Abstract
Carcinoid heart disease (CHD) occurs in 20–70% of the patients with metastatic well-differentiated neuroendocrine tumours (NET). We evaluated whether natriuretic peptides (ANP or NT-proBNP) are useful in early detection of CHD. Blood samples from 32 patients with NET were compared with cardiac ultrasound follow-up. CHD was defined as thickening of the tricuspid valve in the presence of grade III–IV/IV tricuspid valve regurgitation. CHD was found in nine out of 32 patients (28%), all with symptoms of the carcinoid syndrome compared to 65% in the 23 patients without CHD (P=0.04). Median levels of NT-proBNP and 5-HIAA were significantly higher in patients with CHD (894 ng l−1 and 815 μmol 24 h−1) compared to those without (89 and 206 ng l−1, P<0.001 and P=0.007). No significant differences were detected in ANP levels (P=0.11). Dilatation of the right atrium and ventricle as well as thickening of the tricuspid valve and degree of regurgitation were statistically significant correlated with NT-proBNP levels. The accuracy of NT-proBNP in the diagnosis of CHD was higher than that of ANP. A significantly better survival was observed in case of normal NT-proBNP values. In conclusion, NT-proBNP is helpful as a simple marker in the diagnosis of CHD. Survival is better in patients with normal levels of NT-proBNP.
Keywords: carcinoid heart disease, urinary 5-HIAA, ANP, BNP, Chromogranin A
In 1981, De Bold et al (1981) first described the endocrine function of the heart with natriuretic and diuretic effects. These hormonal activities were later linked to peptides such as atrial natriuretic peptide (ANP) and brain natriuretic peptide (BNP). The peptides are produced primarily within the atria and ventricles of the heart and are released into the circulation in response to increased wall tension, thus reflecting increased intravascular volume. Both ANP and BNP are produced as propolypeptides (pro-ANP and pro-BNP) and are cleaved after excretion into the biological active peptides (ANP and BNP) and an inactive N-terminal fragments (NT-proANP and NT-proBNP). Both active and inactive peptides can be isolated from the blood, but the stability of the prohormones and NT-terminal fragments is much higher compared to the activated form. After activation, natriuresis starts and a decrease in blood pressure occurs as a result of shifting intravascular fluid into the extravascular compartment and suppression of the rennin–angiotensin–aldosteron axis.
Well-differentiated neuroendocrine tumours (NET) with liver metastases can give symptoms of the characteristic carcinoid syndrome with diarrhoea and flushes caused by the overproduction of serotonin. Carcinoid heart disease (CHD) is a well-known complication of long-lasting exposure to high levels of serotonin (Tornebrandt et al, 1986; Lundin et al, 1988; Robiolio et al, 1995; Westberg et al, 2001; Zuetenhorst et al, 2003). Many carcinoid patients die from cardiac causes (Ross and Roberts, 1985) and the detection of CHD in an early stage is important to adjust therapy and hence improve prognosis.
Large studies in the general population or in noncardiac patients showed that measuring natriuretic peptides might be an effective screening method for left-ventricular systolic dysfunction (McDonagh et al, 1998; Luchner et al, 2000; Bay et al, 2003). In patients with the suspicion of heart failure several other studies showed natriuretic peptides to be useful indicators for the detection of heart failure (Lerman et al, 1993; Davidson et al, 1996; Cowie et al, 1997; Hammerer-Lercher et al, 2001; Maisel et al, 2002,2003). In the follow-up of patients with an acute cardiac event levels of natriuretic peptides were proved to be of prognostic value for outcome (Hall et al, 1994; Omland et al, 1996; de Lemos et al, 2001; Koglin et al, 2001; Richards et al, 2003).
Studies about the role of natriuretic peptides in patients with NET are rare. In a report of Lundin et al (1989) ultrasound studies were performed in 50 patients and combined with blood atrial natriuretic peptide concentrations. In patients with clinical findings of right ventricular failure significantly higher levels of ANP were found. However, no studies have been performed to determine the diagnostic value of BNP in patients with CHD.
In this study, we investigated the relationship between CHD and the blood levels of NT-proBNP and ANP as markers for cardiac (dys)function. We also examined survival of patients with and without elevated levels of these natriuretic peptides in order to evaluate the prognostic value of these hormones.
PATIENTS AND METHODS
Cardiac ultrasound studies were performed in 32 consecutive patients with NET (18 women and 14 men) who visited the outpatient department of the Netherlands Cancer Institute/Antoni van Leeuwenhoek Hospital in 1999 and 2000 for follow-up. The mean age was 61 years (range 34–77 years). The median interval between the diagnosis of metastatic NET and the cardiac investigation plus laboratory testing was 22 months (range 2–121 months).
Cardiac ultrasound imaging
Two-dimensional echocardiography with continuous wave Doppler and colour flow Doppler studies were performed using standard techniques (Hewlett-Packard Sonos 5500 with 2.0/2.5 MHz probes). Echocardiographic parameters analysed were: valve morphology (normal or thickened), valve mobility (normal, mildly-, moderately-, severely diminished, fixed), valve regurgitation (none, I–IV/IV), valvular stenosis and atrial/ventricular dimensions. The criteria for CHD in our study was: a thickened tricuspid valve with additional III/IV or IV/IV tricuspid valve regurgitation (Zuetenhorst et al, 2003).
Laboratory techniques
Urinary 5-HIAA excretion and levels of NT-proBNP and ANP were determined at the same time as the cardiac investigation. A routine of 24 h urine samples were collected and qualitatively evaluated for 5-HIAA and analysed by reversed-phase HPLC (normal <40 μmol 24 h−1). A fluorescence detector was used for detection and quantification (Stroomer et al, 1990).
Serum levels of NT-proBNP were determined in serum by an electrochemiluminescence immunoassay used on the Modular Analytics E170 (Roche Diagnostics, Mannheim, Germany). Normal levels of NT-proBNP are affected by age (under or above 50 years) and gender. According to instructions of the manufacturer, in patients above 50 years the cutoff value for healthy women is 155 ng l−1 and for men 222 ng l−1. For practical reasons, we decided to use a cutoff value of 200 ng l−1, because all our patients except two were aged above 50 years. Atrial natriuretic peptide (ANP) was measured in plasma samples using an IRMA assay manufactured by CIS bio international, Gif-sur-Yvette, France (normal value <43 ng l−1). Determination of NT-proBNP was performed in all patients, ANP in 27 out of 32 patients (eight with CHD and 19 without CHD).
Chromogranin A levels were determined in serum using a solid-phase two site immunoradiometric assay (normal <120 μg l−1). Two monoclonal antibodies were prepared against sterically remote sites on the CgA molecule. The first one is coated on the tube and the second one, radiolabelled with iodine 125 is used as a tracer (CIS bio international, Gif-sur-Yvette, France) (Degorce et al, 1999).
Histology
Histology was classified into low-grade (<10 mitoses per 2 mm2 without necrosis) and high-grade neuroendocrine tumours (>10 mitoses per 2 mm2 and/or necrosis) according to the revised classification described by Capella et al (1995).
Statistics
Comparisons between the CHD and the non-CHD group were made by the Mann–Whitney test or the Kruskal–Wallis test in case of a continuous variable. Dichotomous variables were tested by means of the Fisher's exact test.
RESULTS
Tricuspid valvular lesions combined with regurgitation as described in our criteria for CHD were found in nine out of 32 patients (28%). Additionally, severe dilatation of the right atrium was present in almost all (eight out of nine) patients with CHD, while severe dilatation of the right ventricle was found in three non-CHD patients (Table 1 ).
Table 1. Echocardiographic findings in carcinoid patients (n=32) according to the presence of heart disease.
| Without carcinoid heart disease (n=23) | Carcinoid heart disease (n=9)a | |
|---|---|---|
| Right atrium | ||
| Normal | 21 (91%) | 0 (0%) |
| Mildly dilated | 2 (9%) | 1 (11%) |
| Severely dilated | 0 (0%) | 8 (89%) |
| Right ventricle | ||
| Normal | 22 (96%) | 1 (11%) |
| Mildly dilated | 1 (4%) | 5 (56%) |
| Severely dilated | 0 (0%) | 3 (33%) |
| Tricuspid valve | ||
| Thickened | 2 (9%) | 9 (100%) |
| Normal | 21 (91%) | 0 (0%) |
| Tricuspid regurgitation | ||
| None | 8 (35%) | 0 (0%) |
| I/IV | 8 (35%) | 0 (0%) |
| II/IV | 7 (30%) | 0 (0%) |
| III/IV | 0 (0%) | 3 (33%) |
| IV/IV | 0 (0%) | 6 (67%) |
Defined as: thickening of the tricuspid valve with additional III/IV or IV/IV tricuspid valve regurgitation.
In 29 out of 32 patients (91%) liver metastases were present. In six patients urinary 5-HIAA excretion was normal, while it was elevated in 26 patients (median 369 μmol 24 h−1, range 54-1185 μmol 24 h−1). Patients with CHD had a significant longer history of liver metastases compared to those without CHD (median duration 40 and 14 months, respectively, P=0.02) (Table 2 ). All CHD patients suffered from the carcinoid syndrome (flushes, diarrhoea or wheezing) compared to 65% of the non-CHD patients (P=0.04). No significant differences were seen between the CHD and non-CHD group in respect to age, gender, presence of liver metastases (Table 2).
Table 2. Clinical characteristics in carcinoid patients according to the presence of heart disease.
| Total group (n=32) | Without carcinoid heart disease (n=23) | Carcinoid heart diseasea (n=9) | P-value | |
|---|---|---|---|---|
| Age at cardiac ultrasound (years) | ||||
| Mean (range) | 61 (34–77) | 61 (34–76) | 65 (51–77) | 0.81 |
| Sex | ||||
| Male | 14 (44%) | 9 (39%) | 5 (55%) | 0.41 |
| Female | 18 (56%) | 14 (61%) | 4 (45%) | |
| Duration of carcinoid disease at echocardiogram (months) | ||||
| Median (range) | 22 (2–121) | 20 (2–121) | 40 (9–96) | 0.08 |
| Liver metastases | 29 (91%) | 21 (88%) | 9 (100%) | 0.36 |
| Duration of liver metastases (months) | ||||
| Median (range) | 31 (2–96) | 14 (2–84) | 40 (9–96) | 0.02 |
| Symptoms of carcinoid syndrome | ||||
| Yes | 24 (76%) | 15 (65%) | 9 (100%) | 0.04 |
| No | 8 (24%) | 8 (35%) | 0 (0%) | |
| Primary tumour | ||||
| Foregut | 2 (6%) | 2 (9%) | 0 (0%) | 0.16 |
| Midgut | 15 (47%) | 11(48%) | 4 (45%) | |
| Hindgut | 1 (3%) | 0 (0%) | 1 (10%) | |
| Unknown | 14 (44%) | 10 (43%) | 4 (45%) | |
| Pathology | ||||
| Low-grade NETb | 24 (76%) | 16 (69%) | 8 (90%) | 0.33 |
| High-grade NET | 5 (16%) | 5 (22%) | 0 (0%) | |
| Cytological function | 3 (5%) | 2 (9%) | 1 (10%) | |
| NT-proBNP (normal <200 ng l−1) | ||||
| Median (range) | 155 (23–4432) | 89 (23–1449) | 894 (328–4432) | <0.001 |
| ANP (normal <43 ng l−1) | ||||
| Median (range) | 26 (10–89) | 25 (10–57) | 41 (12–89) | 0.11 |
| 5-HIAA (normal <40 μmol (24 h)−1) | ||||
| Median (range) | 292 (19–1185) | 206 (19–1116) | 815 (87–1185) | 0.007 |
| CgA (normal <120 μg l−1) | ||||
| Median (range) | 777 (24–22282) | 684 (24–9115) | 1958 (506–22282) | 0.05 |
Defined as: thickening of the tricuspid valve with additional III/IV or IV/IV tricuspid valve regurgitation.
NET=neuroendocrine tumour.
During sample collection a total of 20 out of 32 patients were treated with somatostatin analoga. Pharmacological doses of meta-iodobenzylguanidine (MIBG) were administered in 18 patients, two of them during sample collection. Nine patients received a combination with radioactive labelled MIBG (Taal et al, 1996,2000), all but one at least 3 months before blood collection. In all, 14 patients were treated with interferon, none of them during collection time. There were no significant differences in these treatment modalities between CHD and non-CHD patients.
111In-pentetreotide scintigraphy was available in 31 out of 32 patients. A positive scan was found in 26 out of 32 (81%) patients and five patients had a negative scan. In four of these five patients, the primary tumour was located in the midgut and in one patient in the foregut.
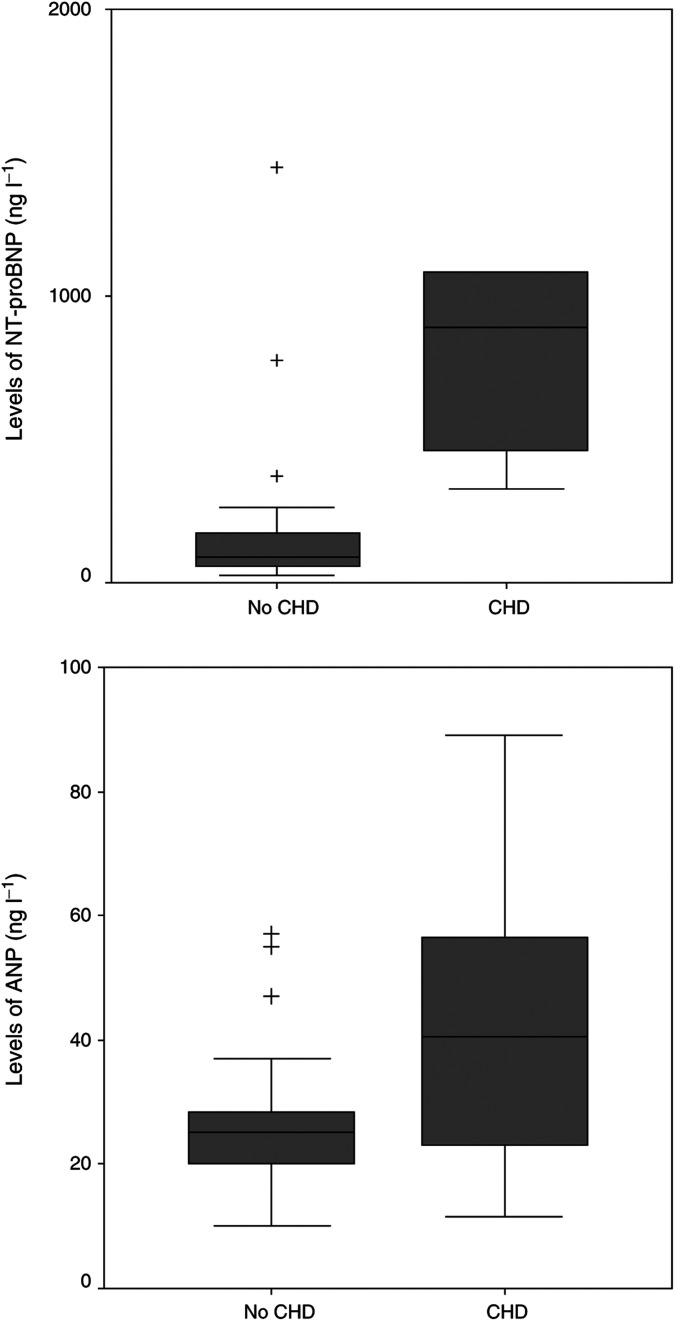
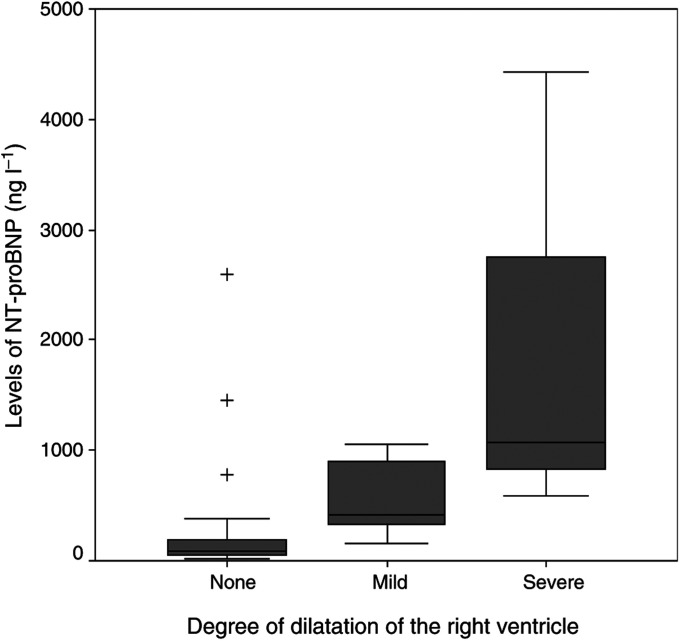
Significantly higher median levels of NT-proBNP and urinary 5-HIAA were found in the patients with CHD (894 ng l−1 and 815 μmol 24 h−1, respectively) compared to those without CHD (89 and 206 ng l−1; P<0.001 and P=0.007, respectively) (Figure 1 and Table 2). Median CgA levels were also found to be significantly higher in patients with CHD (1958 μg l−1) compared with the non-CHD group (684 μg l−1, P=0.05). No significant differences were detected in the levels of ANP between both groups (P=0.11) (Figure 1). Although levels of NT-proBNP are affected by age (under or above 50 years) and gender, we applied a fixed cut-off value of 200 ng l−1 because all our patients except two had an age above 50 years. In two patients (both women) with an age under 50 years (34 and 47, respectively) the NT-proBNP levels were beneath 60 ng l−1. The advised cutoff value for this group is 155 ng l−1, using our cutoff point of 200 ng l−1 did not make any difference in our study population. For ANP, no differences in levels between men and women are described and a correlation with age is weaker than described in BNP (Clerico et al, 2002). The serum concentration of NT-proBNP was elevated in all patients with CHD. ANP levels were elevated in four out of seven CHD patients. Elevated levels of NT-proBNP in patients with reported normal echocardiographic findings were found in four out of 23 patients (median 575 ng l−1, range 266–1449). In three of these patients thickening of the tricuspid valve with grade II/IV tricuspid regurgitation was already present. During follow-up 1 year later, one of these patients met our criteria for CHD. The other two died before a new echocardiography could be performed. The fourth patient suffered from dilatation of the right atrium after a myocardial infarction. NT-proBNP was elevated in all patients with severe dilatation of either right atrium or ventricle and the level of NT-proBNP was correlated with the degree of dilatation (P=0.002 and 0.005, respectively) (Figure 2) (Table 3 ). Elevated NT-proBNP levels were found in four out of 21 patients with normal dimensions of the right atrium (range 266–1449 ng l−1) and in five out of 23 patients with normal right ventricle dimension (range 266–2587 ng l−1). No significant correlation was detected between the median levels of ANP and the existence of atrial or ventricle dilatation (Table 3). Median NT-proBNP levels were higher in patients with pathological thickening of the tricuspid valve (894 ng l−1) compared to those with a normal aspect of the tricuspid valve (84 ng l−1, P<0.001). Elevated levels of NT-proBNP were present in all patients with severe tricuspid valve regurgitation and significantly correlated with the degree of regurgitation (P=0.007). Such significant findings were not found in the levels of ANP (Table 3).
Figure 1.
The median NT-proBNP serum level is significantly higher in patients with CHD compared to those without. The difference in ANP levels is not significant. Boxes are median and interquartiles range, whiskers show ranges excluding outliers. Values beyond the lines are considered outliers (+).
Figure 2.
The median NT-proBNP serum level is significantly correlated with the degree of dilatation of the right ventricle. Boxes are median and interquartiles range, whiskers show ranges. Values beyond the lines are considered outliers (+).
Table 3. Levels of NT-proBNP and ANP according to the echocardiographic findings.
| NT-proBNP level (ng l−1) (normal <200 ng l−1) | ANP (ng l−1) (normal <43 ng l−1) | |
|---|---|---|
| Carcinoid heart disease | ||
| Median (range) | ||
| Absent (n=23) | 89 (23–1449) | 25 (10–57) |
| Present (n=9) | 894 (328–4432) | 41 (12–89) |
| P-value | P<0.001 | P=0.11 |
| Right atrium dilatation | ||
| Median (range) | ||
| None (n=21) | 89 (23–1449) | 26 (10–57) |
| Mildly dilated (n=3) | 195 (62–2587) | 25 (20–30) |
| Severely dilated (n=8) | 738 (328–4432) | 48 (12–89) |
| P-value | P=0.002 | P=0.36 |
| Right ventricle dilatation | ||
| Median (range) | ||
| None (n=23) | 84 (23–2587) | 25 (10–55) |
| Mildly dilated (n=6) | 407 (153–1058) | 49 (12–62) |
| Severely dilated (n=3) | 1081 (581–4432) | 52 (16–89) |
| P-value | P=0.005 | P=0.13 |
| Tricuspid valve morphology | ||
| Median (range) | ||
| Normal (n=21) | 84 (23–372) | 24 (10–57) |
| Thickened (n=11) | 894 (328–4432) | 48 (12–89) |
| P-value | P<0.001 | P=0.39 |
| Tricuspid valve regurgitation | ||
| Median (range) | ||
| None (n=8) | 50 (23–1349) | 18 (10–28) |
| Mild (I/IV & II/IV) (n=15) | 153 (52–1449) | 26 (20–57) |
| Severe (III/IV &IV/IV) (n=9) | 894 (328–4432) | 41 (12–89) |
| P-value | P=0.007 | P=0.13 |
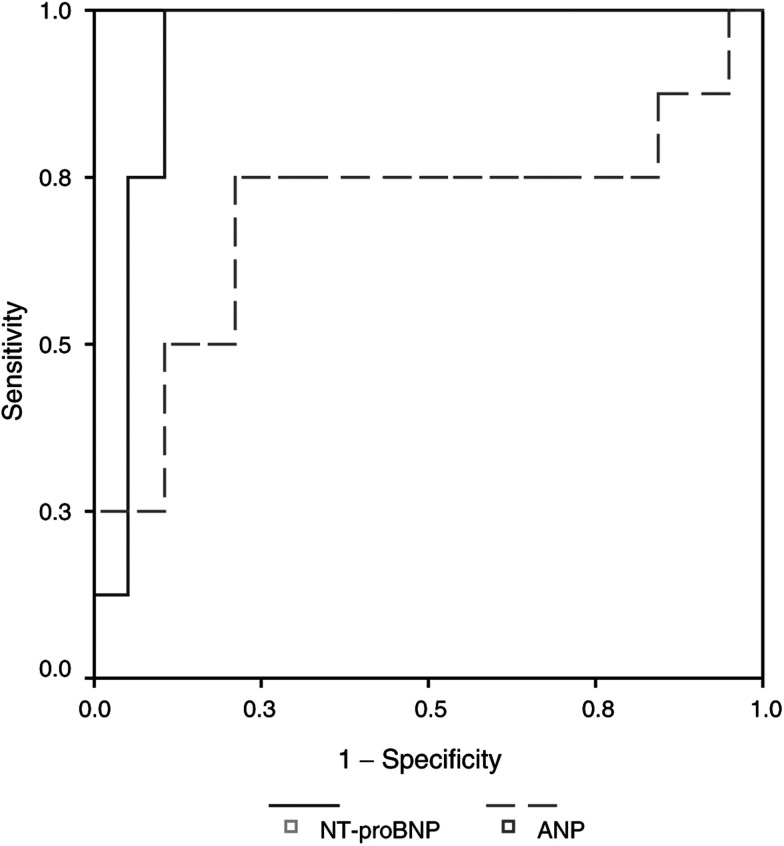
In our patient group NT-proBNP had a positive predictive value (PPV) of 69% at a cutoff value of 200 ng l−1 and a negative predictive value (NPV) of 100%. No additional information was obtained by combining the NT-proBNP values with the ANP levels. To determine the accuracy of both diagnostic tests, a receiver operating characteristic (ROC) curve was used, which showed an area under the curve for NT-proBNP of 0.94 (95% CI 0.85–1.04) and for ANP of 0.69 (95% CI 0.44–0.96) (Figure 3). The highest cutoff value of NT-proBNP with retaining a sensitivity of 100% was 300 ng l−1.
Figure 3.
The ROC curve shows that the accuracy to differentiate between patients with and without heart disease is the best in NT-proBNP compared to ANP levels.
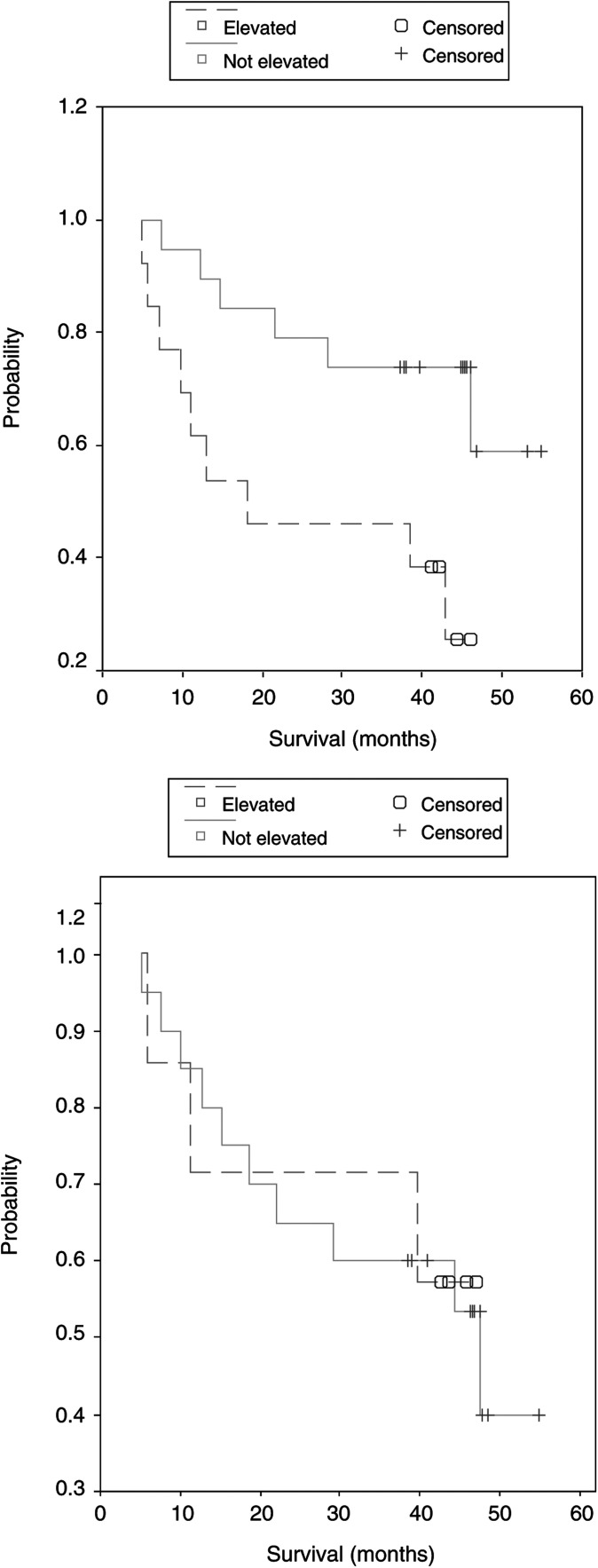
A significantly better survival was observed in patients with a normal NT-proBNP value compared to those with elevated levels (P=0.02). This difference was not seen in the group with a normal compared to an elevated ANP level (P=0.93) (Figure 4).
Figure 4.
Kaplan–Meier curves show a significant better survival in patients with normal levels of NT-proBNP compared to those with elevated levels. This does not apply for the levels of ANP.
DISCUSSION
Thickening of the right heart valves caused by formation of fibrotic plaques eventually followed by regurgitation and right ventricular failure is a characteristic feature of CHD. In metastatic NET with production of hormones the development of CHD is reported in 20–70% of the patients (Tornebrandt et al, 1986; Robiolio et al, 1995; Westberg et al, 2001; Zuetenhorst et al, 2003) and in many patients attributed to the cause of death (Ross and Roberts, 1985). In the present series of 32 patients, the incidence of CHD is 28%, which is rather low compared to the results reported in literature. This might be due to the strict criteria we used for the definition CHD and the availability of octreotide the last decades has improved survival in these patients group with probably a less frequent development of CHD (Quaedvlieg et al, 2001).
In the follow-up and monitoring of carcinoid patients the echocardiography is the cornerstone in the diagnosis of CHD. However, performing an echocardiography is laborious, expensive and not always readily available as referral to a cardiologist is necessary. For these reasons, the cardiac evaluation of carcinoid patients without symptoms of heart failure is often performed less frequently than recommended. Clearly, a screening method allowing rapid and accurate differentiation between patients with and without CHD would be desirable. In this study with 32 patients, we found NT-proBNP to be a reliable marker to make this differentiation with a sensitivity of 100% and a specificity of 83%. This is comparable to the literature for diagnosis of cardiac dysfunction in the general population (McDonagh et al, 1998; Luchner et al, 2000) or in patients suspected to have heart failure (Cowie et al, 1997; Maisel et al, 2002,2003). The PPV of 69% as described in our study is relatively high compared to studies in the general population with a PPV of approximately 30% (Bay et al, 2003), but is in accordance with studies performed in a population with a higher chance of cardiac dysfunction (Cowie et al, 1997; Hammerer-Lercher et al, 2001; Maisel et al, 2002). In our carcinoid population, ANP was less reliable. An explanation could be the application of the activated ANP, which is less stable compared to the prohormone and NT-terminal fragment. However, earlier reports did show diagnostic values for activated ANP in carcinoid patients (Lundin et al, 1989; Zuetenhorst et al, 2003). Tested by a ROC curve, the diagnostic capacities of NT-proBNP were better compared to ANP, and no additional information was obtained by combining NT-proBNP with ANP. Similar to our findings, in earlier studies with a direct comparison between atrial and brain natriuretic peptides, an advantage for brain natriuretic peptides was convincingly proved with no increased predictive power by addition of ANP to BNP determination (Davidson et al, 1996; Cowie et al, 1997; McDonagh et al, 1998; Hammerer-Lercher et al, 2001).
Natriuretic peptides are mainly produced and excreted in the atria of the heart in response to increased wall tension. BNP, in contrast to ANP, is not only secreted from the atria, but also from the ventricles, especially in patients with heart failure. Moreover, there is a correlation between the degree of dilatation and levels of natriuretic peptides (Yasue et al, 1994). Similar to the literature, we also found a significant correlation between the levels of NT-proBNP and the degree of dilatation of the right atrium and ventricle. Although higher levels of ANP were detected in patients with severe dilatation of the right atrium and ventricle compared to those with only mild or no dilatation, this did not reach significance. Most studies about the influence of cardiac dilatation and levels of natriuretic peptides are performed in patients with left-sided heart failure. Information about natriuretic peptide excretion in right ventricular pressure overload, such as in CHD, is scarce and therefore comparison of our findings with other studies is difficult. In two studies of Tulevski et al, 2001a,2001b a relationship between levels of ANP and BNP with right ventricular dysfunction was reported. In our population, elevated levels of NT-proBNP were present in all patients with severe tricuspid valve regurgitation and a significant correlation between degree of regurgitation and NT-proBNP levels was found.
Several studies described the prognostic value of natriuretic peptides in patients with acute coronary syndromes and heart failure (Omland et al, 1996; de Lemos et al, 2001; Koglin et al, 2001; Richards et al, 2003). Patients with elevated levels of BNP were at a higher risk of dying, developing heart failure or undergoing a new myocardial event compared to those with normal levels. As might be expected, we also found a significant better survival for patients with normal levels of NT-proBNP compared to those with elevated levels.
In conclusion, NT-proBNP is a reliable marker to make a rapid and accurate differentiation between patients with and without CHD. Survival of patients with normal levels of NT-proBNP is better compared to those with elevated levels. As many patients with hormonal active NET die from cardiac causes, the detection of CHD in an early stage is important to adjust therapy and improve prognosis. A regular screening of NT-proBNP levels might direct the use of cardiac echography and guide treatment strategies.
References
- Bay M, Kirk V, Parner J, Hassager C, Nielsen H, Krogsgaard K, Trawinski J, Boesgaard S, Aldershvile J (2003) NT-proBNP: a new diagnostic screening tool to differentiate between patients with normal and reduced left ventricular systolic function. Heart 89: 150–154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capella C, Heitz PU, Hofler H, Solcia E, Kloppel G (1995) Revised classification of neuroendocrine tumours of the lung, pancreas and gut. Virchows Arch 425: 547–560 [DOI] [PubMed] [Google Scholar]
- Clerico A, Del Ry S, Maffei S, Prontera C, Emdin M, Giannessi D (2002) The circulating levels of cardiac natriuretic hormones in healthy adults: effects of age and sex. Clin Chem Lab Med 40: 371–377 [DOI] [PubMed] [Google Scholar]
- Cowie MR, Struthers AD, Wood DA, Coats AJ, Thompson SG, Poole-Wilson PA, Sutton GC (1997) Value of natriuretic peptides in assessment of patients with possible new heart failure in primary care. Lancet 350: 1349–1353 [DOI] [PubMed] [Google Scholar]
- Davidson NC, Naas AA, Hanson JK, Kennedy NS, Coutie WJ, Struthers AD (1996) Comparison of atrial natriuretic peptide B-type natriuretic peptide, and N-terminal proatrial natriuretic peptide as indicators of left ventricular systolic dysfunction. Am J Cardiol 77: 828–831 [DOI] [PubMed] [Google Scholar]
- de Bold AJ, Borenstein HB, Veress AT, Sonnenberg H (1981) A rapid and potent natriuretic response to intravenous injection of atrial myocardial extract in rats. Life Sci 28: 89–94 [DOI] [PubMed] [Google Scholar]
- de Lemos JA, Morrow DA, Bentley JH, Omland T, Sabatine MS, McCabe CH, Hall C, Cannon CP, Braunwald E (2001) The prognostic value of B-type natriuretic peptide in patients with acute coronary syndromes. N Engl J Med 345: 1014–1021 [DOI] [PubMed] [Google Scholar]
- Degorce F, Goumon Y, Jacquemart L, Vidaud C, Bellanger L, Pons-Anicet D, Seguin P, Metz-Boutigue MH, Aunis D (1999) A new human chromogranin A (CgA) immunoradiometric assay involving monoclonal antibodies raised against the unprocessed central domain (145–245). Br J Cancer 79: 65–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall C, Rouleau JL, Moye L, de Champlain J, Bichet D, Klein M, Sussex B, Packer M, Rouleau J, Arnold MO (1994) N-terminal proatrial natriuretic factor. An independent predictor of long-term prognosis after myocardial infarction. Circulation 89: 1934–1942 [DOI] [PubMed] [Google Scholar]
- Hammerer-Lercher A, Neubauer E, Muller S, Pachinger O, Puschendorf B, Mair J (2001) Head-to-head comparison of N-terminal pro-brain natriuretic peptide, brain natriuretic peptide and N-terminal pro-atrial natriuretic peptide in diagnosing left ventricular dysfunction. Clin Chim Acta 310: 193–197 [DOI] [PubMed] [Google Scholar]
- Koglin J, Pehlivanli S, Schwaiblmair M, Vogeser M, Cremer P, vonScheidt W (2001) Role of brain natriuretic peptide in risk stratification of patients with congestive heart failure. J Am Coll Cardiol 38: 1934–1941 [DOI] [PubMed] [Google Scholar]
- Lerman A, Gibbons RJ, Rodeheffer RJ, Bailey KR, McKinley LJ, Heublein DM, Burnett Jr JC (1993) Circulating N-terminal atrial natriuretic peptide as a marker for symptomless left-ventricular dysfunction. Lancet 341: 1105–1109 [DOI] [PubMed] [Google Scholar]
- Luchner A, Burnett Jr JC, Jougasaki M, Hense HW, Heid IM, Muders F, Riegger GA, Schunkert H (2000) Evaluation of brain natriuretic peptide as marker of left ventricular dysfunction and hypertrophy in the population. J Hypertens 18: 1121–1128 [DOI] [PubMed] [Google Scholar]
- Lundin L, Norheim I, Landelius J, Oberg K, Theodorsson-Norheim E (1988) Carcinoid heart disease: relationship of circulating vasoactive substances to ultrasound-detectable cardiac abnormalities. Circulation 77: 264–269 [DOI] [PubMed] [Google Scholar]
- Lundin L, Oberg K, Landelius J, Hansson HE, Wilander E, Theodorsson E (1989) Plasma atrial natriuretic peptide in carcinoid heart disease. Am J Cardiol 63: 969–972 [DOI] [PubMed] [Google Scholar]
- Maisel AS, Krishnaswamy P, Nowak RM, McCord J, Hollander JE, Duc P, Omland T, Storrow AB, Abraham WT, Wu AH, Clopton P, Steg PG, Westheim A, Knudsen CW, Perez A, Kazanegra R, Herrmann HC, McCullough PA (2002) Rapid measurement of B-type natriuretic peptide in the emergency diagnosis of heart failure. N Engl J Med 347: 161–167 [DOI] [PubMed] [Google Scholar]
- Maisel AS, McCord J, Nowak RM, Hollander JE, Wu AH, Duc P, Omland T, Storrow AB, Krishnaswamy P, Abraham WT, Clopton P, Steg G, Aumont MC, Westheim A, Knudsen CW, Perez A, Kamin R, Kazanegra R, Herrmann HC, McCullough PA (2003) Bedside B-Type natriuretic peptide in the emergency diagnosis of heart failure with reduced or preserved ejection fraction. Results from the Breathing Not Properly Multinational Study. J Am Coll Cardiol 41: 2010–2017 [DOI] [PubMed] [Google Scholar]
- McDonagh TA, Robb SD, Murdoch DR, Morton JJ, Ford I, Morrison CE, Tunstall-Pedoe H, McMurray JJ, Dargie HJ (1998) Biochemical detection of left-ventricular systolic dysfunction. Lancet 351: 9–13 [DOI] [PubMed] [Google Scholar]
- Omland T, Aakvaag A, Bonarjee VV, Caidahl K, Lie RT, Nilsen DW, Sundsfjord JA, Dickstein K (1996) Plasma brain natriuretic peptide as an indicator of left ventricular systolic function and long-term survival after acute myocardial infarction. Comparison with plasma atrial natriuretic peptide and N-terminal proatrial natriuretic peptide. Circulation 93: 1963–1969 [DOI] [PubMed] [Google Scholar]
- Quaedvlieg PF, Visser O, Lamers CB, Janssen-Heijen ML, Taal BG (2001) Epidemiology and survival in patients with carcinoid disease in The Netherlands. An epidemiological study with 2391 patients. Ann Oncol 12: 1295–1300 [DOI] [PubMed] [Google Scholar]
- Richards AM, Nicholls MG, Espiner EA, Lainchbury JG, Troughton RW, Elliott J, Frampton C, Turner J, Crozier IG, Yandle TG (2003) B-type natriuretic peptides and ejection fraction for prognosis after myocardial infarction. Circulation 107: 2786–2792 [DOI] [PubMed] [Google Scholar]
- Robiolio PA, Rigolin VH, Wilson JS, Harrison JK, Sanders LL, Bashore TM, Feldman JM (1995) Carcinoid heart disease. Correlation of high serotonin levels with valvular abnormalities detected by cardiac catheterization and echocardiography. Circulation 92: 790–795 [DOI] [PubMed] [Google Scholar]
- Ross EM, Roberts WC (1985) The carcinoid syndrome: comparison of 21 necropsy subjects with carcinoid heart disease to 15 necropsy subjects without carcinoid heart disease. Am J Med 79: 339–354 [DOI] [PubMed] [Google Scholar]
- Stroomer AE, Overmars H, Abeling NG, Van Gennip AH (1990) Simultaneous determination of acidic 3,4-dihydroxyphenylalanine metabolites and 5-hydroxyindole-3-acetic acid in urine by high-performance liquid chromatography. Clin Chem 36: 1834–1837 [PubMed] [Google Scholar]
- Taal BG, Hoefnagel C, Boot H, Valdes OR, Rutgers M (2000) Improved effect of 131I-MIBG treatment by predosing with non-radiolabeled MIBG in carcinoid patients, and studies in xenografted mice. Ann Oncol 11: 1437–1443 [DOI] [PubMed] [Google Scholar]
- Taal BG, Hoefnagel CA, Valdes Olmos RA, Boot H, Beijnen JH (1996) Palliative effect of metaiodobenzylguanidine in metastatic carcinoid tumors. J Clin Oncol 14: 1829–1838 [DOI] [PubMed] [Google Scholar]
- Tornebrandt K, Eskilsson J, Nobin A (1986) Heart involvement in metastatic carcinoid disease. Clin Cardiol 9: 13–19 [DOI] [PubMed] [Google Scholar]
- Tulevski II, Groenink M, Der Wall EE, van Veldhuisen DJ, Boomsma F, Stoker J, Hirsch A, Lemkes JS, Mulder BJ (2001a) Increased brain and atrial natriuretic peptides in patients with chronic right ventricular pressure overload: correlation between plasma neurohormones and right ventricular dysfunction. Heart 86: 27–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tulevski II, Hirsch A, Sanson BJ, Romkes H, van der Wall EE, van Veldhuisen DJ, Buller HR, Mulder BJ (2001b) Increased brain natriuretic peptide as a marker for right ventricular dysfunction in acute pulmonary embolism. Thromb Haemost 86: 1193–1196 [PubMed] [Google Scholar]
- Westberg G, Wangberg B, Ahlman H, Bergh CH, Beckman-Suurkula M, Caidahl K (2001) Prediction of prognosis by echocardiography in patients with midgut carcinoid syndrome. Br J Surg 88: 865–872 [DOI] [PubMed] [Google Scholar]
- Yasue H, Yoshimura M, Sumida H, Kikuta K, Kugiyama K, Jougasaki M, Ogawa H, Okumura K, Mukoyama M, Nakao K (1994) Localization and mechanism of secretion of B-type natriuretic peptide in comparison with those of A-type natriuretic peptide in normal subjects and patients with heart failure. Circulation 90: 195–203 [DOI] [PubMed] [Google Scholar]
- Zuetenhorst JM, Bonfrer JMG, Korse CM, Bakker H, Tinteren van H, Taal BG (2003) Carcinoid heart disease; the role of urinary 5-HIAA excretion and plasma levels of ANP, TGF-b and FGF. Cancer 97: 1609–1615 [DOI] [PubMed] [Google Scholar]