Abstract
We previously performed a global analysis of the gene expression of gastric cancer cell lines established from metastases to the peritoneal cavity with the cDNA microarray method, which made it possible to analyse the expression of approximately 21 168 genes for the identification of novel markers for the detection of micrometastases in the peritoneal cavity. One of the upregulated genes is dopa decarboxylase (DDC), which is responsible for the synthesis of the key neurotransmitters dopamine and serotonine. We have examined its potential as a novel marker for the detection of peritoneal micrometastases of gastric cancer.
DDC mRNA in the peritoneal wash from 112 gastric cancer patients was quantified for comparison of carcinoembryonic antigen (CEA) mRNA by means of real-time reverse transcriptase–polymerase chain reaction (RT–PCR) with a fluorescently labelled probe to predict peritoneal recurrence. The quantity of DDC and CEA correlated with wall penetration. Real-time RT–PCR could quantitate 10–106 DDC-expressing gastric cancer cells per 107 mesothelial cells. The cutoff value was set at the upper limit of the quantitative value for noncancer patients, and those above this cutoff value constituted the micrometastasis (MM+) group. Of 15 cases with peritoneal dissemination, 13 were MM+DDC (87% sensitivity), and one of 48 t1 cases was MM+ (98% specificity). DDC levels in peritoneal washes from patients with synchronous peritoneal metastases were more than 50 times higher than in those from patients without metastasis (P<0.01). For 15 cases of peritoneal dissemination (seven cases were cytologically positive), DDC was positive in 13 cases (87% sensitivity), but CEA failed to detect micrometastases in four cases (73% sensitivity), indicating that DDC is in some cases superior to CEA for the detection of peritoneal micrometastases of gastric cancer in terms of sensitivity as well as specificity, especially for poorly differentiated adenocarcinomas. A combination of CEA and DDC improved the accuracy of diagnosis up to 94%.
These results suggest that DDC is potentially a novel marker for peritoneal dissemination of gastric cancer and that quantitative RT–PCR of DDC is reliable and efficient for the selection of patients for adjuvant intraperitoneal chemotherapy to prevent peritoneal recurrence.
Keywords: DDC, real-time RT–PCR, gastric cancer, peritoneal dissemination
The prognosis for gastric cancer that has invaded as far as the gastric serosa is still poor, with a 5-year survival of less than 35% (Yamazaki et al, 1989). Peritoneal dissemination is reported to be the most frequent type of recurrence after curative resection in such gastric cancer cases (Baba et al, 1989; Kodera et al, 1996). Free cancer cells derived from serosal invasion may thus be an indicator of early peritoneal seeding with subsequent formation of metastatic colonies, so that their detection is likely to be a useful tool for predicting the outcome for patients with advanced gastric cancers (Abe et al, 1995; Bonenkamp et al, 1996). Conventional intraperitoneal cytology (CY) has recently become a common procedure for the detection of free gastric cancer cells in the peritoneal cavity. Cytology-positive cases are classified as Stage IV of the UICC gastric cancer classification and curative operation is impossible in such cases. Depending on the cytological results, operative procedures including lymphnode dissection may have to be changed, and intensive chemotherapy may have to be used for such cases. Conventional CY, however, lacks sensitivity and some patients with negative CY results have nevertheless been found to show recurrence in the form of peritoneal dissemination. A previous study has indicated the usefulness of carcinoembryonic antigen (CEA)-specific RT–PCR to detect free cancer cells in the peritoneal fluids (Nakanishi et al, 1997); CEA is not perfect as a marker, so that identification of more reliable markers is needed.
We previously performed a global analysis of the differential gene expression of a gastric cancer cell line established from a primary main tumour and of other cell lines established from metastases to the peritoneal cavity (Sakakura et al, 2002). The application of a high-density cDNA microarray method made it possible to analyse the expression of approximately 21 168 genes. Our investigations showed that 24 genes were upregulated and 17 genes downregulated, in addition to the expression sequence tags (ESTs) in gastric cancer cell lines established from metastases to the peritoneal cavity. One of these upregulated genes is dopa decarboxylase (DDC).
DDC is an enzyme that metabolises DOPA (3,4-dihydroxyphenylalanine) to DA (dopamine), and increased urine excretion of catecholamine metabolites is well known as a key diagnostic feature of neuroblastoma (Eldrup et al, 2001). DDC is responsible for the synthesis of the key neurotransmitters dopamine and serotonin, and is frequently expressed in neuroblastoma and small-cell carcinoma of the lung (Jensen et al, 1990; Gilbert et al, 1999). DOPA is used as a tumour marker for children with neuroblastoma (Ikeda et al, 1996), but the role of DDC in gastric cancer peritoneal dissemination is still unclear.
In the study presented here, even more sensitive detection of micrometastases of cancer cells could be achieved through amplification of the novel marker DDC by means of quantitative reverse transcriptase–polymerase chain reaction (RT–PCR) for peritoneal lavage fluid cells. This detection method can be expected to result in a more accurate prediction of peritoneal recurrence in gastric cancer patients.
MATERIALS AND METHODS
Cell culture, peritoneal washes and RNA preparation
Gastric cancer cell lines SNU-1, SNU-5, SNU-16, SNU-620 cells were established previously by Park et al. KATO-III, GT3TKB, MKN7 and acute myeloid leukaemia cell line HL60 were purchased from the Riken Cell Bank (Tsukuba, Japan), and NUGC-3 cells from the Health Science Research Resources Bank (Osaka, Japan). GT3TKB cells were maintained at 37°C in a humidified atmosphere of 5% CO2 in high-glucose DMEM (Sigma, St Louis, MO, USA), whereas MKN7 and NUGC-3 were maintained in RPMI 1640 (Sigma). Both media were supplemented with 10% foetal bovine serum, penicillin and streptomycin. When they had reached 80–90% confluence, cells were washed with ice-cold PBS and homogenised immediately in Isogen reagent (Nippon Gene, Osaka, Japan), and total RNA was extracted. mRNA was extracted from each cell line with the FAST track kit Ver.2 (Invitrogen), according to the manufacturer's instructions. Mesothelial cell line Met5A was established by Reddel et al. (Duncan et al, 1993; Nakabayashi et al, 1999).
The study population consisted of 112 patients with gastric cancer and 15 patients with benign diseases, including cholecystolithiasis and leiomyoma of the stomach, who underwent surgery at Kyoto Prefectural University of Medicine between 1999 and 2003. Ascitic fluid was collected from the Douglas cavity during laparotomy. Written informed consent was obtained from each patient prior to tissue acquisition. In the absence of ascites, 150 ml of saline was introduced into the Douglas cavity at the beginning of the operation and aspirated after gentle stirring. These washes were centrifuged at 2000 rpm for 10 min to collect intact cells, which were rinsed with PBS, dissolved in ISOGEN RNA extraction buffer (Nippon Gene, Tokyo) and stored at −80°C until use. Samples were centrifuged at 12 000 rpm in a rotor (RA-48J, Kubota Fujioka, Japan) at 4°C for ethanol precipitation.
Northern blot analysis
Northern blot was performed as previously described by us (Sakakura et al, 1994, 1996). In brief, the total cellular RNA was prepared with the guanidine isothiocyanate–phenol–chloroform procedure. Poly(A)+ RNA was selected with an oligo dT column (Cat. No. R500-50, Invitrogen), and then fractionated on 1% agarose/2.2 M formaldehyde gels. Probes were labelled with 32P by random priming. Each blot was then hybridised with the probe for the selected gene and β-actin. Signals were analysed with a BAS 2000 image analyser followed by calculation of the degree of overexpression compared to the control.
Real-time quantitative RT–PCR
cDNA was produced from total RNA by using a Superscript Preamplification System (BRL, Bethesda, MD, USA) in accordance with the procedures suggested by the manufacturer. Total RNA (2 μg) was heated to 70°C for 10 min in 14 μl of duethylpyrocarbonate-treated water containing 0.5 μg oligo (dT). Synthesis buffer (10 ×, 500 mM Tris-HCl, pH 8.3, 750 mM KCl, 30 mM MgCl2), 2 μl 10 mM dNTP mix, 2 μl 0.1 M DTT, and reverse transcriptase (Superscript RT; 200 U μL−1, GIBCO BRL, Gaithersburg, MD, USA) were added to the sample. The resulting reaction mixture was incubated at 42°C for 50 min, and the reaction was terminated by incubating the mixture at 90°C for 5 min.
Quantitative PCR was performed using the real-time'Taqman TM' technology as described by Nakanishi et al (2000) and the results were analysed on a Model 5700 Sequence Detector (Applied Biosystems Corp., Foster City, CA, USA).
The DDC RT–PCR primers were 5′-AAGCACAGCCATCAGGATTCA-3′ and 5′-TGGACATGCTTGCGGATATAAG-3′, and the CEA RT–PCR primers were 5′-TCTGGAACTTCTCCTGGTCTCTCAGCTGG-3′ and 5′-TGAAGCTGTTGCAAATGCTTTAAGGAAGAAGC-3′. Hybridisation probes, which bind to PCR products, were labelled with a reporter dye, FAM, on the 5′ nucleotide and a quenching dye, TAMRA, on the 3′nucleotide. The sequences of hybridisation probes were CEA: 5′-(FAM) CATCTGGAACTTCTCCTGGTCTCTCAGC (TAMRA)-3′ and DDC: 5′-(FAM) AAGCACAGCCATCAGGATTCA (TAMRA)-3′.
In total, 50 μl reactions contained: 1.25 U Amp-Taq DNA polymerase, 1 × PCR reaction buffer, 180 ng of each primer, 200 mM dNTP, 400 mM dUTP, 100 nM Taqman probe and 0.5 U Amplirase (Applied Biosystems Corp.). The Ct value corresponding to the number of cycles at which the fluorescence emission monitored in real time reached a threshold of 10 standard deviations (s.d.) above the mean base line emission from cycles 1 to 40 was measured in serial dilutions of control cDNA, and analysed for each target. CEA and DDC served as standard curves from which the rates of change in Ct values were determined. The cycling parameters were 2 min at 50°C and 10 min at 95°C, followed by 40 cycles of 15 s at 95°C and 1 min at 60°C.
To minimise the errors arising from the validation of the initial amount of RNA in the samples, β-actin mRNA was amplified as an internal reference against which other RNA values could be normalised. The primers and the probe for the β-actin RNA were purchased from Applied Biosystems. Normalised results were expressed as the ratio of copies of each of the genes to copies of the β-actin gene.
Statistical methods for analysis
Statistical analysis was performed using the NAP system programmed by Aoki (Version 4.0). The first objective of the statistical analysis was to examine the influence of DDC expression on clinicopathological factors with the unpaired T-test. The various groups of patients were compared by means of either χ2 test or Mann–Whitney U-test. The results with P-values of less than 0.05 were considered statistically significant.
RESULTS
LightCycler validation of rapid quantitative RT–PCR
Real-time fluorescence PCR monitoring with the LightCycler using hybridisation probes allowed for rapid and sensitive detection of DDC mRNA from the patient samples. With this method, 10–106 DDC-expressing gastric cancer SNU-16 cells per 107 mesothelial cells could be quantitated. No significant level of DDC mRNA was detected in peripheral blood lymphocytes or mesothelial cells from healthy volunteers.
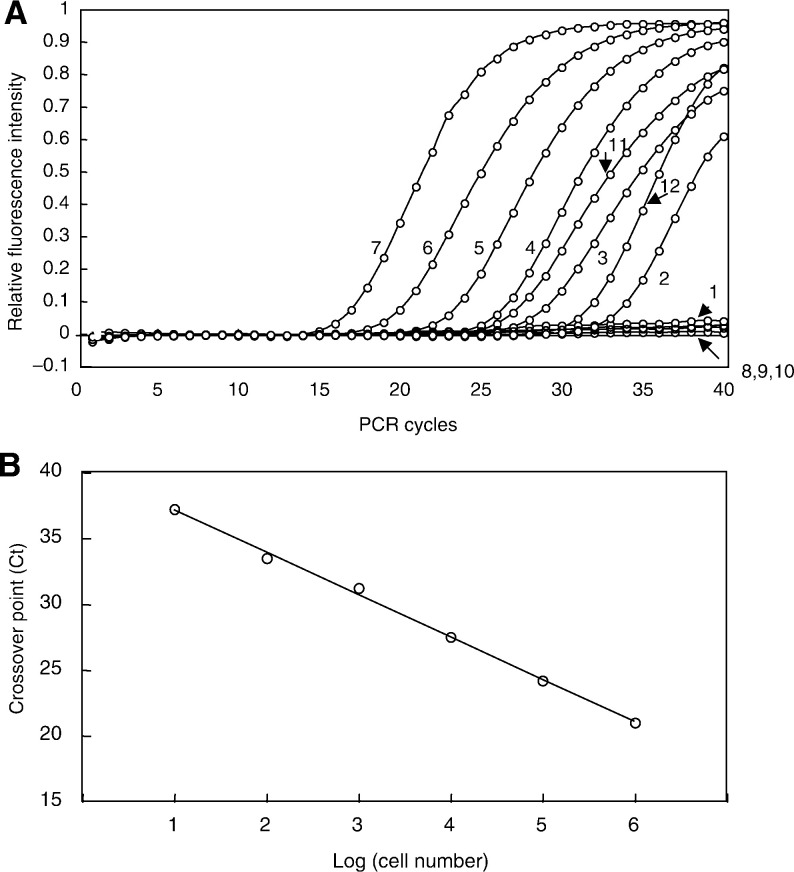
Quantification of messages with the LightCycler was assessed by determination of the crossover point (Ct), the cycle when fluorescence of a given sample rose above the background level to yield the maximal slope with respect to log-linear amplification. Figure 1B illustrates a standard curve constructed by plotting the log number of 10-fold serially diluted SNU-16 cells against their respective Cts. DDC mRNA values for patient samples with unknown concentration were calculated with reference to the calibration curve.
Figure 1.
Representative results of real-time RT–PCR with LightCycler and calibration curve for DDC mRNA estimation. (A) Run profile vs PCR cycles. Six external standards (lines 1–6) were compared with two patient samples (lines 10 and 11) with unknown concentrations, which were amplified with real-time ‘Taqman TM’ technology and analysed with a Model 5700 Sequence Detector: line 1=1; line 2=10; line 3=102; line 4=103; line 5=104; line 6=105; line 7=106 SNU-16 gastric cancer cell equivalent cDNA, line 8=Met 5A; line 9=HL60; line 10=peritoneal wash with negative conventional RT–PCR results; lines 11 and 12=peritoneal wash with positive conventional RT–PCR results. (B) Calibration curve for DDC mRNA estimation constructed from data for external controls (A) by plotting the crossover points (Ct) against the log (SNU-16 cell number). Relative DDC mRNA values in patient samples were calculated with reference to this curve.
Expression of DDC mRNA in gastric cancer cell lines, mesothelial cell line, normal gastric mucosa and cancerous tissues
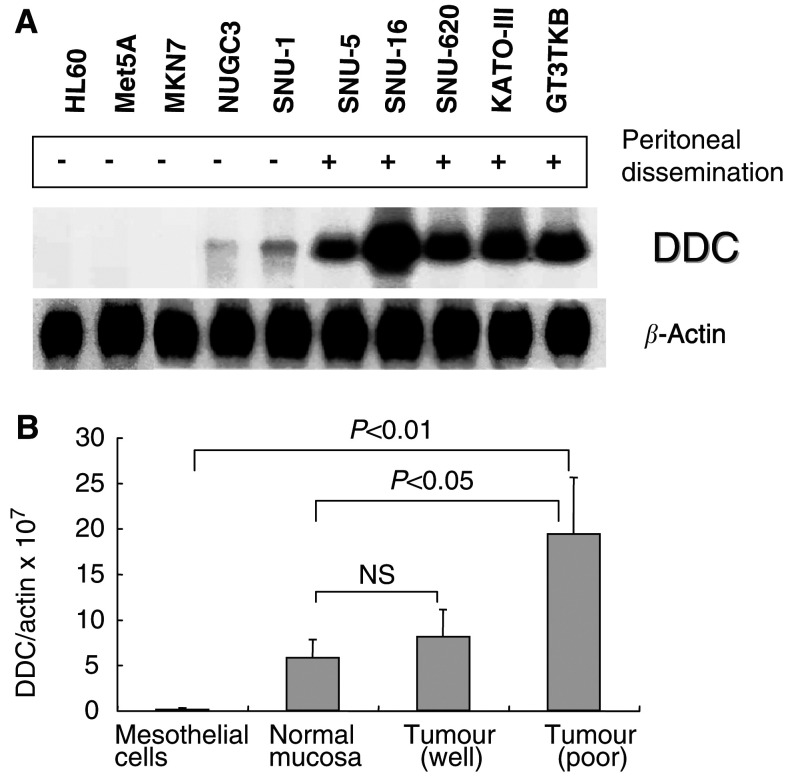
Northern blot analysis showed a high expression level of DDC only in cells with a high potential for peritoneal dissemination and a low expression level in all the cells with a low potential. The mesothelial cell line Met5A and human leukaemia cell line HL-60 showed a very low level of DDC expression (Figure 2A).
Figure 2.
Specificity of DDC expression. (A) DDC mRNA expressions in various gastric cancer cell lines were analysed by Northern blotting. Northern blot analysis of differently expressed genes in eight gastric cancer cells. Upregulated genes in gastric cancer cells from malignant ascites compared to those in the primary lesion. (B) DDC mRNA expression in primary gastric cancers, normal gastric mucosa and mesothelial cells were analysed by quantitative RT–PCR. Well: well-differentiated adenocarcinonoma; poor: poorly differentiated adenocarcinoma.
The DDC expression was detected in both normal gastric mucosa and gastric cancer tissues of clinical specimens, but the DDC expression was higher in the latter than in the former. It was significant in poorly differentiated adenocarcinomas, but the difference was not significant between cancerous tissue and normal mucosa in cases of well-differentiated adenocarcinoma. The DDC expression was significantly higher than in mesothelial cells from peritoneal wash, especially in the case of poorly differentiated adenocarcinoma (Figure 2B). The summary of DDC mRNA and clinicopathological factors in gastric cancer patients is shown in Table 1 . DDC expression correlates with differentiation, depth of invasion, lymphatic invasion and peritoneal dissemination.
Table 1. Summary of DDC mRNA and clinicopathological factors in gastric cancer patients.
|
DDC mRNA |
||||
|---|---|---|---|---|
| Variables | Positive | Negative | P-value* | |
| Sex | Male | 9 | 44 | NS |
| Female | 11 | 48 | (0.7332376) | |
| Differentiation | Differentiated | 4 | 47 | 0.002314** |
| Undifferentiated | 16 | 45 | ||
| Depth of invasion | t1, t2 | 3 | 66 | 0.0073323** |
| t3, t4 | 17 | 26 | ||
| Lymphatic invasion | Negative | 3 | 45 | 0.0322323** |
| Positive | 17 | 47 | ||
| Vascular invasion | Negative | 11 | 48 | N.S. |
| Positive | 9 | 44 | (0.067332376) | |
| Lymph node metastasis | Negative | 3 | 42 | 0.037622** |
| Positive | 17 | 50 | ||
| Peritoneal dissemination | Negative | 2 | 90 | 0.0013339** |
| Positive | 18 | 2 | ||
t = classification: t1=mucosa to submucosa; t2=muscularis propria to subserosa; t3=serosa-exposed; t4=serosa-infiltrating.
Mann–Whitney test;
statistically significant, NS=not significant.
DDC mRNA/β-actin mRNA ratio and the degree of wall invasiveness
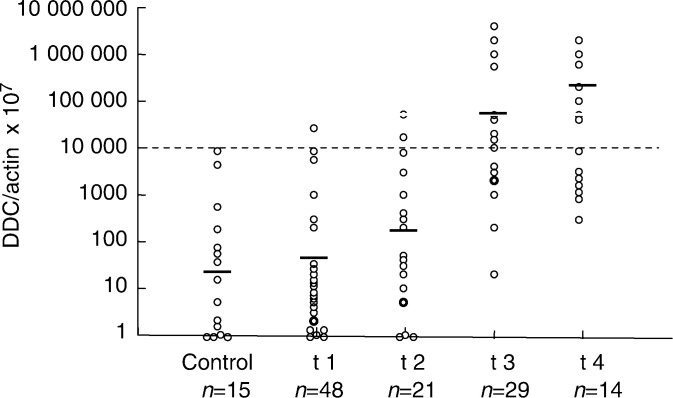
In order to standardise the amount of RNA in each sample, β-actin mRNA was used as an internal control. The value for extracted DDC was determined as the DDC mRNA/β-actin mRNA ratio. The average DDC mRNA/β-actin mRNA ratio (× 107) by t classification was: t1, 39+13; t2, 162+93; t3, 39210+6613; t4, 17300+4139 (average+s.d.). Subjects were further classified into positive cases (t3, t4) and negative (t1, t2) for invasion of the serosa. The results showed that the DDC mRNA/β-actin mRNA ratio correlates with the degree of wall invasiveness. The plot for DDC mRNA/β-actin mRNA (× 107) is shown in Figure 3. The DDC mRNA/β-actin mRNA ratio was significantly higher for cases positive for invasiveness of the serosa than for negative cases.
Figure 3.
Relative values for DCC mRNA/β-actin mRNA ratios in peritoneal washes of gastric carcinoma patients by depth of invasion (pT category). DDC mRNA values correlated with depth of cancer invasion (P<0.01).
DDC as an independent prognostic factor
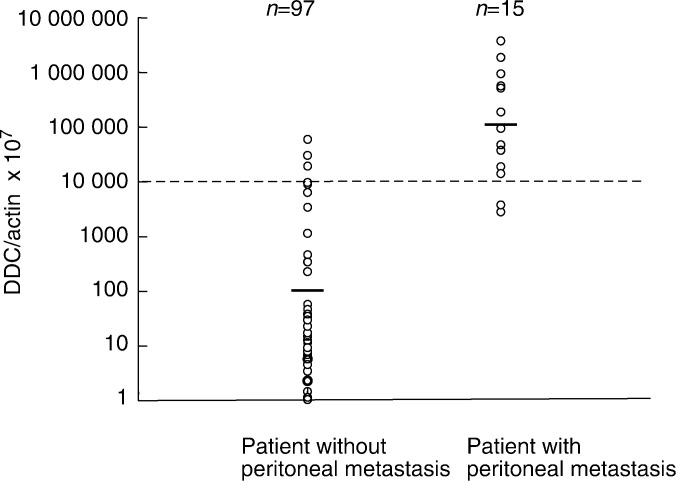
The highest value of the DDC mRNA/β-actin mRNA ratio determined for noncancer cases undergoing gastric surgery was adopted as the cutoff value (broken line on the graph), and values greater than this value were regarded as positive (DDC+). Of the total of 112 cases, 20 were determined to be DDC positive, and 15 showed positive cytology (CY+) or were observed to have peritoneal metastases. Since 13 of these 15 subjects had results above the cutoff value, they were classified as DDC+ (86% sensitivity). Moreover, one of the 48 t1 cases was DDC+ (98% specificity) (Figure 4).
Figure 4.
Relative DDC mRNA values for peritoneal washes from the Douglas cavity measured by real-time RT–PCR with the Light Cycler in gastric cancer patients with or without synchronous peritoneal metastasis. DDC mRNA values for washes from metastasis-positive patients were significantly higher than those for metastasis-negative patients (P<0.001).
Comparison of sensitivity and specificity of DDC and CEA as markers for peritoneal micrometastasis of gastric cancer
CEA mRNA/β-actin mRNA was also measured in all clinical samples, and the results are presented as the CEA mRNA/β-actin mRNA ratio. The results for CEA mRNA/β-actin mRNA (× 1000) were: t1, 5.3+3.5; t2, 8.4+6.8; t3, 71.1+22.8; and t4, 643.9+378.3. A correlation was observed between the degree of wall invasion and the level of CEA mRNA/β-actin mRNA (data not shown). Moreover, when classified, according to the invasion of serosa into positive (t3, t4) and negative (t1, t2), a significant difference was observed between the two groups (data not shown). The highest value for the CEA mRNA/β-actin mRNA ratio in noncancer cases was set as the cutoff value, and the cases showing a value greater than this were regarded as CEA positive. Of the 15 subjects who were also CY+ or were intraoperatively observed to have peritoneal metastasis, 11 were CEA+. However, for four subjects below the cutoff value, (CEA−) metastasis was observed (73% sensitivity), and three of the 48 subjects who were t1 cases were classified as CEA+ (93% specificity) (Tables 2 and 3 ).
Table 2. Summary of –RTPCR results for DDC/CEA expression and peritoneal wash in gastric cancer.
|
No. of positive cases in quantitative RT–PCR |
||||
|---|---|---|---|---|
| Depth of invasion | No. of patients | CEA | DDC | Cytology |
| Primary tumour | ||||
| t1 | 48 | 3 (3%) | 1 (2%) | 0 (0%) |
| t2 | 21 | 2 (9%) | 2 (9%) | 0 (0%) |
| t3 | 29 | 9 (31%) | 10 (34%) | 5 (17%) |
| t4 | 14 | 5 (36%) | 7 (50%) | 2 (15%) |
| Total | 112 | 19 (16%) | 20 (18%) | 14 (20%) |
| Benign disease | 15 | 0 (0%) | 0 (0%) | 0 (0%) |
classification: t1=mucosa to submucosa; t2=muscularis propria to subserosa; t3=serosa-exposed; t4=serosa-infiltrating.
Table 3. Clinicopathological features of cases with peritoneal recurrence of gastric cancer and quantitative results of DDC and CEA for peritoneal washes.
|
Markers |
|||||
|---|---|---|---|---|---|
| Case no. | Stagea | Histologyb | CEA | DDC | CEA and DDC |
| 1 | P1H0N2T3CY1 Stage IV | por | + | + | + |
| 2 | P0H0N2T3CY0 Stage IIIB | sig | − | + | + |
| 3 | P0H0N1T3CY1 Stage IV | por | + | + | + |
| 4 | P0H0N1T3CY0 Stage IIIA | tub | + | + | + |
| 5 | P1H0N2T3CY0 Stage IIIB | sig | + | + | + |
| 6 | P0H1N1T4CY1 Stage IV | sig | + | + | + |
| 7 | P0H1N3T3CY0 Stage IV | por | + | + | + |
| 8 | P1H1N2T3CY0 Stage IV | por | + | + | + |
| 9 | P0H0N2T3CY1 Stage IV | sig | + | + | + |
| 10 | P1H0N1T4CY1 Stage IV | por | − | − | − |
| 11 | P0H0N2T3CY0 Stage IIIB | muc | + | + | + |
| 12 | P0H0N3T3CY0 Stage IV | pap | + | + | + |
| 13 | P0H0N1T3CY0 Stage IIIA | sig | + | + | + |
| 14 | P0H0N2T3CY1 Stage IV | por | − | + | + |
| 15 | P0H0N2T3CY1 Stage IV | por | − | + | + |
| Sensitivity for the detection of MM | 73% (11/15) | 87% (13/15) | 94% (14/15) | ||
These cases were subsequently found to show recurrence of peritoneal carcinomatosa.
Clinical stage according to Japanese Gastric Cancer Classification.
Histology of the primary lesion according to Japanese Gastric Cancer Classification.por=poorly differentiated adenocarcinoma, sig=signet ring cell carcinoma, tub=tubular adenocarcinoma, muc=mucinous carcinoma, pap=papillary adenocarcinoma.
Although four subjects (cases 2, 11, 14 and 15 in Table 3) registered a CEA – a result of poorly differentiated adenocarcinoma, they showed evidence of peritoneal metastasis at an early stage. Three of these cases showed positive DDC mRNA/β-actin mRNA ratio results (Tables 2 and 3). As shown in Figure 2, DDC is frequently overexpressed in poorly differentiated adenocarcinomas, suggesting that DDC is viable as a novel marker for cases that are undetectable with CEA. As shown in Table 3, the combination of CEA and DDC improved the accuracy of diagnosis up to 94%.
DISCUSSION
Peritoneal dissemination is the most frequent pattern of gastric cancer recurrence and prognosis for gastric cancer patients with peritoneal dissemination is invariably poor. Some previous reports have indicated that intraperitoneal chemotherapy improves the survival of these patients, but it can sometimes be life-threatening because of side effects. We developed a novel technique to administer the anticancer agent mitomycin-C adsorbed on activated carbon particles (MMC-CH) (Hagiwara et al, 1992, 1993a, 1993b, 1999; Takahashi et al, 1995). This form of administration can deliver a large amount of the anticancer agent, because the corpuscular particles are not absorbed through the capillary wall. Instead, they are retained in the cavity, which remains closed for a long time and thus can maintain a high concentration of anticancer agents. We previously reported on this new form of drug administration and its therapeutic efficacy for peritonitis carcinomatosa of gastric cancers (Hagiwara et al, 1992). However, sometimes side effects occur such as ileus, fever, leukocytopenia and so on, so that it has been necessary to determine the indication for this therapeutic application to avoid such side effects.
The extreme sensitivity of RT–PCR makes it possible to diagnose micrometastases on the basis of tissue-specific mRNA expression of tumour cells in peripheral blood, bone marrow, lymph nodes and cerebrospinal fluid (Burchill et al, 1995; Johnson et al, 1995; Maehara et al, 1996; Noguchi et al, 1996; Mori et al, 1998). Recently, the use of rapid quantitative RT–PCR-based screening methods for the detection of micrometastasis from clinical specimens has become standard procedure (Nakanishi et al, 2000; Sato et al, 2001; Oki et al, 2002). Many different kinds of molecular markers for the detection of peritoneal micrometastases have been described, such as conventional RT–PCR assay of CEA, keratin 19 and AFP using the peritoneal wash from gastric cancers (Nakanishi et al, 1997; Schmidt et al, 2001). Other reports have dealt with novel markers for the detection of micrometastases. Yonemura et al (2001) increased the sensitivity of detection to 62% by using a combination of CY and RT–PCR of MMP-7 mRNA. Schuhmacher et al (1999) used RT–PCR to show the relationship between the expression of E-cadherin mutation and metastasis to the peritoneum. But any assay using peritoneal wash is inferior in sensitivity and specificity when compared to the real-time RT–PCR for CEA mRNA described by Nakanishi et al. CEA is recently a standard molecular marker for the detection of gastric cancer micrometastasis. However, it is not expressed in all cases of peritoneal metastases, and very weakly in mesothelial cells, so that it is difficult to exclude false-positive or false-negative cases. This means that markers with greater sensitivity and specificity are needed to reduce misdiagnosis. It is very important to choose specifically expressed genes, which are most useful as markers of peritoneal dissemination, in order to exclude false-positive or false-negative readings. In other words, genes with a much higher expression in cancer cells than in mesothelial cells should be chosen. One of the novel markers selected by DNA microarray is DDC, which satisfies the above conditions.
DDC is responsible for the synthesis of the key neurotransmitters dopamine and serotonin, and is frequently expressed in neuroblastoma and small-cell carcinoma of the lung (North and Du, 1998; Gilbert et al, 1999). It is used for the differential diagnosis of neuroblastoma from other paediatric small round cell malignancies (Gilbert et al, 2000). DDC expression is also considered to be a marker for neuroendocrine differentiation in lung cancer cell lines (Jensen et al, 1990). Finally, it is also known as L-amino acid decarboxylase, which catalyses the synthesis of biogenic amines involved in different important functions, such as angiogenesis, cell proliferation, apoptosis and cell proliferation (Berry et al, 1996; Medina et al, 1999), which suggests that it plays an important role in gastric cancer progression.
In conclusion, our results show that DDC-specific RT–PCR is more sensitive than conventional CY or CEA–RT–PCR of peritoneal wash, so that this method may well be useful for the prediction of peritoneal recurrence in gastric cancer patients. In view of the correlation established by our study between PCR results and cancer recurrence, the use of DDC RT–PCR to predict peritoneal recurrence of gastric cancer is likely to be effective. A large-scale, long-term follow-up study is currently underway in our department to ascertain the actual rate of peritoneal recurrence in CY− and PCR-positive patients and to determine whether negative patients in fact remain disease-free. DDC is overexpressed in gastric cancer peritoneal dissemination, but the role of DDC in such dissemination is still unclear, so that further investigation is necessary to clarify it.
Acknowledgments
This work was supported by a Grant-in-Aid for Cancer Research from the Ministry of Health and Welfare and from the Ministry of Education, Science and Culture, and by grants from the Uehara Memorial Foundation and Inamori Foundation, Japan.
References
- Abe S, Yoshimura H, Tabara H, Tachibana M, Monden N, Nakamura T, Nagaoka S (1995) Curative resection of gastric cancer: limitation of peritoneal lavage cytology in predicting the outcome. J Surg Oncol 59: 226–229 [DOI] [PubMed] [Google Scholar]
- Baba H, Korenaga D, Okamura T, Saito A, Sugimachi K (1989) Prognostic factors in gastric cancer with serosal invasion. Univariate and multivariate analyses. Arch Surg 124: 1061–1064 [DOI] [PubMed] [Google Scholar]
- Berry MD, Juorio AV, Li XM, Boulton AA (1996) Aromatic L-amino acid decarboxylase: a neglected and misunderstood enzyme. Neurochem Res 21: 1075–1087 [DOI] [PubMed] [Google Scholar]
- Bonenkamp JJ, Songun I, Hermans J, van de Velde CJ (1996) Prognostic value of positive cytology findings from abdominal washings in patients with gastric cancer. Br J Surg 83: 672–674 [DOI] [PubMed] [Google Scholar]
- Burchill SA, Bradbury MF, Pittman K, Southgate J, Smith B, Selby P (1995) Detection of epithelial cancer cells in peripheral blood by reverse transcriptase–polymerase chain reaction. Br J Cancer 71: 278–281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan EL, Whitaker NJ, Moy EL, Reddel RR (1993) Assignment of SV40-immortalized cells to more than one complementation group for immortalization. Exp Cell Res 205: 337–344 [DOI] [PubMed] [Google Scholar]
- Eldrup E, Clausen N, Scherling B, Schmiegelow K (2001) Evaluation of plasma 3,4-dihydroxyphenylacetic acid (DOPAC) and plasma 3,4-dihydroxyphenylalanine (DOPA) as tumor markers in children with neuroblastoma. Scand J Clin Lab Invest 61: 479–490 [DOI] [PubMed] [Google Scholar]
- Gilbert JA, Frederick LM, Ames MM (2000) The aromatic-L-amino acid decarboxylase inhibitor carbidopa is selectively cytotoxic to human pulmonary carcinoid and small cell lung carcinoma cells. Clin Cancer Res 6: 4365–4372 [PubMed] [Google Scholar]
- Gilbert J, Haber M, Bordow SB, Marshall GM, Norris MD (1999) Use of tumor-specific gene expression for the differential diagnosis of neuroblastoma from other pediatric small round-cell malignancies. Am J Pathol 155: 17–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagiwara A, Takahashi T, Kojima O, Kitamura K, Sakakura C, Shoubayashi S, Osaki K, Iwamoto A, Lee M, Fujita K (1993a) Endoscopic local injection of a new drug-delivery format of peplomycin for superficial esophageal cancer: a pilot study. Gastroenterology 104: 1037–1043 [DOI] [PubMed] [Google Scholar]
- Hagiwara A, Takahashi T, Kojima O, Sawai K, Yamaguchi T, Yamane T, Taniguchi H, Kitamura K, Noguchi A, Seiki K, Sokakura C (1992) Prophylaxis with carbon-adsorbed mitomycin against peritoneal recurrence of gastric cancer. Lancet 339: 629–631 [DOI] [PubMed] [Google Scholar]
- Hagiwara A, Takahahi T, Sawai K, Taniguchi H, Shimotsuma M, Okano S, Sakakura C, Tsujimoto H, Osaki K, Sasaki S, Shirasu M (1993b) Milky spots as the implantation site for malignant cells in peritoneal dissemination in mice. Cancer Res 53: 687–692 [PubMed] [Google Scholar]
- Hagiwara A, Togawa T, Yamasaki J, Ohgaki M, Imanishi T, Shirasu M, Sakakura C, Yamaguchi T, Sawai K, Yamagishi H (1999) Extensive gastrectomy and carbon-adsorbed mitomycin C for gastric cancer with peritoneal metastases. Case reports of survivors and their implications. Hepatogastroenterology 46: 1673–1677 [PubMed] [Google Scholar]
- Ikeda H, Suzuki N, Takahashi A, Kuroiwa M, Matsuyama S (1996) 3,4-dihydroxyphenylalanine (DOPA) metabolism in screening-detected and non-screening-detected neuroblastoma. Pediatr Hematol Oncol 13: 21–32 [DOI] [PubMed] [Google Scholar]
- Jensen SM, Gazdar AF, Cuttitta F, Russell EK, Linnoila RI (1990) A comparison of synaptophysin, chromogranin, and L-dopa decarboxylase as markers for neuroendocrine differentiation in lung cancer cell lines. Cancer Res 50: 6068–6074 [PubMed] [Google Scholar]
- Johnson PW, Burchill SA, Selby PJ (1995) The molecular detection of circulating tumour cells. Br J Cancer 72: 268–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodera Y, Yamamura Y, Torii A, Uesaka K, Hirai T, Yasui K, Morimoto T, Kato T, Kito T (1996) Postoperative staging of gastric carcinoma. A comparison between the UICC stage classification and the 12th edition of the Japanese General Rules for Gastric Cancer Study. Scand J Gastroenterol 31: 476–480 [DOI] [PubMed] [Google Scholar]
- Maehara Y, Yamamoto M, Oda S, Baba H, Kusumoto T, Ohno S, Ichiyoshi Y, Sugimachi K (1996) Cytokeratin-positive cells in bone marrow for identifying distant micrometastasis of gastric cancer. Br J Cancer 73: 83–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina MA, Quesada AR, Nunez de Castro I, Sanchez-Jimenz F (1999) Histamine, polyamines, and cancer. Biochem Pharmacol 57: 1341–1344 [DOI] [PubMed] [Google Scholar]
- Mori M, Mimori K, Ueo H, Tsuji K, Shiraishi T, Barnard GF, Sugimachi K, Akiyoshi T (1998) Clinical significance of molecular detection of carcinoma cells in lymph nodes and peripheral blood by reverse transcription–polymerase chain reaction in patients with gastrointestinal or breast carcinomas. J Clin Oncol 16: 128–132 [DOI] [PubMed] [Google Scholar]
- Nakabayashi K, Ogimo H, Michishita E, Satoh N, Ayusawa D (1999) Introduction of chromosome 7 suppresses telomerase with shortening of telomeres in a human mesothelial cell line. Exp Cell Res 252: 376–382 [DOI] [PubMed] [Google Scholar]
- Nakanishi H, Kodera Y, Torii A, Hirai T, Yamamura Y, Kato T, Kito T, Tatematsu M (1997) Detection of carcinoembryonic antigen-expressing free tumor cells in peritoneal washes from patients with gastric carcinoma by polymerase chain reaction. Jpn J Cancer Res 88: 687–692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakanishi H, Kodera Y, Yamamura Y, Ito S, Kato T, Ezaki T, Tatematsu M (2000) Rapid quantitative detection of carcinoembryonic antigen-expressing free tumor cells in the peritoneal cavity of gastric-cancer patients with real-time RT–PCR on the lightcycler. Int J Cancer 89: 411–417 [DOI] [PubMed] [Google Scholar]
- Noguchi S, Hiratsuka M, Furukawa H, Aihara T, Kasugai T, Tamura S, Imaoka S, Koyama H, Iwanaga T (1996) Detection of gastric cancer micrometastases in lymph nodes by amplification of keratin 19 mRNA with reverse transcriptase–polymerase chain reaction. Jpn J Cancer Res 87: 650–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- North WG, Du J (1998) Key peptide processing enzymes are expressed by a variant form of small-cell carcinoma of the lung. Peptides 19: 1743–1747 [DOI] [PubMed] [Google Scholar]
- Oki E, Maehara Y, Tokunaga E, Schibahara K, Hasuda S, Kakeji Y, Sugimachi K (2002) Detection of disseminated cancer cells in bone marrow of gastric cancer using real time quantitative reverse transcriptase chain reaction. Cancer Lett 188: 191–198 [DOI] [PubMed] [Google Scholar]
- Sakakura C, Hagiwara A, Nakanishi M, Shimomura K, Takagi T, Yasuoka R, Fujita Y, Abe T, Ichikawa Y, Takahashi S, Ishikawa T, Nishizuka I, Morita T, Shimada H, Okazaki Y, Hayashizaki Y, Yamagishi H (2002) Differential gene expression profiles of gastric cancer cells established from primary tumour and malignant ascites. Br J Cancer 87: 1153–1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakakura C, Sweeney EA, Shirahama T, Igarashi Y, Hakomori S, Nakatani H, Tsujimoto H, Imanishi T, Ohgaki M, Ohyama T, Yamazaki J, Hagiwara A, Yamaguchi T, Sawai K, Takahashi T (1996) Overexpression of bax sensitizes human breast cancer MCF-7 cells to radiation-induced apoptosis. Int J Cancer 67: 101–105 [DOI] [PubMed] [Google Scholar]
- Sakakura C, Yamaguchi-Iwai Y, Satake M, Bae SC, Takahashi A, Ogawa E, Hagiwara A, Takahashi T, Murakami A, Makino K, Nakagawa T, Kamada N, Ito Y (1994) Growth inhibition and induction of differentiation of t(8;21) acute myeloid leukemia cells by the DNA-binding domain of PEBP2 and the AML1/MTG8(ETO)-specific antisense oligonucleotide. Proc Natl Acad Sci USA 91: 11723–11727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato F, Shimada Y, Li Z, Watanabe G, Maeda M, Imamura M (2001) Lymph node micrometastasis and prognosis in patients with oesophageal squamous cell carcinoma. Br J Surg 88: 426–432 [DOI] [PubMed] [Google Scholar]
- Schmidt P, Thiele M, Rudroff C, Vaz A, Schilli M, Friedrich K, Scheele J (2001) Detection of tumor cells in peritoneal lavages from patients with gastrointestinal cancer by multiplex reverse transcriptase PCR. Hepatogastroenterology 48: 1675–1679 [PubMed] [Google Scholar]
- Schuhmacher C, Becker KF, Reich U, Schenk U, Mueller J, Siewert JR, Hofler H (1999) Rapid detection of mutated E-cadherin in peritoneal lavage specimens from patients with diffuse-type gastric carcinoma. Diagn Mol Pathol 8: 66–70 [DOI] [PubMed] [Google Scholar]
- Takahashi T, Hagiwar A, Shimotsuma M, Sawai K, Yamaguchi T (1995) Prophylaxis and treatment of peritoneal carcinomatosis: intraperitoneal chemotherapy with mitomycin C bound to activated carbon particles. World J Surg 19: 565–569 [DOI] [PubMed] [Google Scholar]
- Yamazaki H, Oshima A, Murakami R, Endoh S, Ubukata T (1989) A long-term follow-up study of patients with gastric cancer detected by mass screening. Cancer 63: 613–617 [DOI] [PubMed] [Google Scholar]
- Yonemura Y, Fujimura T, Ninomiya I, Kim S-B, Bandou E, Sawa T, Kinoshita K, Endo Y, Sugiyama K, Sasaki T (2001) Prediction of peritoneal micrometastasis by peritoneal lavaged cytology and reverse transcriptase polymerase chain reaction by matrix metalloproteinase-7 mRNA. Clin Cancer Res 7: 1647–1653 [PubMed] [Google Scholar]