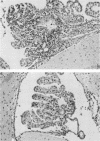
Abstract
The role of antiviral antibody in resistance to acute lymphocytic choriomeningitis virus infection has been examined by passive transfer of monoclonal antibodies and intracerebral challenge infection. Protection of mice from lethal T-cell-mediated acute disease was observed following passive administration of antibodies either 1 day before or up to 2 days after infection. Viral replication was suppressed in protected mice, and the cytotoxic T-cell response to virus was also diminished. Virus was cleared from the brain and other tissues of protected mice without development of lethal immunopathology, suggesting that preexisting antibody may play a significant role in modulating potentially destructive effects of T-cell-mediated immune responses to pathogens.
Full text
PDF

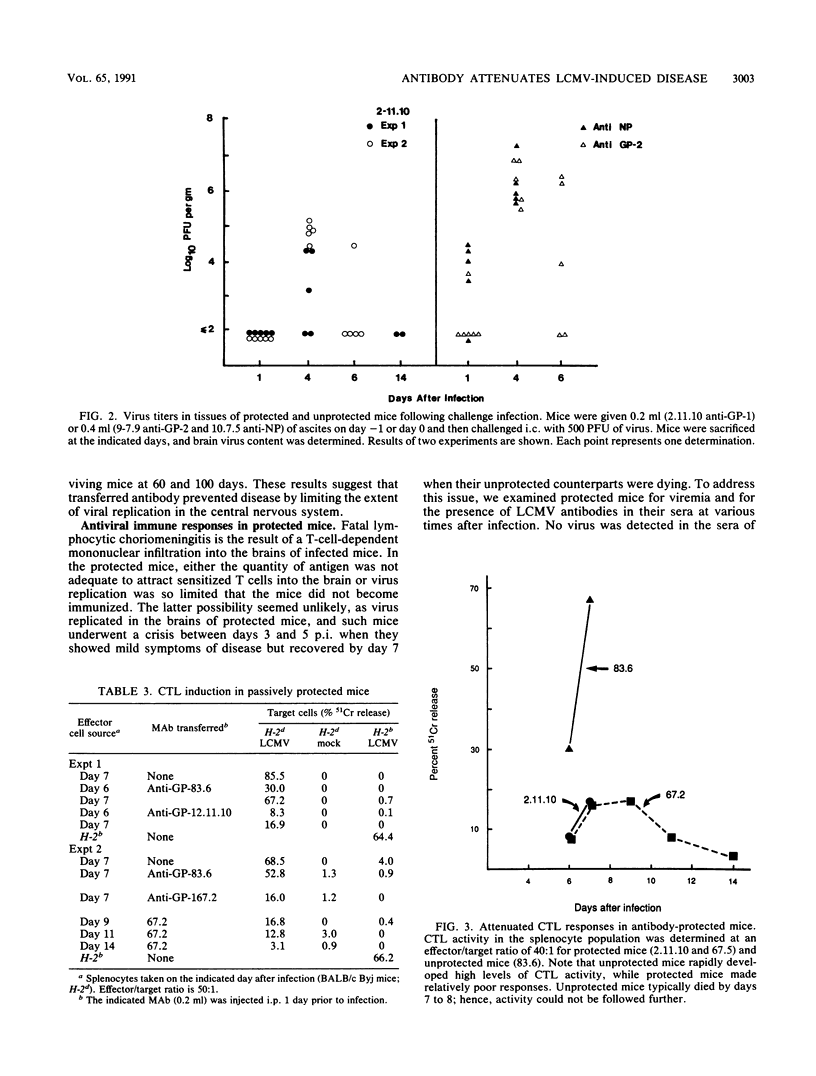
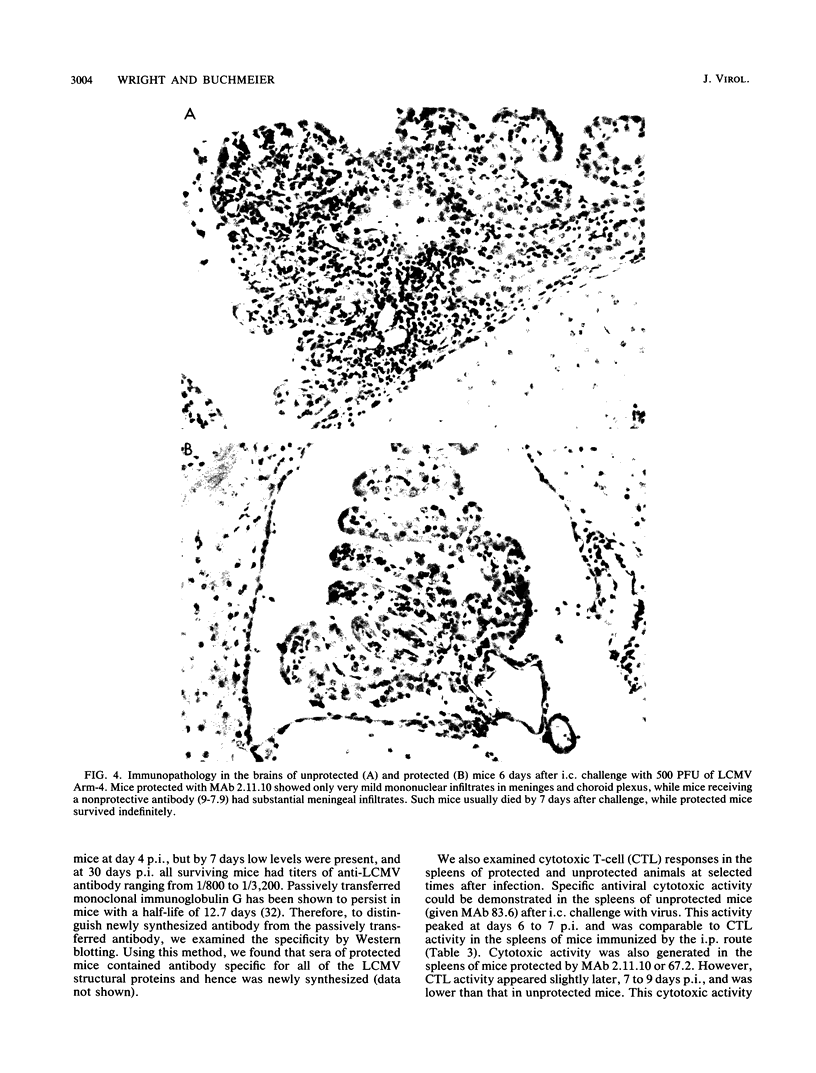


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan J. E., Doherty P. C. Immune T cells can protect or induce fatal neurological disease in murine lymphocytic choriomeningitis. Cell Immunol. 1985 Feb;90(2):401–407. doi: 10.1016/0008-8749(85)90204-7. [DOI] [PubMed] [Google Scholar]
- Baenziger J., Hengartner H., Zinkernagel R. M., Cole G. A. Induction or prevention of immunopathological disease by cloned cytotoxic T cell lines specific for lymphocytic choriomeningitis virus. Eur J Immunol. 1986 Apr;16(4):387–393. doi: 10.1002/eji.1830160413. [DOI] [PubMed] [Google Scholar]
- Balachandran N., Bacchetti S., Rawls W. E. Protection against lethal challenge of BALB/c mice by passive transfer of monoclonal antibodies to five glycoproteins of herpes simplex virus type 2. Infect Immun. 1982 Sep;37(3):1132–1137. doi: 10.1128/iai.37.3.1132-1137.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boere W. A., Benaissa-Trouw B. J., Harmsen M., Kraaijeveld C. A., Snippe H. Neutralizing and non-neutralizing monoclonal antibodies to the E2 glycoprotein of Semliki Forest virus can protect mice from lethal encephalitis. J Gen Virol. 1983 Jun;64(Pt 6):1405–1408. doi: 10.1099/0022-1317-64-6-1405. [DOI] [PubMed] [Google Scholar]
- Bruns M., Cihak J., Müller G., Lehmann-Grube F. Lymphocytic choriomeningitis virus. VI. Isolation of a glycoprotein mediating neutralization. Virology. 1983 Oct 15;130(1):247–251. doi: 10.1016/0042-6822(83)90135-6. [DOI] [PubMed] [Google Scholar]
- Buchmeier M. J., Oldstone M. B. Protein structure of lymphocytic choriomeningitis virus: evidence for a cell-associated precursor of the virion glycopeptides. Virology. 1979 Nov;99(1):111–120. doi: 10.1016/0042-6822(79)90042-4. [DOI] [PubMed] [Google Scholar]
- Buchmeier M. J., Oldstone M. B. Virus-induced immune complex disease: identification of specific viral antigens and antibodies deposited in complexes during chronic lymphocytic choriomeningitis virus infection. J Immunol. 1978 Apr;120(4):1297–1304. [PubMed] [Google Scholar]
- Buchmeier M. J., Welsh R. M., Dutko F. J., Oldstone M. B. The virology and immunobiology of lymphocytic choriomeningitis virus infection. Adv Immunol. 1980;30:275–331. doi: 10.1016/s0065-2776(08)60197-2. [DOI] [PubMed] [Google Scholar]
- Byrne J. A., Oldstone M. B. Biology of cloned cytotoxic T lymphocytes specific for lymphocytic choriomeningitis virus: clearance of virus in vivo. J Virol. 1984 Sep;51(3):682–686. doi: 10.1128/jvi.51.3.682-686.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerny A., Huegin A. W., Sutter S., Bazin H., Hengartner H. H., Zinkernagel R. M. Immunity to lymphocytic choriomeningitis virus in B cell-depleted mice: evidence for B cell and antibody-independent protection by memory T cells. Eur J Immunol. 1986 Aug;16(8):913–917. doi: 10.1002/eji.1830160807. [DOI] [PubMed] [Google Scholar]
- Cerny A., Sutter S., Bazin H., Hengartner H., Zinkernagel R. M. Clearance of lymphocytic choriomeningitis virus in antibody- and B-cell-deprived mice. J Virol. 1988 May;62(5):1803–1807. doi: 10.1128/jvi.62.5.1803-1807.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole G. A., Gilden D. H., Monjan A. A., Nathanson N. Lymphocytic choriomeningitis virus: pathogenesis of acute central nervous system disease. Fed Proc. 1971 Nov-Dec;30(6):1831–1841. [PubMed] [Google Scholar]
- Doherty P. C. Some problem areas in the interaction between viruses and the immune system. Immunol Cell Biol. 1987 Aug;65(Pt 4):279–286. doi: 10.1038/icb.1987.32. [DOI] [PubMed] [Google Scholar]
- Dutko F. J., Oldstone M. B. Genomic and biological variation among commonly used lymphocytic choriomeningitis virus strains. J Gen Virol. 1983 Aug;64(Pt 8):1689–1698. doi: 10.1099/0022-1317-64-8-1689. [DOI] [PubMed] [Google Scholar]
- Edwards S. A., Fan H. Immunoselection and characterization of Moloney murine leukemia virus-infected cell lines deficient in surface gag antigen expression. Virology. 1981 Aug;113(1):95–108. doi: 10.1016/0042-6822(81)90139-2. [DOI] [PubMed] [Google Scholar]
- Geschwender H. H., Rutter G., Lehmann-Grube F. Lymphocytic choriomeningitis virus. II. Characterization of extractable complement-fixing activity. Med Microbiol Immunol. 1976 Jun 1;162(2):119–131. doi: 10.1007/BF02121321. [DOI] [PubMed] [Google Scholar]
- Hirsch R. L. The complement system: its importance in the host response to viral infection. Microbiol Rev. 1982 Mar;46(1):71–85. doi: 10.1128/mr.46.1.71-85.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmig B., Lehmann-Grube F. The immune response of the mouse to lymphocytic choriomeningitis virus. I. Circulating antibodies. J Gen Virol. 1979 Dec;45(3):703–710. doi: 10.1099/0022-1317-45-3-703. [DOI] [PubMed] [Google Scholar]
- Lehmann-Grube F., Assmann U., Löliger C., Moskophidis D., Löhler J. Mechanism of recovery from acute virus infection. I. Role of T lymphocytes in the clearance of lymphocytic choriomeningitis virus from spleens of mice. J Immunol. 1985 Jan;134(1):608–615. [PubMed] [Google Scholar]
- Lehmann-Grube F. Persistent infection of the mouse with the virus of lymphocytic choriomeningitis. J Clin Pathol Suppl (R Coll Pathol) 1972;6:8–21. [PMC free article] [PubMed] [Google Scholar]
- Moskophidis D., Cobbold S. P., Waldmann H., Lehmann-Grube F. Mechanism of recovery from acute virus infection: treatment of lymphocytic choriomeningitis virus-infected mice with monoclonal antibodies reveals that Lyt-2+ T lymphocytes mediate clearance of virus and regulate the antiviral antibody response. J Virol. 1987 Jun;61(6):1867–1874. doi: 10.1128/jvi.61.6.1867-1874.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldstone M. B., Blount P., Southern P. J., Lampert P. W. Cytoimmunotherapy for persistent virus infection reveals a unique clearance pattern from the central nervous system. Nature. 1986 May 15;321(6067):239–243. doi: 10.1038/321239a0. [DOI] [PubMed] [Google Scholar]
- Oldstone M. B., Dixon F. J. Pathogenesis of chronic disease associated with persistent lymphocytic choriomeningitis viral infection. I. Relationship of antibody production to disease in neonatally infected mice. J Exp Med. 1969 Mar 1;129(3):483–505. doi: 10.1084/jem.129.3.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parekh B. S., Buchmeier M. J. Proteins of lymphocytic choriomeningitis virus: antigenic topography of the viral glycoproteins. Virology. 1986 Sep;153(2):168–178. doi: 10.1016/0042-6822(86)90020-6. [DOI] [PubMed] [Google Scholar]
- Perrin L. H., Joseph B. S., Cooper N. R., Oldstone M. B. Mechanism of injury of virus-infected cells by antiviral antibody and complement: participation of IgG, F(ab')2, and the alternative complement pathway. J Exp Med. 1976 May 1;143(5):1027–1041. doi: 10.1084/jem.143.5.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmaljohn A. L., Johnson E. D., Dalrymple J. M., Cole G. A. Non-neutralizing monoclonal antibodies can prevent lethal alphavirus encephalitis. Nature. 1982 May 6;297(5861):70–72. doi: 10.1038/297070a0. [DOI] [PubMed] [Google Scholar]
- Sissons J. G., Oldstone M. B. Antibody-mediated destruction of virus-infected cells. Adv Immunol. 1980;29:209–260. doi: 10.1016/S0065-2776(08)60045-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot P. J., Buchmeier M. J. Catabolism of homologous murine monoclonal hybridoma IgG antibodies in mice. Immunology. 1987 Apr;60(4):485–489. [PMC free article] [PubMed] [Google Scholar]
- Thomsen A. R., Marker O. The complementary roles of cellular and humoral immunity in resistance to re-infection with LCM virus. Immunology. 1988 Sep;65(1):9–15. [PMC free article] [PubMed] [Google Scholar]
- Thomsen A. R., Volkert M., Marker O. Different isotype profiles of virus-specific antibodies in acute and persistent lymphocytic choriomeningitis virus infection in mice. Immunology. 1985 Jun;55(2):213–223. [PMC free article] [PubMed] [Google Scholar]
- Thomsen A. R., Volkert M., Marker O. The timing of the immune response in relation to virus growth determines the outcome of the LCM infection. Acta Pathol Microbiol Scand C. 1979 Feb;87C(1):47–54. [PubMed] [Google Scholar]
- Volkert M., Lundstedt C. The provocation of latent lymphocytic choriomeningitis virus infections in mice by treatment with antilymphocytic serum. J Exp Med. 1968 Feb 1;127(2):327–339. doi: 10.1084/jem.127.2.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright K. E., Salvato M. S., Buchmeier M. J. Neutralizing epitopes of lymphocytic choriomeningitis virus are conformational and require both glycosylation and disulfide bonds for expression. Virology. 1989 Aug;171(2):417–426. doi: 10.1016/0042-6822(89)90610-7. [DOI] [PubMed] [Google Scholar]
- Zinkernagel R. M., Welsh R. M. H-2 compatibility requirement for virus-specific T cell-mediated effector functions in vivo. I. Specificity of T cells conferring antiviral protection against lymphocytic choriomeningitis virus is associated with H-2K and H-2D. J Immunol. 1976 Nov;117(5 Pt 1):1495–1502. [PubMed] [Google Scholar]