshaw & Holzapfel 2001). So, also for these genotypes, climate change leads to an uncoupling of environmental variables and microevolution is needed to adapt to their new range. Thus, immigration will lead to more genetic diversity and will thus speed up adaptation to climate change but these immigrants are not already fully adapted to their new environment as is often assumed (c.f. Bridle & Vines 2007).
3. Long-term pedigrees
In the assessment of the rate of adaptation to climate change, long-term studies on wild populations with individually known animals play an essential role. These studies can be used to detect and understand temporal trends in life-history traits. Many of the examples of phenotypic shift due to climate change come from such studies (Dunn & Winkler 1999; Visser et al. 2003; Both et al. 2004).
Furthermore, long-term pedigrees also can be used to estimate heritability of traits (van Noordwijk et al. 1981; van der Jeugd & McCleery 2002). Under climate change, heritabilities may well not be constant. When the environment changes, the additive genetic variation will change for those traits for which there is a genetic basis of among-individual variation in plasticity, i.e. an environment×genes interaction (Hoffmann & Merilä 1999; Charmantier & Garant 2005). This is especially important if there is a correlation between the strength of selection and the amount of additive variation in a year. A negative correlation will slow down the response to selection (Wilson et al. 2006) while a positive correlation will speed up microevolution. As it is unlikely that the additive variance within a population will be constant over decades, and even more unlikely that it will remain constant under climate change, long-term studies can be used to detect long-term trends in heritability and to quantify any correlation between the strength of selection and the heritability.
Finally, long-term pedigrees can be used to assess the (changing) selection pressures on life-history traits. Such documentation of shifts in selection due to climate change have been few. Interestingly, even within species there are differences in how selection is affected: while in Dutch great tits there is increased selection for earlier laying (Visser et al. 1998; Nussey et al. 2005; Gienapp et al. 2006), in UK great tits, selection on early laying has become less intense (Cresswell & McCleery 2003). To understand intra- and interspecific differences in how selection intensity changes over time, additional measurements on other trophic levels of the food chain are needed (Visser & Both 2005). When a species shifts in phenology faster than the phenology of its prey (as may be the case in the UK great tits), this may lead to selection for a later timing of reproduction (or in the UK case less strong selection for earlier laying) while if they shift to a lesser extent than their prey (as in the case of the Dutch great tits) this will lead to stronger selection on early laying. Thus, the value of long-term pedigreed populations increases if additional measurements on their food chain are done.
A major problem with climate change is that we need to extrapolate outside the natural range, or at least outside the range that has been observed over the past decades. In that sense, even long-term data have little predictive value if the environment keeps getting warmer. To put this into perspective, the difference between very warm and very cold springs in The Netherlands is just 4°C, while some of the climate scenarios predict temperature increases of up to 6°C for 2100. Can we still extrapolate along, for instance, a linear reaction norm? To determine whether a reaction norm is nonlinear, long-term data from long-lived animals are needed. Other possibilities are to use controlled conditions (i.e. climate-controlled aviaries, etc.) or to move animals to other parts of their range (with a different climate) and study their phenotypes there (cf. genetically identical trees that are planted in botanical garden throughout Europe, Menzel (2000)).
A second major problem is that because climatic variables become uncoupled due to climate change, it becomes very important to identify the right environmental variables that affect the phenotypes (i.e. the x-axis of the reaction norm). Ultimately, only detailed knowledge on mechanisms will inform us about this but this is a challenging task and will require close collaboration between evolutionary ecologists and physiologists (Visser et al. 2004; Wingfield et al. 2008). An alternative route is to use statistical models that correspond much better to the underlying (unknown) mechanism. For instance, while in reaction norms of timing of reproduction laying date is often regressed against the mean temperature over some specific time period of the year, it is also possible to statistically model this as a temperature-dependent daily probability of starting reproduction, using proportional hazard models, which also allows for interactions between, for instance, temperature and photoperiod (Gienapp et al. 2005).
To complicate matters further, there is not of course a single environmental variable that affects the phenotype, there are many. A final complication is that these multi-dimensional reaction norms are not only affected by a number of environmental variables that directly affect the genotype–phenotype relationship but also the environment experienced earlier in life (learning) and the environment as under control of the mother (maternal effects). These all need to be integrated into a single ‘response mechanism’ (Visser et al. 2004).
In addition, in order to understand the variation in reaction norm slopes, the environmental variables the organisms respond to need to be precisely identified. The variation in the laying date versus temperature reaction norm in great tits (Nussey et al. 2005) may be due to differences in the sensitivity to temperature among individuals (temperature on the x-axis). However, it could also be differences in the cost of egg production, which may enable some individuals to lay early under cold conditions, but not others (food intake on the x-axis). Yet another possibility is that there is variation in the photoperiodic sensitivity (Silverin et al. 1993) and some individuals are simply not ready to lay early in spring as they have not completed their gonadal development (photoperiod on the x-axis). Especially in the phenology in vertebrates, photoperiod plays an important role (Gwinner 1986). The annual pattern in photoperiod is of course not changing, leading to an uncoupling of photoperiodic and temperature patterns, and this will mean that the rate of microevolution in sensitivity to photoperiod may at some point become more important than the rate of microevolution in temperature sensitivity (Bradshaw & Holzapfel 2008).
To resolve these two problems, long-term pedigreed population studies need to be accompanied by physiological studies on the mechanisms underlying phenotypic plasticity. These mechanisms will tell us which variables actually play a role in plasticity, and what their ‘weight’ is within the response mechanism. Although it is less likely that species start using different environmental variables (cues), it is more probable that these ‘weights’ are under selection. Knowledge of the genetic variation in, and selection on, the components of the mechanism underlying plasticity is crucial to make the step towards predicting the rate of adaptation to climate change (see also Wingfield et al. 2008).
4. Linking climate scenarios to biodiversity loss
Climate change is threatening biodiversity (McLaughlin et al. 2002; Thomas et al. 2004) as organisms are no longer adapted to their changed environment. There are still relatively few examples that link disrupted life-history traits of organisms to their population viability or even extinction risks. One of the clear examples is the decline of pied flycatchers (Ficedula hypoleuca) in areas where the birds are most severely mistimed with their nestlings' food (Both et al. 2006). Another example is the decline of black grouse (Tetrao tetrix) population numbers that is linked in with mistimed reproduction (Ludwig et al. 2006). In, also mistimed, great tits, there is a decline in fitness (number of surviving offspring produced, Nussey et al. (2005)). However, there is no clear decline in population numbers, probably because population density is strongly affected by winter food in the form of beech nuts (Perdeck et al. 2000). The same is found in song sparrows (Melospiza melodia) where, despite increasing selection for early breeding, there is no effect on population numbers as numbers may be determined by juvenile survival, which is not affected by climate (Wilson & Arcese 2003). The development of a theoretical framework to link life-history traits under natural selection and population dynamics (ecoevolutionary dynamics), such as described above, has only recently been initiated (Hairston et al. 2005; Saccheri & Hanski 2006; Caroll et al. 2007; Pelletier et al. 2007) but is much needed to assess the ecological consequences of climate change.
The slow rate of adaptation will have large population consequences as populations will lag behind in their phenotype distribution. I want to briefly mention that climate change may also have population consequences even when the rate of adaptation is high enough (figure 1d). As discussed in the introduction, the rate of adaptation can be viewed as sufficiently high if there is stabilizing selection throughout the period of the mean phenotype shift. It is important to realize that this does not necessarily mean that there is no decline in the mean reproductive output per individual or that population numbers stay stable. For instance, if climate change leads to a lower quality habitat because some prey species disappear, birds are less able to rear large broods and thus this will lead to a decline in the average clutch size via microevolution. Even if this microevolution is instantaneous, and thus there is a high rate of adaptation and there is always stabilizing selection, the reproductive output (and population numbers) will go down simply because in these poorer conditions fewer offspring can be reared to independence. But obviously, when the rate of adaptation is low, the shift in clutch size will lag behind and the impact of climate change will be even more severe.
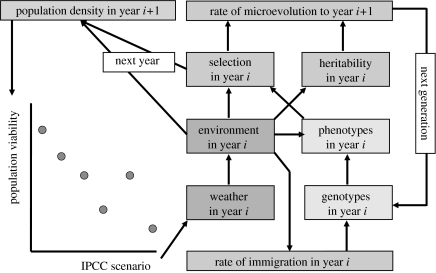
With knowledge of both the rate of adaptation in different environments, and of the population consequences of disrupted life-history traits, we will be able to link population viability to IPCC socio-economic scenarios (IPCC 2007). From the socio-economic scenarios, emissions are calculated and from there climatic effects such as temperature and rainfall follow. From these, evolutionary ecologists need to predict the rate of genetic change in life-history traits, and as a final step, the effects on population viability for those scenarios where this rate of adaptation is insufficient. As is depicted in figure 2, we can then use these insights to link population viability to socio-economic scenarios.
Figure 2.
Diagram highlighting the importance of assessing the rate of microevolution when determining the ecological consequences of climate change, here depicted as the way population viability depends on the socio-economic development of the planet (as captured in the six scenarios of the IPCC). Each IPCC scenario predicts how the climate will change up to 2100. This will determine the weather in a specific year, which in turn will affect the biotic and abiotic environment for a species. This environment, combined with the set of genotypes, determines the set of phenotypes as these are phenotypically plastic. The environment also affects the heritability of the trait and, combined with the set of phenotypes, will determine the strength of selection on the trait. This selection and heritability determine the rate of microevolution, and thus, in combination with the immigration rate, the set of genotypes in the following year. The environment (via its (density dependent) effect on the average survival of adults and juveniles) and the strength of selection affect the population numbers in the next year, making the link to population viability. It is expected that the IPCC scenarios that will lead to a slow rate in temperature increase will have a small impact on population viability as the rate of microevolution will be sufficient for species to keep up with the warming world.
In order to make this link, evolutionary ecologists will need climate predictions with a resolution of days as, especially the phenology of organisms, life-history traits correlate with temperatures in very specific periods of the year (Visser et al. 2006). Climatologists use IPCC socio-economic and emission scenarios (IPCC 2007) to calculate such climate scenarios (using general circulation models like the ECHAM4 and the HadCM3, Pope et al. (2000)), and predict minimum/maximum temperatures, rainfall etc. on a daily basis for 1960–2100. Ideally, climatologists need to provide evolutionary biologists with three climate scenarios for each of the six IPCC emission scenarios (which in turn are base on socio-economic scenarios). This set of 18 climate scenarios can then be used to make predictions on the rate of microevolution. An example for an insect herbivore, the winter moth, for just a single climate scenario shows a microevolution rate sufficiently fast to match the climate change as predicted by that scenario (van Asch et al. 2007). Predicting the rate of microevolution for other emission scenarios for the winter moth was hampered by a lack of such a set of climate scenarios.
Climate change is one of the largest threats to biodiversity of our times. Only when we, as a planet, adopt a socio-economic strategy that will allow organisms to adapt in pace with the changes in their environment can we prevent severe loss of species due to global climate change. Determining the rate of climate change that populations can cope with is, therefore, information that is urgently needed. Quantitative geneticist and evolutionary ecologists, analysing long-term pedigreed datasets of wild populations, will play a crucial role in providing this key insight. We have work to do.
Acknowledgments
The author would like to thank Dan Nussey, Phillip Gienapp and three anonymous referees for their comments on a previous version of the paper and Loeske Kruuk for inviting him to contribute to this special issue, for initial discussions on the topic of the paper and for comments on all previous versions of the paper.
Footnotes
One contribution of 18 to a Special Issue ‘Evolutionary dynamics of wild populations’.
References
- Austin G.E, Rehfisch M.M. Shifting nonbreeding distributions of migratory fauna in relation to climatic change. Glob. Change Biol. 2005;11:31–38. doi:10.1111/j.1529-8817.2003.00876.x [Google Scholar]
- Beebee T.J.C. Amphibian breeding and climate. Nature. 1995;374:219–220. doi:10.1038/374219a0 [Google Scholar]
- Berthold P, Helbig A.J, Mohr G, Querner U. Rapid microevolution of migratory behavior in a wild bird species. Nature. 1992;360:668–670. doi:10.1038/360668a0 [Google Scholar]
- Bertness M.D, Ewanchuk P.J. Latitudinal and climate-driven variation in the strength and nature of biological interactions in New England salt marshes. Oecologia. 2002;132:392–401. doi: 10.1007/s00442-002-0972-y. doi:10.1007/s00442-002-0972-y [DOI] [PubMed] [Google Scholar]
- Both C, Visser M.E. Adjustment to climate change is constrained by arrival date in a long-distance migrant bird. Nature. 2001;411:296–298. doi: 10.1038/35077063. doi:10.1038/35077063 [DOI] [PubMed] [Google Scholar]
- Both C, et al. Large-scale geographical variation confirms that climate change causes birds to lay earlier. Proc. R. Soc. B. 2004;271:1657–1662. doi: 10.1098/rspb.2004.2770. doi:10.1098/rspb.2004.2770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Both C, Bouwhuis S, Lessells C.M, Visser M.E. Climate change and population declines in a long-distance migratory bird. Nature. 2006;441:81–83. doi: 10.1038/nature04539. doi:10.1038/nature04539 [DOI] [PubMed] [Google Scholar]
- Bradshaw W.E, Holzapfel C.M. Genetic shift in photoperiodic response correlated with global warming. Proc. Natl Acad. Sci. USA. 2001;98:14 509–14 511. doi: 10.1073/pnas.241391498. doi:10.1073/pnas.241391498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradshaw W.E, Holzapfel C.M. Evolutionary responses to rapid climate change. Science. 2006;312:1477–1478. doi: 10.1126/science.1127000. doi:10.1126/science.1127000 [DOI] [PubMed] [Google Scholar]
- Bradshaw W.E, Holzapfel C.M. Genetic response to raped climate change: it's seasonal timing that matters. Mol. Ecol. 2008;17:157–166. doi: 10.1111/j.1365-294X.2007.03509.x. doi:10.1111/j.1365-294X.2007.03509.x [DOI] [PubMed] [Google Scholar]
- Bridle J.R, Vines T.H. Limits to evolution at range margins: when and why does adaptation fail? Trends Ecol. Evol. 2007;22:140–147. doi: 10.1016/j.tree.2006.11.002. doi:10.1016/j.tree.2006.11.002 [DOI] [PubMed] [Google Scholar]
- Brommer J.E, Merila¨ J, Sheldon B.C, Gustafsson L. Natural selection and genetic variation for reproductive reaction norms in a wild bird population. Evolution. 2005;59:1362–1371. [PubMed] [Google Scholar]
- Caroll S.P, Hendry A.P, Reznick D.N, Fox C.W. Evolution on ecological time-scales. Funct. Ecol. 2007;21:387–393. doi:10.1111/j.1365-2435.2007.01289.x [Google Scholar]
- Charmantier A, Garant D. Environmental quality and evolutionary potential: lessons from wild populations. Proc. R. Soc. B. 2005;272:1415–1425. doi: 10.1098/rspb.2005.3117. doi:10.1098/rspb.2005.3117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coppack T, Pulido F. Photoperiodic response and the adaptability of avian life cycles to environmental change. Adv. Ecol. Res. 2004;35:131–150. [Google Scholar]
- Coppack T, Pulido F, Berthold P. Photoperiodic response to early hatching in a migratory bird species. Oecologia. 2001;128:181–186. doi: 10.1007/s004420100652. doi:10.1007/s004420100652 [DOI] [PubMed] [Google Scholar]
- Cresswell W, McCleery R. How great tits maintain synchronization of their hatch date with food supply in response to long-term variability in temperature. J. Anim. Ecol. 2003;72:356–366. doi:10.1046/j.1365-2656.2003.00701.x [Google Scholar]
- Crick H.Q.P, Dudley C, Glue D.E, Thomson D.L. UK birds are laying eggs earlier. Nature. 1997;388:526. doi:10.1038/41453 [Google Scholar]
- Davis M.B, Shaw R.G, Etterson J.R. Evolutionary responses to changing climate. Ecology. 2005;86:1704–1714. doi:10.1890/03-0788 [Google Scholar]
- Dunn P.O, Winkler D.W. Climate change has affected the breeding date of tree swallows throughout North America. Proc. R. Soc. B. 1999;266:2487–2490. doi: 10.1098/rspb.1999.0950. doi:10.1098/rspb.1999.0950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falconer D.S, Mackay T.F.C. Longman; New York, NY: 1996. Introduction to quantitative genetics. [Google Scholar]
- Garant D, Forde S.E, Hendry A.P. The multifarious effects of dispersal and gene flow on contemporary adaptation. Funct. Ecol. 2007;21:434–443. doi:10.1111/j.1365-2435.2006.01228.x [Google Scholar]
- Garant, D., Hadfield, J. D., Kruuk, L. E. B. & Sheldon, B. C. In press. Stability of genetic variance and covariance for reproductive characters in the face of climate change in a wild bird population. Mol. Ecol [DOI] [PubMed]
- Gienapp P, Visser M.E. Possible fitness consequences of experimentally advanced laying dates in great tits: differences between populations in different habitats. Funct. Ecol. 2006;20:180–185. doi:10.1111/j.1365-2435.2006.01079.x [Google Scholar]
- Gienapp P, Hemerik L, Visser M.E. A new statistical tool to predict phenology under climate change scenarios. Glob. Change Biol. 2005;11:600–606. doi:10.1111/j.1365-2486.2005.00925.x [Google Scholar]
- Gienapp P, Postma E, Visser M.E. Why breeding time has not responded to selection for earlier breeding in a songbird population. Evolution. 2006;60:2381–2388. [PubMed] [Google Scholar]
- Gienapp P, Teplitsky C, Alho J.S, Mills J.A, Merilä J. Climate change and evolution: disentangling environmental and genetic responses. Mol. Ecol. 2008;17:167–178. doi: 10.1111/j.1365-294X.2007.03413.x. doi:10.1111/j.1365-294X.2007.03413.x [DOI] [PubMed] [Google Scholar]
- Grieco F, van Noordwijk A.J, Visser M.E. Evidence for the effect of learning on timing of reproduction in blue tits. Science. 2002;296:136–138. doi: 10.1126/science.1068287. doi:10.1126/science.1068287 [DOI] [PubMed] [Google Scholar]
- Gwinner E. Springer; Berlin, Germany: 1986. Circannual rhythms. [Google Scholar]
- Hairston N.G, Ellner S.P, Geber M.A, Yoshida T, Fox J.A. Rapid evolution and the convergence of ecological and evolutionary time. Ecol. Lett. 2005;8:1114–1127. doi:10.1111/j.1461-0248.2005.00812.x [Google Scholar]
- Haywood S, Perrins C.M. Is clutch size in birds affected by environmental-conditions during growth? Proc. R. Soc. B. 1992;249:195–197. doi: 10.1098/rspb.1992.0103. doi:10.1098/rspb.1992.0103 [DOI] [PubMed] [Google Scholar]
- Hoffmann A.A, Merilä J. Heritable variation and evolution under favourable and unfavourable conditions. Trends Ecol. Evol. 1999;14:96–101. doi: 10.1016/s0169-5347(99)01595-5. doi:10.1016/S0169-5347(99)01595-5 [DOI] [PubMed] [Google Scholar]
- Holt R.D. The microevolutionary consequences of climate change. Trends Ecol. Evol. 1990;5:311–315. doi: 10.1016/0169-5347(90)90088-U. doi:10.1016/0169-5347(90)90088-U [DOI] [PubMed] [Google Scholar]
- Hüppop O, Hüppop K. North Atlantic oscillation and timing of spring migration in birds. Proc. R. Soc. B. 2003;270:233–240. doi: 10.1098/rspb.2002.2236. doi:10.1098/rspb.2002.2236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- IPCC. Summary for policymakers. In: Solomon S, Qin D, Manning M, Chen Z, Marquis M, editors. Climate change 2007: the physical science basis. Contribution of working group I to the fourth assessment report of the intergovernmental panel on climate change. Cambridge University Press; Cambridge, UK: 2007. pp. 1–18. [Google Scholar]
- Jiang L, Morin P.J. Temperature-dependent interactions explain unexpected responses to environmental warming in communities of competitors. J. Anim. Ecol. 2004;73:569–576. doi:10.1111/j.0021-8790.2004.00830.x [Google Scholar]
- Jonzen N, Hedenstrom A, Lundberg P. Climate change and the optimal arrival of migratory birds. Proc. R. Soc. B. 2007;274:269–274. doi: 10.1098/rspb.2006.3719. doi:10.1098/rspb.2006.3719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkpatrick M, Lande R. The evolution of maternal characters. Evolution. 1989;43:485–503. doi: 10.1111/j.1558-5646.1989.tb04247.x. doi:10.2307/2409054 [DOI] [PubMed] [Google Scholar]
- Ludwig G.X, Alatalo R.V, Helle P, Linden H, Lindstrom J, Siitari H. Short- and long-term population dynamical consequences of asymmetric climate change in black grouse. Proc. R. Soc. B. 2006;273:2009–2016. doi: 10.1098/rspb.2006.3538. doi:10.1098/rspb.2006.3538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin J.F, Hellmann J.J, Boggs C.L, Ehrlich P.R. Climate change hastens population extinctions. Proc. Natl Acad. Sci. USA. 2002;99:6070–6074. doi: 10.1073/pnas.052131199. doi:10.1073/pnas.052131199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNamara J.M, Houston A.I. Optimal annual routines: behaviour in the context of physiology and ecology. Phil. Trans. R. Soc. B. 2008;363:301–319. doi: 10.1098/rstb.2007.2141. doi:10.1098/rstb.2007.2141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzel A. Trends in phenological phases in Europe between 1951 and 1996. Int. J. Biometeorol. 2000;44:76–81. doi: 10.1007/s004840000054. doi:10.1007/s004840000054 [DOI] [PubMed] [Google Scholar]
- Menzel A, Fabian P. Growing season extended in Europe. Nature. 1999;397:659. doi:10.1038/17709 [Google Scholar]
- Merilä J, Sheldon B.C, Kruuk L.E.B. Explaining stasis: microevolutionary studies in natural populations. Genetica. 2001;112:199–222. doi:10.1023/A:1013391806317 [PubMed] [Google Scholar]
- Millien V, Lyons S.K, Olson L, Smith F.A, Wilson A.B, Yom-Tov Y. Ecotypic variation in the context of global climate change: revisiting the rules. Ecol. Lett. 2006;9:853–869. doi: 10.1111/j.1461-0248.2006.00928.x. doi:10.1111/j.1461-0248.2006.00928.x [DOI] [PubMed] [Google Scholar]
- Mousseau T.A, Fox C.W. Oxford University Press; New York, NY: 1998. Maternal effects as adaptations. [Google Scholar]
- Nager R.G, Van Noordwijk A.J. Proximate and ultimate aspects of phenotypic plasticity in timing of great tit breeding in a heterogeneous environment. Am. Nat. 1995;146:454–474. doi:10.1086/285809 [Google Scholar]
- Nussey D.H, Postma E, Gienapp P, Visser M.E. Selection on heritable phenotypic plasticity in a wild bird population. Science. 2005;310:304–306. doi: 10.1126/science.1117004. doi:10.1126/science.1117004 [DOI] [PubMed] [Google Scholar]
- Nussey D.H, Wilson A.J, Brommer J.E. The evolutionary ecology of individual phenotypic plasticity in wild populations. J. Evol. Biol. 2007;20:831–844. doi: 10.1111/j.1420-9101.2007.01300.x. doi:10.1111/j.1420-9101.2007.01300.x [DOI] [PubMed] [Google Scholar]
- Parmesan C. Ecological and evolutionary responses to recent climate change. Annu. Rev. Ecol. Evol. Syst. 2006;37:637–669. doi:10.1146/annurev.ecolsys.37.091305.110100 [Google Scholar]
- Parmesan C, Yohe G. A globally coherent fingerprint of climate change impacts across natural systems. Nature. 2003;421:37–42. doi: 10.1038/nature01286. doi:10.1038/nature01286 [DOI] [PubMed] [Google Scholar]
- Parmesan C, et al. Poleward shifts in geographical ranges of butterfly species associated with regional warming. Nature. 1999;399:579–583. doi:10.1038/21181 [Google Scholar]
- Pelletier F, Clutton-Brock T, Pemberton J, Tuljapurkar S, Coulson T. The evolutionary demography of ecological change: linking trait variation and population growth. Science. 2007;315:1571–1574. doi: 10.1126/science.1139024. doi:10.1126/science.1139024 [DOI] [PubMed] [Google Scholar]
- Perdeck A.C, Visser M.E, Van Balen J.H. Great tit Parus major survival, and the beech-crop cycle. Ardea. 2000;88:99–108. [Google Scholar]
- Perrins C.M. The timing of birds' breeding season. Ibis. 1970;112:242–255. [Google Scholar]
- Pigliucci M. Syntheses in ecology and evolution. John Hopkins University Press; Baltimore, MD: 2001. Phenotypic plasticity; beyond nature and nurture. [Google Scholar]
- Pope V.D, Gallani M.L, Rowntree P.R, Stratton R.A. The impact of new physical parametrizations in the Hadley Centre climate model: HadAM3. Clim. Dyn. 2000;16:123–146. doi:10.1007/s003820050009 [Google Scholar]
- Postma E. Implications of the difference between true and predicted breeding values for the study of natural selection and micro-evolution. J. Evol. Biol. 2006;19:309–320. doi: 10.1111/j.1420-9101.2005.01007.x. doi:10.1111/j.1420-9101.2005.01007.x [DOI] [PubMed] [Google Scholar]
- Postma E, van Noordwijk A.J. Gene flow maintains a large genetic difference in clutch size at a small spatial scale. Nature. 2005;433:65–68. doi: 10.1038/nature03083. doi:10.1038/nature03083 [DOI] [PubMed] [Google Scholar]
- Price T, Kirkpatrick M, Arnold S.J. Directional selection and the evolution of breeding date in birds. Science. 1988;240:798–799. doi: 10.1126/science.3363360. doi:10.1126/science.3363360 [DOI] [PubMed] [Google Scholar]
- Przybylo R, Sheldon B.C, Merila J. Climatic effects on breeding and morphology: evidence for phenotypic plasticity. J. Anim. Ecol. 2000;69:395–403. doi:10.1046/j.1365-2656.2000.00401.x [Google Scholar]
- Reale D, McAdam A.G, Boutin S, Berteaux D. Genetic and plastic responses of a northern mammal to climate change. Proc. R. Soc. B. 2003;270:591–596. doi: 10.1098/rspb.2002.2224. doi:10.1098/rspb.2002.2224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root T.L, Price J.T, Hall K.R, Schneider S.H, Rosenzweig C, Pounds J.A. Fingerprints of global warming on wild animals and plants. Nature. 2003;421:57–60. doi: 10.1038/nature01333. doi:10.1038/nature01333 [DOI] [PubMed] [Google Scholar]
- Saccheri I, Hanski I. Natural selection and population dynamics. Trends Ecol. Evol. 2006;21:341–347. doi: 10.1016/j.tree.2006.03.018. doi:10.1016/j.tree.2006.03.018 [DOI] [PubMed] [Google Scholar]
- Sgro C.M, Hoffmann A.A. Genetic correlations, tradeoffs and environmental variation. Heredity. 2004;93:241–248. doi: 10.1038/sj.hdy.6800532. doi:10.1038/sj.hdy.6800532 [DOI] [PubMed] [Google Scholar]
- Sheldon B.C, Kruuk L.E.B, Merila J. Natural selection and inheritance of breeding time and clutch size in the collared flycatcher. Evolution. 2003;57:406–420. doi: 10.1111/j.0014-3820.2003.tb00274.x. [DOI] [PubMed] [Google Scholar]
- Silverin B, Massa R, Stokkan K.A. Photoperiodic adaptation to breeding at different latitudes in great tits. Gen. Comp. Endocrinol. 1993;90:14–22. doi: 10.1006/gcen.1993.1055. doi:10.1006/gcen.1993.1055 [DOI] [PubMed] [Google Scholar]
- Sparks T.H, Roy D.B, Dennis R.L.H. The influence of temperature on migration of Lepidoptera into Britain. Glob. Change Biol. 2005;11:507–514. doi:10.1111/j.1365-2486.2005.00910.x [Google Scholar]
- Stenseth N.C, Mysterud A. Climate, changing phenology, and other life history and traits: nonlinearity and match–mismatch to the environment. Proc. Natl Acad. Sci. USA. 2002;99:13 379–13 381. doi: 10.1073/pnas.212519399. doi:10.1073/pnas.212519399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson I.R, Bryant D.M. Climate change and constraints on breeding. Nature. 2000;406:366–367. doi: 10.1038/35019151. doi:10.1038/35019151 [DOI] [PubMed] [Google Scholar]
- Thomas C.D, Lennon J.J. Birds extend their ranges northwards. Nature. 1999;399:213. doi:10.1038/20335 [Google Scholar]
- Thomas C.D, et al. Extinction risk from climate change. Nature. 2004;427:145–148. doi: 10.1038/nature02121. doi:10.1038/nature02121 [DOI] [PubMed] [Google Scholar]
- van Asch M, van Tienderen P.H, Holleman L.J.M, Visser M.E. Predicting shifts in phenology in response to climate change, an insect herbivore example. Glob. Change Biol. 2007;13:1596–1604. doi:10.1111/j.1365-2486.2007.01400.x [Google Scholar]
- van der Jeugd H.P, McCleery R. Effects of spatial autocorrelation, natal philopatry and phenotypic plasticity on the heritability of laying date. J. Evol. Biol. 2002;15:380–387. doi:10.1046/j.1420-9101.2002.00411.x [Google Scholar]
- van Noordwijk A.J. Are unseen effects of early environment negligible? Three examples in great tits (Parus major) Acta Zool. Sin. 2006;52(Suppl.):675–677. [Google Scholar]
- van Noordwijk A.J, Muller C.B. On adaptive plasticity in reproductive traits, illustrated with laydate in the great tit and colony inception in a bumble bee. In: Jarman P, Rossiter A, editors. Animal societies individuals, interactions and organization. Kyoto University Press; Kyoto, Japan: 1994. pp. 180–194. [Google Scholar]
- van Noordwijk A.J, van Balen J.H, Scharloo W. Genetic variation in the timing of reproduction in the great tit. Oecologia. 1981;49:158–166. doi: 10.1007/BF00349183. doi:10.1007/BF00349183 [DOI] [PubMed] [Google Scholar]
- Visser M.E, Both C. Shifts in phenology due to global climate change: the need for a yardstick. Proc. R. Soc. B. 2005;272:2561–2569. doi: 10.1098/rspb.2005.3356. doi:10.1098/rspb.2005.3356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser M.E, Holleman L.J.M. Warmer springs disrupt the synchrony of oak and winter moth phenology. Proc. R. Soc. B. 2001;268:289–294. doi: 10.1098/rspb.2000.1363. doi:10.1098/rspb.2000.1363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser, M. E. & Lambrechts, M. M. 1999 Information contraints in the timing of reproduction in temperate zone birds: great and blue tits. In Proc. 22nd Int. Ornithol. Congr. Durban (eds N. J. Adams & R. H. Slotow), pp. 249–264. Johannesburg, South Africa: BirdLife.
- Visser M.E, Lessells C.M. The costs of egg production and incubation in great tits (Parus major) Proc. R. Soc. B. 2001;268:1271–1277. doi: 10.1098/rspb.2001.1661. doi:10.1098/rspb.2001.1661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser M.E, van Noordwijk A.J, Tinbergen J.M, Lessells C.M. Warmer springs lead to mistimed reproduction in great tits (Parus major) Proc. R. Soc. B. 1998;265:1867–1870. doi:10.1098/rspb.1998.0514 [Google Scholar]
- Visser M.E, et al. Variable responses to large-scale climate change in European Parus populations. Proc. R. Soc. B. 2003;270:367–372. doi: 10.1098/rspb.2002.2244. doi:10.1098/rspb.2002.2244 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser M.E, Both C, Lambrechts M.M. Global climate change leads to mistimed avian reproduction. Adv. Ecol. Res. 2004;35:89–110. [Google Scholar]
- Visser M.E, Holleman L.J.M, Gienapp P. Shifts in caterpillar biomass phenology due to climate change and its impact on the breeding biology of an insectivorous bird. Oecologia. 2006;147:164–172. doi: 10.1007/s00442-005-0299-6. doi:10.1007/s00442-005-0299-6 [DOI] [PubMed] [Google Scholar]
- Walther G.R, Post E, Convey P, Menzel A, Parmesan C, Beebee T.J.C, Fromentin J.M, Hoegh-Guldberg O, Bairlein F. Ecological responses to recent climate change. Nature. 2002;416:389–395. doi: 10.1038/416389a. doi:10.1038/416389a [DOI] [PubMed] [Google Scholar]
- Wijngaarden P.J, Brakefield P.M. Lack of response to artificial selection on the slope of reaction norms for seasonal polyphenism in the butterfly Bicyclus anynana. Heredity. 2001;87:410–420. doi: 10.1046/j.1365-2540.2001.00933.x. doi:10.1046/j.1365-2540.2001.00933.x [DOI] [PubMed] [Google Scholar]
- Wilson S, Arcese P. El Nino drives timing of breeding but not population growth in the song sparrow (Melospiza melodia) Proc. Natl Acad. Sci. USA. 2003;100:11 139–11 142. doi: 10.1073/pnas.1931407100. doi:10.1073/pnas.1931407100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson A.J, Pemberton J.M, Pilkington J.G, Coltman D.W, Mifsud D.V, Clutton-Brock T.H, Kruuk L.E.B. Environmental coupling of selection and heritability limits evolution. PLoS Biol. 2006;4:1270–1275. doi: 10.1371/journal.pbio.0040216. doi:10.1371/journal.pbio.0040216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingfield J.C, Visser M.E, Williams T.D. Integration of ecology and endocrinology in avian reproduction: a new synthesis. Phil. Trans. R. Soc. B. 2008;363:425–441. doi: 10.1098/rstb.2007.0012. doi:10.1098/rstb.2007.2149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yom-Tov Y, Yom-Tov S, Wright J, Thorne C.J.R, Du Feu R. Recent changes in body weight and wing length among some British passerine birds. Oikos. 2006;112:91–101. doi:10.1111/j.0030-1299.2006.14183.x [Google Scholar]