Abstract
Modular tissue-engineered constructs are assembled from sub-mm sized cylindrical collagen gel modules which are covered with a surface layer of human umbilical vein endothelial cells (HUVEC). The resulting construct is permeated by a network of interconnected endothelial cell lined channels to facilitate blood perfusion and nutrient delivery. This design strategy relies critically on the endothelial cells layer behaving in a non-thrombogenic manner on the module surface and the objective here was to characterize this thrombogenity. HUVEC prolonged clotting times in whole blood-module mixtures, and enabled slightly heparinized whole blood perfusion of an assembled modular construct in vitro with no increase in platelet loss compared to background levels. Flow cytometry and scanning electron microscopy indicated that HUVEC seeded modules reduced platelet activation and deposition but not leukocyte activation, compared to collagen only modules. Plasma recalcification times on non-stimulated HUVEC were longer compared to stimulated HUVEC but not different than on collagen only modules films and were not prolonged by incubation with a tissue factor blocking antibody. Together these data suggest that a functional nonthrombogenic layer of EC was generated on the module surface and that this layer should be sufficient to maintain continuous blood flow through an engineered modular tissue. In/ex vivo studies are warranted to confirm this conclusion.
Introduction
Large tissue engineered constructs must be vascularised to enable sufficient nutrient delivery to all regions within the construct [1]. We recently proposed a modular design strategy, in which tissue constructs are assembled from sub-mm sized cylindrical modules [2]. The resulting construct is permeated by a network of interconnected channels which facilitate nutrient delivery (Figure 1). Because the surface of each module is covered with a layer of endothelial cells prior to construct assembly, the resulting interconnected channel network is lined with an endothelial cell layer which is expected to enable blood to be continuously perfused through the construct with minimal thrombosis. This modular design strategy relies critically on the endothelial layer behaving in a non-thrombogenic manner on the module surface.
Figure 1.
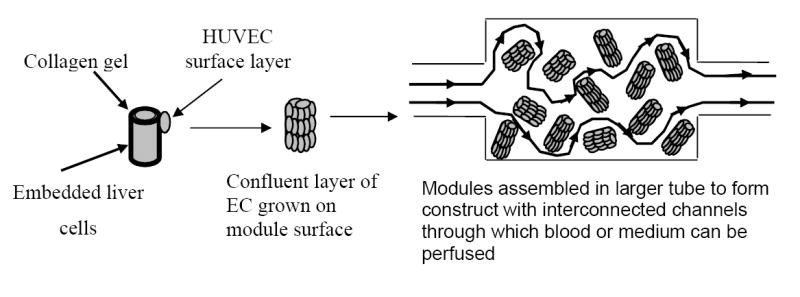
Schematic illustration of modular tissue engineering. Adapted from reference 2.
Thrombosis involves the activation of three interconnected regulatory systems, the coagulation cascade, the complement cascade and the cellular components of the blood such as platelets and leukocytes. Due to interactions among these three regulatory systems [3, 4] activation of one system typically results in some level of disruption of the other two thus creating a multifaceted cascade to facilitate wound repair, the ultimate “goal” of thrombosis. Endothelial cells (EC) line the vasculature and regulate thrombosis through the expression and secretion of a spectrum of molecules [5]. Specifically, EC mediate coagulation [6,7], through the surface expression of tissue factor (TF) [8,9], a key coagulation initiation molecule, and by the expression/secretion of thrombomodulin (TM) [10,11] and tissue factor pathway inhibitor (TFPI) [12], two key negative feeback control molecules. Surface bound heparan sulphate [13], which supports antithrombin binding, also modulate the output of active thrombin. Leukocyte and platelet adhesion and aggregation are regulated through the expression of several agonists such as nitric oxide (NO) [14] and prostacyclin (PGI2) [15] and adhesion molecules on the EC surface [16,17] such as ICAM-1, P-selectin, and VCAM-1. In addition EC express molecules involved in complement regulation and receptors for a number of complement system proteins [18,19,20]. The relative levels of all these mediators dictate whether an endothelium surface is pro- or anti-thrombogenic and thus the endothelium behaves as a dynamic interface which actively maintains blood circulation until tissue damage occurs, at which point the EC drive local thrombosis and inflammation enabling efficient wound repair.
The relative balance of the different pro- and anti-thrombogenic molecules is determined by chemical [21] and mechanical cues (e.g., fluid shear [22,23] acting on the EC through interactions with the surrounding blood, extracellular matrix (ECM) [24] and peripheral cells [25,26].The underlying substrate material on which the EC are grown may also alter cell thrombogenicity.
The objective of this particular study was to characterize the thrombogenicity of human umbilical vein endothelial cells (HUVEC) cultured on collagen modules, without any other cell embedded within the collagen, using human whole blood and plasma. Previous work demonstrated that tissue factor expression was low and thrombomodulin expression was high in HUVEC cultured on collagen modules, suggesting a non-thrombogenic phenotype [27]. It is unclear however, the extent to which such molecular expression levels translate into EC thrombogenicity since it is a combination of many molecules that determines whether the EC is indeed nonthrombogenic. The focus here was therefore to characterise thrombogenicity more directly using leukocyte and platelet adhesion and activation, clotting time, and construct perfusion assays. We were also interested in parsing which of the many interrelated systems was primarily involved in the observed thrombogenicity. Our data are consistent with the molecular data published previously and suggest that the HUVEC exhibit a non-thrombogenic phenotype on the module surface and that their presence reduced thrombosis compared to collagen modules without endothelial cells.
Methods
HUVEC culture
HUVEC (Cambrex Bio Science Walkersville, Inc), were cultured in 75 cm2 tissue culture flasks in EMB-2 medium supplemented with EMB-2 bullet kit (Cambrex Bio Science, Walkersville, Inc - contains hEGF, hydrocortisone, GA-1000, fetal bovine serum, VEGF, hFGF-β, R3–IGF-1, ascorbic acid, heparin) at 37 °C in a 5% CO2/95% air humidified atmosphere.
Module fabrication
Module fabrication using Vitrogen collagen solution (Type I, bovine dermal, 3.1 mg collagen per mL; Cohesion technologies, Palo Alto, CA) is described in detail elsewhere [28, 29]. Briefly, collagen solution was gelled (30 minutes) inside ethylene oxide gas sterilized polyethylene tubing (0.76 mm ID × 1.22 mm OD) and cut into 2 mm lengths using an automated cutter (FCS Technology, London ON). Sections were vortexed gently in cell culture medium to remove the collagen cores from the lumen of the polyethylene tubing. HUVEC between passages 1 and 8 (1.5 - 2.0 × 106 cells per mL of settled modules) were added to modules in a 15 mL centrifuge tube and incubated for 60 minutes with gentle shaking every 10 minutes. Modules were then transferred into a non-tissue culture polystyrene petri dish (Falcon). Medium was replaced every 1-3 days. Except where stated otherwise, collagen modules that had not been seeded with HUVEC were used as the control. Previous studies have shown that EC thrombogenicity is dependent on the level of cell confluence [30]; therefore HUVEC covered modules were incubated for 7 days before experiments were performed to ensure complete coverage of the surface with HUVEC.
Whole blood collection and plasma preparation
Fresh whole blood (10 mL) was collected from consenting donors (with ethics approval by the University of Toronto), who had not taken medication within 72 hours of phlebotomy, into a syringe containing heparin (0.75 - 5 U/mL depending on experiment, Organon, Roseland, NJ.), after discarding the first mL. Each test was repeated using 2 - 4 different donors.
Fresh plasma, used within 30 minutes of collection, was prepared by centrifugation of freshly collected citrated human whole blood: platelet poor plasma (PPP) at 2000g and platelet rich plasma (PRP) at 1500g.
Whole blood - platelet and leukocyte activation
Anticoagulated whole blood was exposed to modules using a modified version of the rocking platform test described previously [31]. Briefly, 400 μL of whole blood (final heparin concentration 5 U/ml) was added to a microcentrifuge tube containing either, 50 μL collagen modules, 50 μL of HUVEC covered modules or no modules. A 400 μL sample of the blood-module mixture was then transferred into the lumen of a 25 cm length of polypropylene tubing (1.57mm ID) connected at either end via Silastic™ tubing (1.57mm ID) to 200 μL pipette tips connected to a rocking platform at 37 °C. Blood was rocked for 1 h (13 oscillations per minute) and then collected in EDTA (5 mM final concentration) for flow cytometry analysis of microparticle formation, leukocyte activation and platelet-leukocyte aggregation. A sample of blood, termed “resting control”, was left at rest in a microcentrifuge tube (without modules) at 37 °C for one hour.
Samples were analyzed on a flow cytometer (FACScan, Becton Dickinson, Mountain View, CA) using CELLQuest software as described previously (32). Briefly small aliquots (5-20 μL) of blood, diluted in PBS (Invitrogen), were incubated with saturating concentrations of fluorescently labeled antibodies for 30 minutes at 4 °C. Monoclonal antibodies to CD11b (Immunotech-Coulter, Marseilles, France), and CD41a (Southern Biotechnology, Birmingham, AL) were FITC conjugates. The monoclonal antibody to CD45, (Caltag, Burlingam, CA) was a PE conjugate. After incubation, erythocytes were lysed using FACSlyse (Becton-Dickinson). Cells were then washed, diluted and fixed with paraformaldehyde (1% final concentration, Polysciences, Warrington, PA). Platelet-specific events, including microparticles were identified by gating on GPIIb/IIIa (CD41/CD61) (FITC-P2) positive events, and microparticles were distinguished by forward scatter analysis. The forward scatter cutoff was set to the immediate left of the single intact platelet population of a resting whole blood sample. Microparticles were reported as the percentage of CD41 positive events within their size window.
Leukocyte activation was assessed by quantifying the average CD11b fluorescence. CD11b up-regulation was expressed as a percentage relative to the maximum, where the maximum up-regulation was determined by the average fluorescence intensity of blood incubated with phorbol 12-myristate 13-acetate (PMA) (Sigma-Aldrich Canada). To determine the percentage of leukocytes with bound platelets, CD45 positive events (leukocytes) were gated for acquisition and then the second colour channel was used to determine the linearized fluorescent intensity of platelets (CD41) associated with the leukocytes. Platelet-leukocyte associations were expressed as the percentage of leukocytes positive for CD41.
Whole blood clotting time
Clotting times were measured with a sub-therapeutic level of heparin (0.75 U/mL). This concentration was identified through pilot studies as that which prevented clotting of the blood during collection from the donor and setting up of the experiment, but which enabled blood clotting on exposure to collagen modules. As is discussed below, this optimal concentration appeared to vary among different donors.
Two means of exposing blood to modules were used. In one, collagen modules or HUVEC covered modules were washed in pre-warmed PBS and then a 100 μL sample was transferred into a 600 μL microcentrifuge tube. Whole blood (final heparin concentration 0.75 U/mL) was added until the total volume in the microcentrifuge tube was 600 μL. The tube lid was closed creating a mobile air bubble within the tube when the tube was rocked. The microcentrifuge tube was placed on a rocking platform (13 oscillations per minute) in a 37 °C oven which produced movement of the air bubble within the tube. Clotting times were defined as the time when bubble motion ceased.
The second test system used the rocking platform as used for platelet and leukocyte activation (see above). Fresh whole blood (350 μL, final heparin concentration 0.75 U/mL) was mixed with 200 μL of collagen modules or HUVEC coated modules in a microcentrifuge tube. A 400 μL sample of this was then pipetted into a 25 cm length of polypropylene tubing (1.57mm ID) connected to a rocking platform. Rocking was initiated and the time when blood motion ceased or significant clot deposition occurred within the tubing was recorded as the clotting time.
Platelet and fibrin deposition visualization by SEM
A 100 μL sample of collagen only or HUVEC covered modules was added to a 1.5 mL microcentrifuge tube. A 1 mL sample of fresh whole blood (final heparin concentration 1 U/mL) was added to each tube and allowed to sit under static conditions for 1 h at 37 °C (or at room temperature). Collagen modules incubated at 37 °C for 1h produced complete blood gelation (despite the 1 U/mL heparin) making sample analysis difficult and hence some samples were incubated at room temperature. After exposure to blood, modules were prepared for SEM analysis using a method slightly modified from that of Wissemann et al [33]. Modules were washed in PBS, fixed in 5% glutaraldehyde (Sigma-Aldrich Canada), at 4 °C for 30 minutes, and then transferred to a 10% solution of glutaraldehyde for an additional 30 min. Samples were serially dehydrated in ethanol, frozen in liquid nitrogen, and lyophilized or critically point dried. Dried specimens were mounted on aluminium SEM stubs using carbon paper, gold coated, and examined using a Hitachi S-570 scanning electron microscope at an accelerating voltage of 20 kV.
Whole blood perfusion of constructs
Constructs were assembled from HUVEC covered modules or control collagenase-dispase treated modules. For the latter, HUVEC covered modules were treated for approximately 15 minutes at 37 °C in 100 mg/mL collagenase-dispase solution (Roche, Mississauga, Ontario) to remove all HUVEC from the surface, yet retain the size and stiffness of the contracted collagen. The short treatment time ensured module dissolution did not occur and microscope observation confirmed removal of the HUVEC layer. Unfortunately collagen modules that had not been seeded with HUVEC and then contracted by the HUVEC were too soft to be useable in a perfused construct. Constructs were assembled within a 0.2 mL length of a 1 mL graduated pipette (ID = 3.1 mm) and held in place at both ends by 1 cm2 sections of polypropylene mesh (PPM-3, Biomedical Materials, Slatersville, RI), inserted within the pipette. Silastic™ tubing (10 cm, 3.18 mm ID) was used to connect the pipette section to the syringe pump (824E Infusion pump model A-99, Razel Scientific Instruments Inc., Fairfax VT). The construct was pre-filled with PBS to prevent air bubble formation.
The Silastic™ tubing was filled with freshly collected whole blood (final heparin concentration 0.75 U/mL) from the syringe before being connected to the PBS pre-filled pipette/construct section. Initial studies indicated that this heparin concentration was sufficient to prevent premature clotting during the blood draw or while the blood was sitting in the syringe pump. The syringe was then placed on the syringe pump located on a rocking platform (to minimize blood settling) within a 37 °C oven and blood was perfused through the construct at a rate of 0.334 mL/min, equivalent to ~ 7 dynes/cm2 [2, 34]. Samples (400 μL) of the perfusate were collected in 0.6 mL graduated microcentrifuge tubes containing 8 μL of 200 mM EDTA at regular intervals during perfusion. An initial sample was collected from the syringe before connecting it to the construct. The experiment was terminated when the syringe was empty or if circuit blockage occurred. Constructs were removed and dissected for visual evidence of thrombus formation.
Plasma recalcification time
This assay was done on thin collagen films (not modules) formed in 96 well plates (Nunc brand) on tissue culture polystyrene (TCPS). Collagen films were prepared from Vitrogen collagen solution (Cohesion technologies) by adding 8 μL of the neutralized collagen to each well of a 96 well plate and incubating for 30 minutes to allow gelation. Films were thin enough to stick well to the bottom of the wells. HUVEC (5 × 104 cells/well) were seeded on to the collagen films and incubated for 2 days to attain confluence. Prior to coagulation testing, EC were incubated for 4 hours in normal medium or in medium containing 10 μg/mL lipopolysaccharide (LPS, Sigma-Aldrich Canada). The culture medium was removed and each well washed twice with PBS, pre-warmed to 37 °C before human plasma addition and recalcification.
To measure coagulation, 100 μL of citrated plasma was added to each well of the 96 well plate and recalcified with 100 μL 0.025M CaCl2 (Sigma-Aldrich Canada) (both pre-warmed to 37 °C). Well absorbance (a surrogate measure for coagulation/fibrin formation) was read every 20 or 25 seconds for 40 minutes at 37 °C using a Sunrise (96 well, Tecan, Maennedorf, Switzerland) plate reader at 400 nm in kinetic mode. “Clot initiation time” was determined as the time at which absorbance began to increase continuously. “Half max. time” was determined as the time at which the absorbance reading was half the difference between initial and maximum absorbance.
In some cases samples were incubated with solutions of polyclonal goat anti-human tissue factor antibodies (#4501, American Diagnostica, Stamford, CT) to block HUVEC tissue factor activity. Wells were washed in PBS and incubated for 30 min with a 1 mg/mL antibody solution, diluted in PBS. Coagulation testing was performed as above after two washes with PBS.
Statistics
The Students t-test was used to determine significance between 2 test groups. Analysis of variance (ANOVA) was used to test for significant differences among multiple test groups. The Levene’s test for homogeneity was used to test for equal variance among samples [35]. When equal variance could be assumed the Tukey HSD post-hoc test was used to identify significant differences among multiple test groups. When equal variance could not be assumed the Games-Howell [36] post-hoc test was used to identify significant differences among multiple test groups. In all tests a p-value of 0.05 was considered significant.
Results
Flow cytometry analysis
After exposure of whole blood to modules in PE tubing on a rocking platform, the level of blood activation (Table 1) was assessed by measuring platelet activation (microparticle formation, Figure 2), leukocyte activation (CD11b up-regulation), and leukocyte-platelet associations (fraction of platelet positive events in a leukocyte gated sample). Microparticle formation in the presence of HUVEC covered modules was significantly less than for collagen modules without HUVEC (Tukey HSD test, p = 0.01, n = 4-8) but significantly greater than for PE tubing alone (Tukey HSD test, p = 0.02, n = 4-8). Furthermore, in three out of the four trials, HUVEC covered modules had lower or even much lower levels of platelet-leukocyte associations than did the collagen control modules. On the other hand, resting blood levels of CD11b, were higher than expected and significantly more variable among trials, suggesting variable levels of background activation. Comparing within trials there was little if any effect of HUVEC on CD11b upregulation by collagen modules.
Table 1.
Flow cytometry analysis of microparticles, leukocyte activation and platelet-leukocyte association in whole blood (5 U/mL heparin) exposed to modules for 1 hour in rocking platform
| Samples | ||||
|---|---|---|---|---|
| Treatment | Resting (n = 4) | PE tubing (n = 4) | Collagen modules (n = 8#) | HUVEC covered modules (n = 8) |
| % microparticles* (platelet activation) | 2.6 ± 0.7 | 8.8 ± 2.5 | 37.5 ± 9.3 | 23.7 ± 8.9 |
| CD11b upregulation** (leukocyte activation) | 27.5 ± 9.4 | 23.3 ± 6.5 | 38.9 ± 2.4 | 34.1 ± 2.2 |
| Platelet-Leukocyte association*** | 2.6 ± 2.6 | 4.4 ± 2.7 | 11.5 ± 3.1 | 8.1 ± 4.6 |
Number of GPIIb/IIIa positive events below platelet size gating line as a percentage of all events.
Percent of maximum upregulation relative to whole blood activated with PMA
Percent of leukocytes with bound platelets
Note n = 8 from 3 donors (multiple trials were conducted using the same donor)
Figure 2.
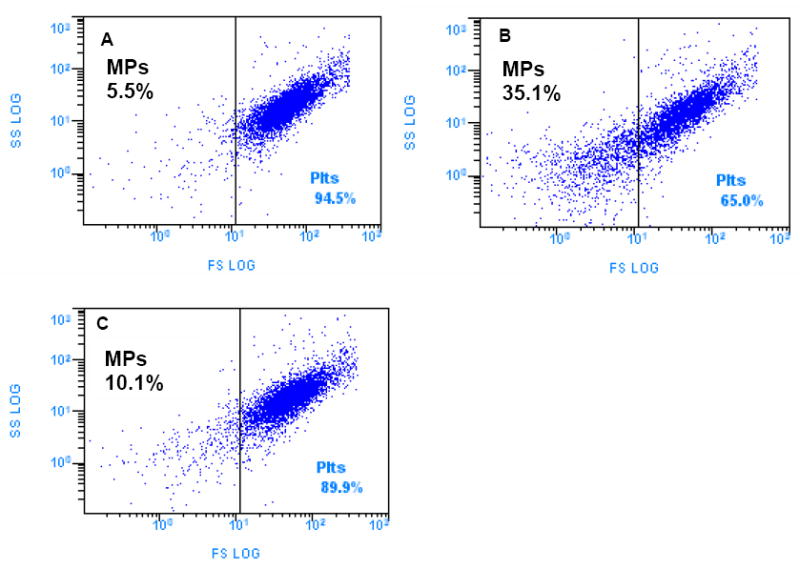
Microparticle formation after exposure of modules to human whole blood (5 U/mL heparin) for one hour at 37 C in the rocking platform. Forward versus side light scatter dot plots of GPIIb/IIIa positive events for one donor: (A) PE tubing, no modules (B) Collagen only modules (never seeded with HUVEC) in PE tubing (C) HUVEC covered modules in PE tubing. HUVEC covered modules produced significantly fewer microparticles (Tukey HSD test, p = 0.01, n=4-8) than collagen modules but significantly more microparticles than PE tubing only (Tukey HSD test, p = 0.02, n=4-8).
Whole blood clotting time
Modules and whole blood (0.75 U/mL heparin), contained either within a microcentrifuge tube, or within the lumen of a PE tube, were rocked gently and clot formation was identified as the time when blood motion ceased or significant clot deposition had occurred. Within either a microcentrifuge tube (Figure 3A) or the lumen of PE tubing (Figure 3B) the presence of HUVEC on the modules significantly increased the time to clot formation (Students t test, p = 0.004 and p < 10−4 respectively). In some cases clot formation never actually occurred and the test was terminated between 60 and 90 minutes. For tests conducted in microcentrifuge tubes, 8 out of 12 samples containing HUVEC modules never clotted compared to 2 out of 12 samples containing collagen modules, while for tests conducted in PE tubing, 9 out of 14 samples containing HUVEC modules never clotted compared to 1 out of 15 samples containing collagen modules.
Figure 3.
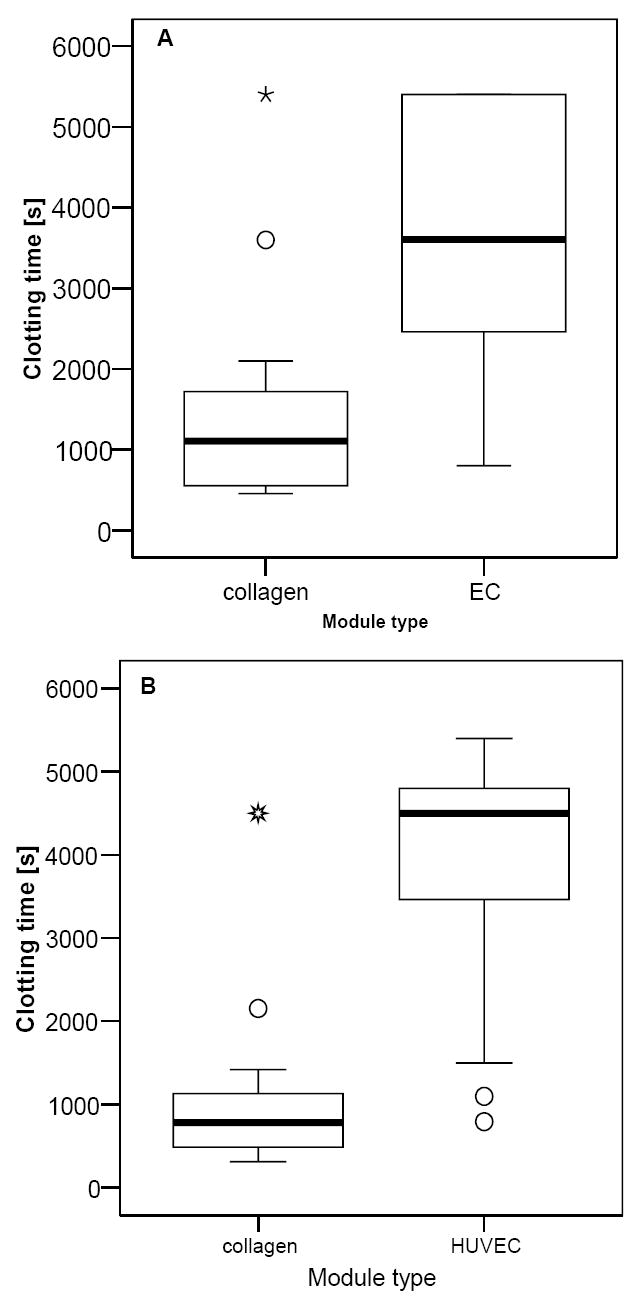
Whole blood clotting times for collagen and HUVEC modules mixed with slightly heparinized whole blood (0.75 U/mL) blood. The presence of HUVEC on the modules significantly increased the clotting time of blood in (A) microcentrifuge tubes placed on a rocking platform (Students t test, p =0.004, n=12) and (B) in a rocking platform test conducted in PE tubing (Students t test, p < 10−4, n= 14 for HUVEC and n=15 for collagen). In some cases clotting never actually occurred and the test was terminated between 60 and 90 minutes; in these instances the recorded time was the test termination time. Median clot time is represented by the thick central line within the box. Open circles and stars represent outliers and extreme outliers respectively. Data is based on multiple blood collections from 3 different donors.
Platelet and fibrin deposition
Scanning electron micrographs of modules after exposure to 1 U/mL whole blood for 1 h under static conditions are shown in Figure 4. On HUVEC covered modules incubated for 1 h at room temperature (Figure 4 B,C) or 37 °C (Figure 4D), fibrin was not visible or limited, and few platelets appeared to have been deposited. On the other hand there was a large cellular deposit on the collagen modules at room temperature (Figure 4A). When incubated at 37 °C with collagen modules, complete gelation of the blood occurred rendering it difficult to image properly.
Figure 4.
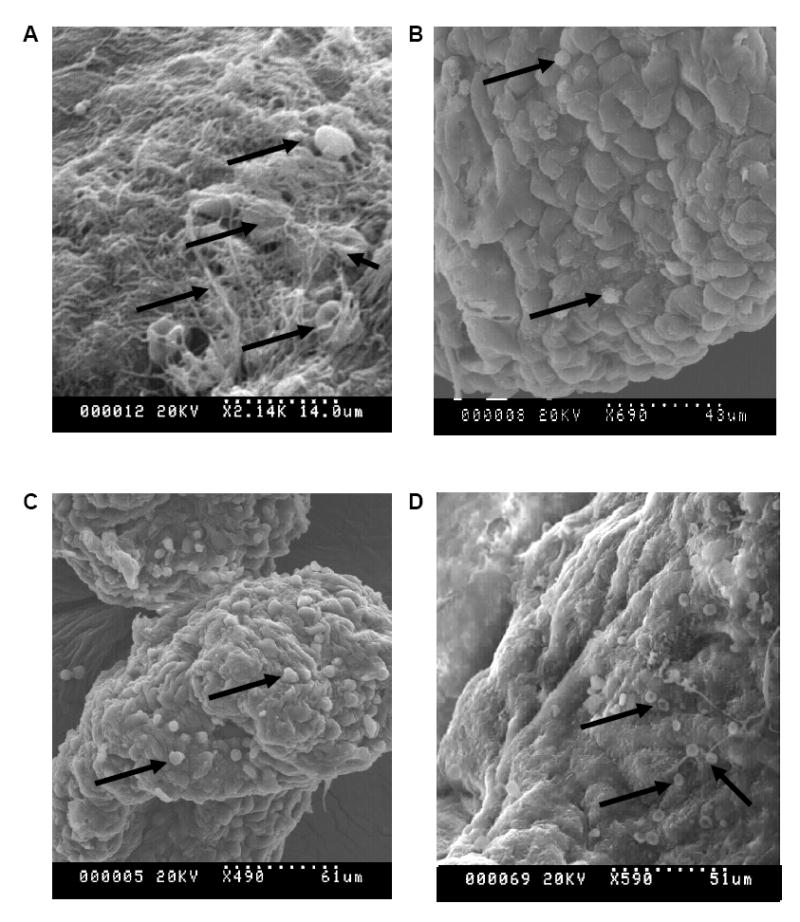
Scanning electron micrographs of modules after exposure to blood (1 U/mL heparin, static incubation, 1 hour). (A) collagen modules, room temperature (B,C) HUVEC covered modules, room temperature, and (D) HUVEC covered modules at 37 °C. Significantly less fibrin and cellular deposition (examples of deposition indicated by arrows) was visible on the HUVEC covered modules compared to the collagen modules. Blood clotted at 37 °C when exposed to collagen modules.
Construct perfusion
Whole blood was perfused through assembled constructs and the effluent analyzed for platelet concentration (Figure 5). When constructs assembled from HUVEC covered modules were perfused, there was no difference in effluent platelet concentration relative to the background changes associated with the flow circuit itself (i.e. measured in the absence of modules). Blood perfusion through collagenase-dispase treated HUVEC-free modules however, significantly reduced platelet concentration in the collected effluent. Obvious thrombus formation (at 30 minutes) was seen for two of the three trials (three different donors) of flow circuits without endothelial cells, but in only three of the seven trials when endothelial cells were present. This variability, as is discussed below, reflects the difficulty of executing this experiment. The presence of the HUVEC appeared to significantly reduce the thrombogenicity of the construct.
Figure 5.
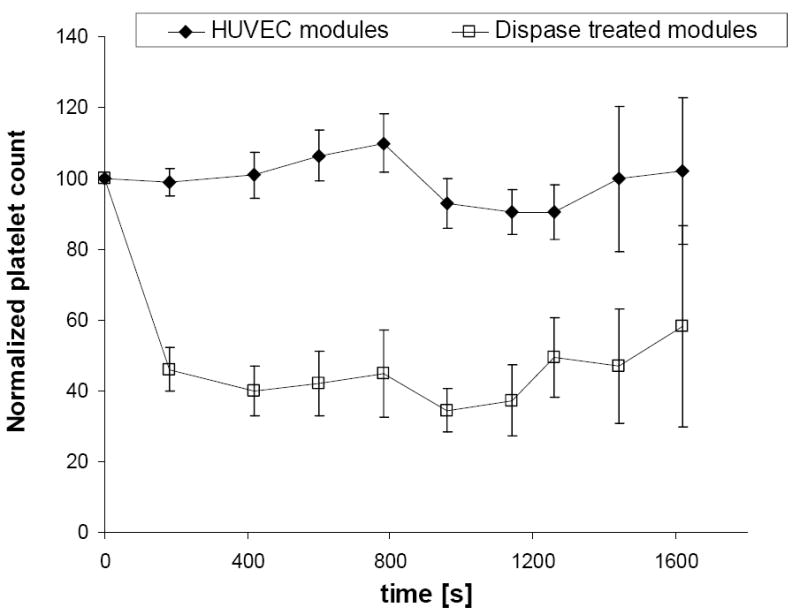
Normalized platelet count in fresh whole blood (0.75 U/mL heparin, 37 °C) after perfusion through a modular construct, with and without HUVEC, as a function of time. The effluent platelet count was ratioed to the initial platelet count for the whole blood used for each perfusion run and then the average of those ratios (at each time) was normalized to the corresponding average ratio for a blank flow circuit containing no modules. Fresh whole blood perfused through a HUVEC-covered modular construct (solid circles) maintained platelet levels essentially no different to those measured in the absence of modules. Blood perfusion through a modular construct in which HUVEC have been removed by treatment with collagenase-dispase (open squares), however, resulted in significant reductions in platelet number. Data was pooled from 3-7 trials using 4 different donors (2 male and 2 female). Error bars indicate the standard error of the mean, also normalized to the corresponding average ratio for a blank flow circuit containing no modules.
Plasma recalcification time
While significant differences were seen in thrombogenicity in whole blood studies, these were not evident when only plasma (PRP or PPP) was used with HUVEC covered collagen films. There was no significant difference (Games-Howell test, p= 0.43 and 0.48 for PRP and PPP respectively) in clotting half max times using PRP or PPP in comparing collagen and HUVEC covered collagen films (Figure 6, grey bars). Activation of the HUVEC with LPS however reduced the half max time. Incubating HUVEC with an anti-TF antibody prior to the recalcification test increased clotting half-max time for LPS activated HUVEC but not for non-stimulated HUVEC or for collagen only substrates for both PPP and PRP.
Figure 6.
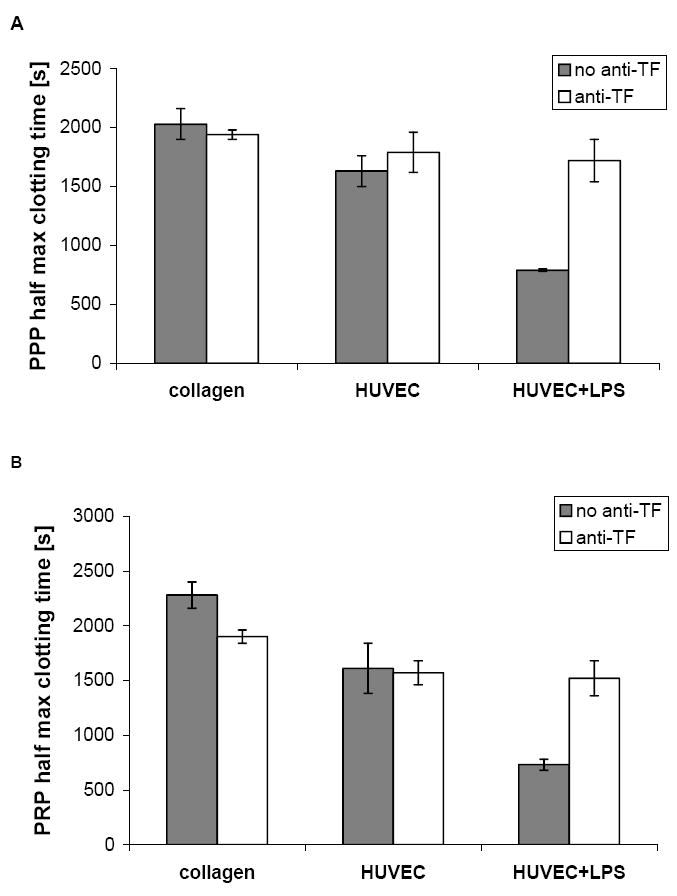
Plasma recalcification times of PPP and PRP exposed to collagen, HUVEC and LPS activated HUVEC after incubation with anti-TF. Plasma recalcification test using PPP (A) and PRP (B) comparing collagen, HUVEC on collagen, and HUVEC on collagen activated with LPS, with or without anti-TF antibody. Half max time (time to half maximum absorbance time), in both PPP and PRP on collagen and non-stimulated HUVEC was not altered by incubation with anti-TF. On LPS treated HUVEC however, incubation with anti-TF increased half max time in both PPP and PRP. Error bars represent the range/2 (n= 2).
Discussion
Modular tissue engineering enables the assembly of large tissue constructs which are permeated with a network of interconnected, EC lined channels to enable blood perfusion. This strategy relies on the EC exhibiting a non-thrombogenic phenotype. EC are dynamic in nature and when unstimulated, actively prevent thrombosis, but when activated, drive thrombosis utilizing a number of mediating molecules which influence coagulation, blood cell activation and adhesion, and complement activation [4]. The activation state of the blood on an EC surface at any time reflects the combined effects of these mediating molecules, and the interactions which occur among blood cells and the coagulation and complement cascades. Molecular characterization studies previously suggested that confluent HUVEC exhibit a non-thrombogenic phenotype on collagen modules [27]: TF expression and activity was low and TM expression was high on non-stimulated EC consistent with others who have previously found low or undetectable levels of TF and high levels of TM on non-stimulated confluent HUVEC cultured on TCPS. Here, we showed that HUVEC covered modules incubated with human whole blood reduced platelet microparticle formation and platelet-leukocyte association, delayed clotting time, reduced cellular deposition and most importantly enabled whole blood perfusion through a modular construct with minimal loss of platelets. On the other hand, the HUVEC did not appear to affect leukocyte CD11b upregulation nor did it increase platelet rich (or poor) plasma recalcification time.
Thrombogenicity of HUVEC on modules
Taken together the results presented here support the conclusion that HUVEC on collagen gel modules are nonthrombogenic consistent with the literature. For example, HUVEC cultured on gelatin [37,38,39] or fibrillar collagen [40] coated thermanox slides connected directly to a non-anticoagulated blood source exhibited low levels of fibrin, platelet and leukocyte deposition, and TAT and FPA generation. Similarly HUVEC cultured on microcarriers, which enable a high EC surface to blood volume ratio to be obtained, reduced levels of TAT, F1+2, FPA, thrombin activity, FXa and kallikrein [41,42,43].
Consistent with the prolonged clotting times, flow cytometry and SEM indicated that the HUVEC reduced platelet activation and cellular deposition compared to unseeded modules. HUVEC seeded modules did however, produce greater activation of the blood than in PE tubing without modules. This was perhaps due to differences in surface area exposed to blood and blood flow (on the rocking platform) within the lumen of the tubing in the presence and absence of modules. Alternatively the HUVEC may not be performing in an optimal manner due to the static culture condition in which they were maintained prior to conducting the blood experiments. Future studies using flow conditioned modules will be of benefit to better assess the effect of HUVEC seeded modules on blood cell activation. That there was little effect on CD11b upregulation is interpreted to suggest that HUVEC have little effect on inhibiting leukocyte activation caused by other means. Background CD11b levels were high and variable (see below) and this tempers the ability to make unequivocal conclusions. Platelet-leukocyte associations appear in other studies [31] to be more a marker of platelet activation than of leukocyte activation.
Most importantly whole blood perfusion of constructs, assembled from HUVEC covered modules, at shear rates equivalent to ~7 dynes/cm2 resulted in no significant decreases in platelet loss above background levels, while constructs assembled from modules from which endothelial cells were removed, showed a significant platelet loss throughout the perfusion period. Complete platelet depletion was not observed in the latter case, perhaps due to the use of short construct lengths and a single pass experimental design (instead of blood recirculation). Collagen gel modules that had never been exposed to HUVEC were used as controls in the other whole blood studies. However, these were too soft and compacted too easily under pressure in the construct. Thus it was necessary to use HUVEC to shrink (and stiffen) the collagen gel and then remove the HUVEC with gentle enzyme (collagenase-dispase) treatment. This was an effective means of generating a HUVEC-free control module to compare against the perfusion behavior of the intact HUVEC covered modules. We are exploring stiffer alternatives to the collagen gel (based on poloxamine, [44]) as a means of circumventing the issue of compaction.
Experimental constraints
Assessing thrombogenicity using whole blood presents a number of experimental difficulties which influence the results. Surface to volume ratio, choice of anticoagulant, and flow regime are chosen as compromises among logistical feasibility, modeling of the in vivo phenomena, and the goal of distinguishing between experimental conditions.
The flow regime implemented during whole blood experiments is a dominant factor in determining the thrombosis response measured. Studies performed under static conditions have been popular due to their logistical simplicity, but are not necessarily reflective of the surface thrombogenicity under flow conditions where reactant and product transport is occurring. At lower flow rates thrombi tend to be rich in fibrin [45], while at high flow rates fibrin deposition is reduced [46,39]. Thus the end point chosen for a particular experiment must be appropriate to the type of response expected under the flow regime of the test system. For this reason several whole blood systems and endpoint measurements were used to characterize EC thrombogenicity on the modules. The presence or absence of flow (perfusion vs rocking vs static incubation) did not alter the conclusion regarding the low thrombogenicity of the HUVEC covered modules relative to modules without HUVEC. Given the large literature on the effects of flow on HUVEC morphology and the expression of adhesion molecules, it is reasonable to expect some benefit to be obtained by flow conditioning the HUVEC or by further analysis of thrombogenicity under flow conditions (e.g in a shunt model). It is likely that the thrombogenicity endpoints used here were insufficient to see differences in thrombogenicity due to flow effects; unfortunately, it is not clear that there are indeed better in vitro systems for this purpose, given the other constraints that exist.
In vitro studies require an anticoagulant to prevent premature coagulation during blood collection or experimental set-up. We used 5 U/mL in the platelet and leukocyte activation studies since coagulation is to be prevented in order to isolate such cellular activation phenomena. On the other hand clotting time studies are precluded at this heparin concentration. In preliminary studies, in the presence of 1 U/mL heparin, clotting was often not observed in any samples, while in the presence of 0.5 U/mL clotting was often observed in all samples. Furthermore variations in the susceptibility of the blood to clot existed among donors. The 0.75 U/mL dose was selected because clotting occurred in the presence of collagen but not HUVEC for the majority of donors. This heparin concentration allowed us to see differences in in vitro behaviour that could be attributed to the presence or absence of HUVEC; in/ex vivo systems are preferred for thrombogenicity studies because even this minimal use of anti-coagulant is not required. The significance of this “optimum” in terms of mechanism of thrombogenicity is discussed below.
In vitro (and often in/ex vivo as well) the test material must be contained within (e.g., polyethylene tubing for the clotting time studies) or connected to other materials (e.g., the syringe for the perfusion studies) which themselves can promote thrombosis. The surface area of the test material must greatly exceed that of the container/connectors in order to detect the effect of the test material. Modules do provide a high surface area and this is likely one reason we are able to distinguish between HUVEC covered modules and control modules, despite the presence of these other materials. Adding small amounts of heparin as discussed above further obviates the effects of these other materials. The surface-to-volume ratio issue associated with plasma recalcification experiments is discussed below.
In this context, it should be noted that we compared clotting times and most other thrombogenicity parameters with equal volumes of modules, not equal surface areas; collagen gel control modules were not contracted by the presence of HUVEC and so were about six times the volume of HUVEC covered modules. When packed, equivalent volumes of collagen gel modules had 15-20 times less surface than HUVEC covered modules, which biased the studies against the HUVEC, if surface area was important. Since we are comparing HUVEC to collagen and the mechanisms of thrombogenicity are likely different and we don’t know the relative contributions of bulk and surface mediated mechanisms (in the limited volumes of in vitro assays) to the final outcome, comparing equal surface areas is to some extent as arbitrary as comparing equal volumes. Volume had the advantage of being the most relevant parameter when comparing constructs and minimized inadevertent effects on blood composition. It is important in future studies to keep this issue in mind, regardless of what comparator is used.
Donor variability (both donor itself and the variability in drawing blood) is another factor that confounds thrombogenicity studies. For example, greater visual evidence of thrombosis was seen in the perfusion studies with collagen constructs in which the HUVEC were removed as compared to the HUVEC covered modules. Although similar patterns of platelet loss were seen for all donors, different amounts of thrombus formation occurred in different runs. Although heparin was added to minimize the impact of activation during blood collection, minimizing the time between blood collection and the start of perfusion was likely also important to prevent significant background activation occurring prior to starting the experiment. Donor variability also influenced the flow cytometry results. The average platelet-leukocyte associations shown in Table 1 were largely but not totally masked by the variation. The beneficial effect of HUVEC was seen in this parameter when results were compared on an individual donor/trial basis. However, even looking at CD11b results on an individual donor basis did not reveal any effect of HUVEC on this parameter, suggesting that HUVEC had little detectable effect on leukocyte activation.
The distinct channel architectures associated with different constructs was a possible contributor to the variable amounts of thrombus formation observed. The non-uniform nature of the perfusion channels in a construct lead to a non-uniform percolating flow and the nature of this flow likely differed among constructs, promoting thrombosis to variable extents.
The perfusion experiment
The shear stress on the endothelial cells at the perfusion flow rate used was estimated at ~7 dynes/cm2. This assumed no construct compaction, full perfusion of all the channels within the construct, and round pores of constant diameter. Pores were not round and of constant diameter and were likely reduced in size over time due to compaction. Some regions within the channels therefore, were likely exposed to significantly higher shear stress than 7 dynes/cm2 during perfusion. Future studies should look for signs of activation (by immunohistochemistry) or detachment by examination of constructs at the end of the experiment. Construct compaction probably occurred, to some extent, during perfusion and likely resulted in closure of some channels within the construct and reduction in the pore diameter of other channels. It was expected that both HUVEC covered modules and the modules from which HUVEC were removed had similar mechanical properties and therefore a similar tendency to undergo compaction.
The logistics of the blood perfusion experiment proved to be difficult and illustrate the issues with blood exposure studies that involve even limited amounts of flow. Experiments were performed at 37 °C but some temperature variation occurred due to opening and closing of the door to collect effluent samples. All circuit components were washed with ethanol and PBS prior to use, and pre-warmed to 37 °C. The syringe and heparin used for blood collection were also pre-warmed to 37 °C before use. The experimental apparatus was located on a rocking platform to prevent settling of the blood over the course of the experiment. Even with rocking, the syringe had to be rotated within the syringe pump periodically to prevent settling of the cells. A further constraint was that each experiment required a fresh blood draw and so paired experiments with the same starting blood sample could not be performed.
Plasma recalcification
We also strove to isolate some key mechanisms with a view to further understanding how HUVEC enhance thrombogenicity relative to collagen modules without HUVEC. Hence we measured plasma recalcification times with both platelet rich and platelet poor plasma on HUVEC covered films. Not surprisingly, recalcification times were shorter when HUVEC were LPS activated. This was attributed to increased Tissue Factor expression for the LPS activated cells since anti-TF antibodies abrogated this reduction in recalcification time.
Once initiated, clotting on collagen proceeded faster (similar half-max times despite longer initiation time (relative to HUVEC covered collagen) in PPP, data not shown); presumably without HUVEC, there were no negative feedback mechanisms, such as activated protein C (binds to thrombomodulin), to reduce coagulation rate. This is consistent with a report by Joosten et al. [47] who showed that while EC initiate coagulation earlier they reduced coagulation rate through the action of protein C. Blocking tissue factor (TF) had essentially no effect on the recalcification time with non-activated LPS indicating that TF had little contribution to clotting with these cells; this is consistent with the low TF measured by immunofluorescence [27] suggesting that in this context, TF has little role in the clotting observed on the non-stimulated HUVEC.
The limited utility of the plasma recalcification assay to assess the functional attributes of HUVEC is probably a consequence of the limited surface-to-volume ratio in the 96 well plate to test films. In such wells most of the surface exposed to the plasma is the side of the well and not the test surface. In vivo the average EC surface-to-blood volume ratio is between 560 and 1440 cm2/mL [41]; here the EC surface to volume ratio was 1.6 cm2/mL. Presumably, the small number of EC in a 96 well plate are unable to generate a sufficient quantity of anti-thrombogenic molecules to maintain the anti-thrombogenic balance of factors in the blood and prevent propagation of the coagulation cascade. The consequence is rapid coagulation without apparent control by the EC.
The use of small amounts of heparin (above) to enable differences to be seen between HUVEC covered and control modules reflects a similar issue. Unstimulated HUVEC do not promote thrombosis but they do not have unlimited capacity to prevent it once other factors have initiated it. There is only a finite amount of thrombomodulin and prostacyclin, to focus on two anticoagulant/thrombotic molecules for example, and if there are too few HUVEC or too much platelet activation or fibrin formation occurring then it will appear that the HUVEC are ineffective. The presence of 0.75 U/mL heparin is too low to complete inhibit fibrin formation but it was enough for most blood collections to inhibit it enough to allow the effect of the HUVEC to be detected. The question then remains as to whether HUVEC on modules will be sufficiently nonthrombogenic in ex/in vivo constructs and this is the focus of current studies.
Conclusions
We have characterized the thrombogenicity of HUVEC seeded collagen modules, both individually and within assembled constructs, using several experimental strategies. Whole blood studies and especially the results from the perfusion study suggest that HUVEC on the module surface function in a non-thrombogenic manner and improve the thrombogenicity of the module surface relative to collagen only modules. The complexities of in vitro studies (need for some anticoagulant in some cases and limited surface volume ratios in other cases) makes for limited predictions of the in vivo situation. Nonetheless, the in vitro data presented here is an encouraging platform from which to proceed to such ex vivo or in vivo experiments.
Acknowledgments
We would like to acknowledge C. Lo and E. Cheng for technical assistance and the National Institute of Health (EB001013, co-investigators, E. Yeo, A. Gotlieb) and the Natural Sciences and Engineering Research Council for funding, We also wish to thank the contributions of the blood donors. AM acknowledges the fellowship support of the Province of Ontario and the Canadian Institutes of Health Research Training Program in Regenerative Medicine.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Nomi M, Atala A, Coppi PD, Soker S. Principals of neovascularization for tissue engineering. Mol Aspects Med. 2002;23(6):463–83. doi: 10.1016/s0098-2997(02)00008-0. [DOI] [PubMed] [Google Scholar]
- 2.McGuigan AP, Sefton MV. Vascularized organoid engineered by modular assembly enables blood perfusion. Proc Natl Acad Sci U S A. 2006;103(31):11461–6. doi: 10.1073/pnas.0602740103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gorbet MB, Sefton MV. Biomaterial-associated thrombosis: roles of coagulation factors, complement, platelets and leukocytes. Biomaterials. 2004;25(26):5681–703. doi: 10.1016/j.biomaterials.2004.01.023. [DOI] [PubMed] [Google Scholar]
- 4.McGuigan AP, Sefton MV. The influence of biomaterials on endothelial cell thrombogenicity. Biomaterials. 2007;28(16):2547–71. doi: 10.1016/j.biomaterials.2007.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pearson JD. Endothelial cell function and thrombosis. Baillieres Best Pract Res Clin Haematol. 1999;12(3):329–41. doi: 10.1053/beha.1999.0028. [DOI] [PubMed] [Google Scholar]
- 6.Davie EW, Fujikawa K, Kisiel W. The coagulation cascade: initiation, maintenance, and regulation. Biochemistry. 1991;30(43):10363–70. doi: 10.1021/bi00107a001. [DOI] [PubMed] [Google Scholar]
- 7.Esmon CT. Coagulation inhibitors in inflammation. Biochem Soc Trans. 2005;33(Pt 2):401–5. doi: 10.1042/BST0330401. [DOI] [PubMed] [Google Scholar]
- 8.Slupsky JR, Kalbas M, Willuweit A, Henn V, Kroczek RA, Muller-Berghaus G. Activated platelets induce tissue factor expression on human umbilical vein endothelial cells by ligation of CD40. Thromb Haemost. 1998;80(6):1008–14. [PubMed] [Google Scholar]
- 9.Schorer AEMCF. Endothelial cells. Ryan US: CRC Press; 1988. Production of tissue factor. [Google Scholar]
- 10.Esmon CT. Thrombomodulin as a model of molecular mechanisms that modulate protease specificity and function at the vessel surface. Faseb J. 1995;9(10):946–55. doi: 10.1096/fasebj.9.10.7615164. [DOI] [PubMed] [Google Scholar]
- 11.Esmon CT. Regulation of blood coagulation. Biochim Biophys Acta. 2000;1477(12):349–60. doi: 10.1016/s0167-4838(99)00266-6. [DOI] [PubMed] [Google Scholar]
- 12.Sandset PM. Tissue factor pathway inhibitor (TFPI)--an update. Haemostasis. 1996;26(Suppl 4):154–65. doi: 10.1159/000217293. [DOI] [PubMed] [Google Scholar]
- 13.Marcum JA, McKenney JB, Rosenberg RD. Acceleration of thrombin-antithrombin complex formation in rat hindquarters via heparinlike molecules bound to the endothelium. J Clin Invest. 1984;74(2):341–50. doi: 10.1172/JCI111429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Radomski MW, Palmer RM, Moncada S. The role of nitric oxide and cGMP in platelet adhesion to vascular endothelium. Biochem Biophys Res Commun. 1987;148(3):1482–9. doi: 10.1016/s0006-291x(87)80299-1. [DOI] [PubMed] [Google Scholar]
- 15.Moncada S. Eighth Gaddum Memorial Lecture. University of London Institute of Education, December 1980. Biological importance of prostacyclin. Br J Pharmacol. 1982;76(1):3–31. doi: 10.1111/j.1476-5381.1982.tb09186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Andrews RK, Berndt MC. Platelet physiology and thrombosis. Thromb Res. 2004;114(56):447–53. doi: 10.1016/j.thromres.2004.07.020. [DOI] [PubMed] [Google Scholar]
- 17.Garlanda C, Dejana E. Heterogeneity of endothelial cells. Specific markers. Arterioscler Thromb Vasc Biol. 1997;17(7):1193–202. doi: 10.1161/01.atv.17.7.1193. [DOI] [PubMed] [Google Scholar]
- 18.Tedesco F, Fischetti F, Pausa M, Dobrina A, Sim RB, Daha MR. Complement-endothelial cell interactions: pathophysiological implications. Mol Immunol. 1999;36(45):261–8. doi: 10.1016/s0161-5890(99)90054-8. [DOI] [PubMed] [Google Scholar]
- 19.Lidington EA, Haskard DO, Mason JC. Induction of decay-accelerating factor by thrombin through a protease-activated receptor 1 and protein kinase C-dependent pathway protects vascular endothelial cells from complement-mediated injury. Blood. 2000;96(8):2784–92. [PubMed] [Google Scholar]
- 20.Hamilton KK, Ji Z, Rollins S, Stewart BH, Sims PJ. Regulatory control of the terminal complement proteins at the surface of human endothelial cells: neutralization of a C5b-9 inhibitor by antibody to CD59. Blood. 1990;76(12):2572–7. [PubMed] [Google Scholar]
- 21.Schwartz SM, Heimark RL, Majesky MW. Developmental mechanisms underlying pathology of arteries. Physiol Rev. 1990;70(4):1177–209. doi: 10.1152/physrev.1990.70.4.1177. [DOI] [PubMed] [Google Scholar]
- 22.Grabowski EF, Reininger AJ, Petteruti PG, Tsukurov O, Orkin RW. Shear stress decreases endothelial cell tissue factor activity by augmenting secretion of tissue factor pathway inhibitor. Arterioscler Thromb Vasc Biol. 2001;21(1):157–62. doi: 10.1161/01.atv.21.1.157. [DOI] [PubMed] [Google Scholar]
- 23.Malek AM, Jackman R, Rosenberg RD, Izumo S. Endothelial expression of thrombomodulin is reversibly regulated by fluid shear stress. Circ Res. 1994;74(5):852–60. doi: 10.1161/01.res.74.5.852. [DOI] [PubMed] [Google Scholar]
- 24.Schnaper HW, Kleinman HK. Regulation of cell function by extracellular matrix. Pediatr Nephrol. 1993;7(1):96–104. doi: 10.1007/BF00861587. [DOI] [PubMed] [Google Scholar]
- 25.Hordijk PL, Anthony E, Mul FP, Rientsma R, Oomen LC, Roos D. Vascular-endothelial-cadherin modulates endothelial monolayer permeability. J Cell Sci. 1999;112(Pt 12):1915–23. doi: 10.1242/jcs.112.12.1915. [DOI] [PubMed] [Google Scholar]
- 26.Gulino D, Delachanal E, Concord E, Genoux Y, Morand B, Valiron MO, et al. Alteration of endothelial cell monolayer integrity triggers resynthesis of vascular endothelium cadherin. J Biol Chem. 1998;273(45):29786–93. doi: 10.1074/jbc.273.45.29786. [DOI] [PubMed] [Google Scholar]
- 27.She M, McGuigan AP, Sefton MV. Tissue factor and thrombomodulin expression on endothelial cell-seeded collagen modules for tissue engineering. J Biomed Mater Res A. 2007;80(2):497–504. doi: 10.1002/jbm.a.31083. [DOI] [PubMed] [Google Scholar]
- 28.McGuigan AP, Sefton MV. Design and fabrication of sub-mm-sized modules containing encapsulated cells for modular tissue engineering. Tissue Eng. 2007;13(5):1069–78. doi: 10.1089/ten.2006.0253. [DOI] [PubMed] [Google Scholar]
- 29.McGuigan AP, Leung B, Sefton MV. Fabrication of cell-containing gel modules to assemble modular tissue-engineered constructs. Nat Protoc. 2006;1(6):2963–9. doi: 10.1038/nprot.2006.443. corrected. [DOI] [PubMed] [Google Scholar]
- 30.Wissink MJ, Beernink R, Poot AA, Engbers GH, Beugeling T, van Aken WG, et al. J. Relation between cell density and the secretion of von Willebrand factor and prostacyclin by human umbilical vein endothelial cells. Biomaterials. 2001;22(16):2283–90. doi: 10.1016/s0142-9612(00)00417-8. [DOI] [PubMed] [Google Scholar]
- 31.Gemmell CH, Ramirez SM, Yeo EL, Sefton MV. Platelet activation in whole blood by artificial surfaces: identification of platelet-derived microparticles and activated platelet binding to leukocytes as material-induced activation events. J Lab Clin Med. 1995;125(2):276–87. [PubMed] [Google Scholar]
- 32.Gorbet MB, Sefton MV. Leukocyte activation and leukocyte procoagulant activities after blood contact with polystyrene and polyethylene glycol-immobilized polystyrene beads. J Lab Clin Med. 2001;137(5):345–55. doi: 10.1067/mlc.2001.114677. [DOI] [PubMed] [Google Scholar]
- 33.Wissemann KW, Jacobson BS. Pure gelatin microcarriers: synthesis and use in cell attachment and growth of fibroblast and endothelial cells. In Vitro Cell Dev Biol. 1985;21(7):391–401. doi: 10.1007/BF02623470. [DOI] [PubMed] [Google Scholar]
- 34.McGuigan AP, Sefton MV. Design criteria for a modular tissue-engineered construct. Tissue Eng. 2007;13(5):1079–89. doi: 10.1089/ten.2006.0245. [DOI] [PubMed] [Google Scholar]
- 35.Levene H. In: Contributions to Probability and Statistics. Olkin I, editor. Palo Alto, CA: Stanford University Press; 1960. [Google Scholar]
- 36.Zolman JF. Biostatistics. Oxford: Oxford University Press; 1993. p. 151. [Google Scholar]
- 37.Kirchhofer D, Sakariassen KS, Clozel M, Tschopp TB, Hadvary P, Nemerson Y, et al. Relationship between tissue factor expression and deposition of fibrin, platelets, and leukocytes on cultured endothelial cells under venous blood flow conditions. Blood. 1993;81(8):2050–8. [PubMed] [Google Scholar]
- 38.Kirchhofer D, Tschopp TB, Hadvary P, Baumgartner HR. Endothelial cells stimulated with tumor necrosis factor-alpha express varying amounts of tissue factor resulting in inhomogenous fibrin deposition in a native blood flow system. Effects of thrombin inhibitors. J Clin Invest. 1994;93(5):2073–83. doi: 10.1172/JCI117202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Diquelou A, Dupouy D, Gaspin D, Constans J, Sie P, Boneu B, et al. Relationship between endothelial tissue factor and thrombogenesis under blood flow conditions. Thromb Haemost. 1995;74(2):778–83. [PubMed] [Google Scholar]
- 40.Diquelou A, Lemozy S, Dupouy D, Boneu B, Sakariassen K, Cadroy Y. Effect of blood flow on thrombin generation is dependent on the nature of the thrombogenic surface. Blood. 1994;84(7):2206–13. [PubMed] [Google Scholar]
- 41.Biedermann B, Rosenmund A, Muller M, Kohler HP, Haeberli A, Straub PW. Human endothelial cells suppress prothrombin activation in nonanticoagulated whole blood in vitro. J Lab Clin Med. 1994;124(3):339–47. [PubMed] [Google Scholar]
- 42.Bombeli T, Muller M, Straub PW, Haeberli A. Cyclosporine-induced detachment of vascular endothelial cells initiates the intrinsic coagulation system in plasma and whole blood. J Lab Clin Med. 1996;127(6):621–34. doi: 10.1016/s0022-2143(96)90153-5. [DOI] [PubMed] [Google Scholar]
- 43.Kohler HP, Muller M, Bombeli T, Straub PW, Haeberli A. The suppression of the coagulation of nonanticoagulated whole blood in vitro by human umbilical endothelial cells cultivated on microcarriers is not dependent on protein C activation. Thromb Haemost. 1995;73(4):719–24. [PubMed] [Google Scholar]
- 44.Sosnik A, Sefton MV. Semi-synthetic collagen/poloxamine matrices for tissue engineering. Biomaterials. 2005;26(35):7425–35. doi: 10.1016/j.biomaterials.2005.05.086. [DOI] [PubMed] [Google Scholar]
- 45.Hedeman Joosten PP, Verhagen HJ, Heijnen-Snyder GJ, van Vroonhoven TJ, Sixma JJ, de Groot PG, et al. Thrombogenesis of different cell types seeded on vascular grafts and studied under blood-flow conditions. J Vasc Surg. 1998;28(6):1094–103. doi: 10.1016/s0741-5214(98)70036-9. [DOI] [PubMed] [Google Scholar]
- 46.Zwaginga JJ, de Boer HC, MJ IJ, Kerkhof A, Muller-Berghaus G, Gruhlichhenn J, et al. Thrombogenicity of vascular cells. Comparison between endothelial cells isolated from different sources and smooth muscle cells and fibroblasts. Arteriosclerosis. 1990;10(3):437–48. doi: 10.1161/01.atv.10.3.437. [DOI] [PubMed] [Google Scholar]
- 47.Hedeman Joosten PP, Verhagen HJ, Heijnen-Snyder GJ, Sixma JJ, de Groot PG, Eikelboom BC, et al. Thrombomodulin activity of fat-derived microvascular endothelial cells seeded on expanded polytetrafluorethylene. J Vasc Res. 1999;36(2):91–9. doi: 10.1159/000025630. [DOI] [PubMed] [Google Scholar]


