Abstract
In an earlier study, we have demonstrated a high clinical and pathologic response rate of neoadjuvant paclitaxel (P) and cisplatin (C) for patients with locally advanced breast cancer (LABC). The current phase II study includes larger number of patients who had longer follow-up. A total of 126 consecutive patients with noninflammatory LABC (T2 >4 cm, T3 or T4, N0–N3, M0) were included in the study. Patients were scheduled to receive three to four cycles of the neoadjuvant PC (paclitaxel 135 mg m−2 and cisplatin 75 mg m−2 on day 1) every 21 days. Patients were then subjected to surgery and subsequently received six cycles of FAC (5-fluorouracil 500 mg m−2, doxorubicin 50 mg m−2, and cyclophosphamide 500 mg m−2) or four cycles of AC (doxorubicin 60 mg m−2 and cyclophosphamide 600 mg m−2); all drugs were administered intravenously on day 1 with cycles repeated every 21 days. Patients then received radiation therapy, and those with hormone receptor-positive tumours were given adjuvant tamoxifen intended for 5 years. The median age was 41 years. Clinically, 12, 52, and 37% of patients had T2 >4 cm, T3, and T4, respectively. The mean tumour size was 7 cm (95% CI, 7.3–8.5). The clinical nodal status was N0, N1, and N2–N3 in 32, 52, and 17% of patients, respectively. Disease stage at diagnosis was IIA (2%), IIB (32%), IIIA (28%), and IIIB (39%). Clinical assessment of the primary tumour and the axillary nodal status after primary chemotherapy showed that 35 patients (28%) achieved complete response (cCR), while 80 (63%) demonstrated partial response to PC. Of patients with evaluable pathologic data of the primary tumour (123 patients), complete pathologic response (pCR) was achieved in 29 patients (24%), and an additional nine (7%) only had a microinvasive disease. Moreover, 20 of the 122 patients (16%) had no residual disease in the primary tumour or in the axillary nodes. Failure to attain cCR predicted failure to achieve pCR. At a median follow-up of 37.5 months (95% CI, 31.5–43.3), 71% were alive with no recurrence, 16% were alive with evidence of disease, and 13% were dead. Of the 122 patients who had surgery, 36 (29%) developed recurrence including one of the patients who attained pCR. The median overall or disease-free survival has not been reached with a projected 5-year overall survival (OS) and disease-free survival (DFS) of 85% (±4%) and 63% (±5%), respectively. On multivariate analysis, clinical response of the primary tumour, pathological response of the primary tumour, and the pathological nodal status were identified as independent prognostic variables for DFS. No variable, however, was identified to prognosticate OS. PC was acceptably safe. Neoadjuvant PC as used in this phase II study in a multidisciplinary strategy was highly effective. Clinical and pathologic responses remain the most important variables that predict outcome.
Keywords: breast cancer, locally advanced, neoadjuvant, chemotherapy, paclitaxel, cisplatin
Neoadjuvant chemotherapy has been used as the initial treatment in locally advanced breast cancer (LABC). The Milan Group, using a combination of doxorubicin and vincristine, achieved an 80% response rate, with 15% of patients attaining complete clinical response (De Lena et al, 1981). Other groups have achieved comparable results (Hortobagyi et al, 1988; Kuerer et al, 1999). With this approach, 5-year survival rates of approximately 40% have been reported (Valagussa et al, 1990), while significantly higher survival rates have been reported by others (Hortobagyi et al, 1988; Smith and al-Moundhri, 1998; Kuerer et al, 1999). Likewise, primary chemotherapy has been used successfully for large operable breast cancer in nonrandomised and randomised trials (Anderson et al, 1991; Smith et al, 1993; Scholl et al, 1994; Powles et al, 1995; Smith et al, 1995; Bonadonna et al, 1998; Fisher et al, 1998; Von Minckwitz et al, 1999). Moreover, the National Surgical Adjuvant Breast and Bowel Project (NSABB) B-18, one of the largest reported studies including 1523 patients, has clearly shown that at 9 years of follow-up, the outcome of patients who received primary chemotherapy was equal to that for patients treated with adjuvant chemotherapy (Wolmark et al, 2001). However, a marginally statistically significant survival advantage from neoadjuvant chemotherapy was noted for younger patients, whereas the reverse may be true for older patients.
We have demonstrated the significant efficacy of the combination of paclitaxel and cisplatin (PC) in the management of patients with metastatic breast cancer disease where an objective response of 80% was achieved (Ezzat et al, 1997). The results of this phase II study were in concordance with the reported efficacy of paclitaxel and cisplatin used in various settings in the management of breast cancer (Hortobagyi, 1997; Comella et al, 1998; Henderson et al, 1998; Kourousis et al, 1998; Perez et al, 1998; Volm et al, 1998). Furthermore, both drugs have demonstrated potency on anthracycline-resistant tumours (Lau et al, 1998, Ray-Coquard et al, 1998). The lack of apparent crossresistance between paclitaxel and cisplatin, the minimal overlapping toxicity, and the potential angiogenesis antagonistic effect of paclitaxel that appears to be independent of its antiproliferative action, make this combination very appealing for the management of LABC.
In an earlier phase II report that included 72 patients with LABC, we have demonstrated that the PC regimen was very effective and reasonably tolerant (Ezzat et al, 2000). Complete and partial clinical response to PC was demonstrated in 18 and 72% patients, respectively, for an overall response of 90% (95% confidence interval (CI), 79–100%). Moreover, complete pathologic response (pCR) and partial pathologic response (pPR) was achieved in 15 (22%) and 51 (75%) of patients, respectively, for an overall response of 97% (95% CI, 95–99%).
The current series update the outcome of 126 patients at a median follow-up of approximately 4 years. The primary end point was to assess the effect of that regimen on clinical and pathological response, while the secondary end point was to analyse the effect on survival, and to identify predictors of response and prognostic factors of survival.
PATIENTS AND METHODS
Between May 1995 and December 2000, consecutive patients seen at our institution with locally advanced noninflammatory breast cancer (T2>4 cm, T3 or T4, N0–N2, M0) confirmed on fine needle aspirate (FNA) and/or tru-cut biopsy were considered eligible. Those with bilateral breast cancer, or documented evidence of metastatic disease, including supraclavicular lymph node involvement, were excluded. Also excluded were patients with a history of renal, hepatic or heart failure, history of malignancy except basal cell carcinoma of the skin or cervical carcinoma in situ, or history of psychiatric disorder that might interfere with the administration of protocol drugs or follow-up. The study intended to exclude patients who developed breast cancer during pregnancy or those who became pregnant during therapy; however, those events were not encountered. Patients who experienced any grade 4 toxicity, grade 3 or 4 neurotoxicity, those who requested withdrawal from the study, and those who demonstrated tumour progression after at least two cycles of the neoadjuvant regimen were removed from the study, but were included in the analysis.
Staging procedures included complete history and physical examination, laboratory studies, bilateral mammography, chest and abdominal computerised tomography, and radionuclide bone scan. Hormone receptor assay was available for most patients.
Clinical sizes of primary breast cancers and axillary nodes, if the latter were palpable, were determined separately before the administration of each cycle of PC and also before surgery. At each assessment, the product of the two greatest perpendicular diameters of the tumours in the breast and axilla was measured.
The study was approved by King Faisal Specialist Hospital and Research Center's Research Advisory and Ethics Committees, Riyadh. All patients gave a written informed consent.
Therapy
Patients received three to four cycles of the neoadjuvant PC therapy. Paclitaxel 135 mg m−2 on day 1 at 0600 hours as 3-h infusion with the standard premedication (dexamethasone, anti-histamine, and H2 antagonist) was initiated the night before therapy. Cisplatin was administered as 75 mg m−2 on day 1 at 1800 hours as a 1-h infusion with standard pre- and posthydration. The timing of drug administration was chosen in conformity with the circadian rhythm concept in the first 42 patients (Harmanek and Sobin, 1987). Cycles were repeated at 3-week intervals.
Following the neoadjuvant regimen, response was assessed clinically and mammographically. Within 4 weeks after the last cycle, patients were scheduled to undergo conservative surgery or modified radical mastectomy upon the discretion of the surgeon guided by the clinical response. Axillary lymph node dissection to levels I and II was carried out aiming for excision of at least 10 lymph nodes.
Within 2–3 weeks following surgery, patients received six cycles of FAC (5-fluorouracil 500 mg m−2, doxorubicin 50 mg m−2, and cyclophosphamide 500 mg m−2) or four cycles of AC (doxorubicin 60 mg m−2 and cyclophosphamide 600 mg m−2); all drugs were administered intravenously on day 1 with cycles repeated at 3-week intervals. The choice between FAC 6 × vs AC 4 × was made based on the primary oncologist's discretion. In recent years, some patients who demonstrated pCR were given two additional cycles of PC. Any grade 1 toxicity was treated symptomatically and therapy was allowed to continue. However, grade 3 myelotoxicity required a delay in chemotherapy until bone marrow recovery, with granulocyte colony-stimulating factor (G-CSF) support on subsequent cycles.
Following adjuvant chemotherapy, patients received radiation therapy to the chest wall and axilla or to the breast and the axilla, but the supraclavicular fossa and internal mammary chains were not routinely irradiated. Adjuvant tamoxifen 20 mg orally daily intended for 5 years was given to patients shown to have oestrogen receptor (ER)- and/or progesterone receptor (PR)-positive tumour.
Data capture
A computerised database was created to capture prospectively the following information: patients’ demographic and clinical data, laboratory and radiologic studies, disease characteristics, and neoadjuvant therapy details including clinical response and toxicity. The database also included surgery details, axillary lymph node dissection, and pathologic data including response. Also captured were postsurgery further chemotherapy, hormonal therapy, radiation therapy, recurrence, and survival status.
Definitions
Staging was defined according to the criteria determined by the International Union Against Cancer (UICC) (Harmanek and Sobin, 1987) with group clinical and pathological staging according to the American Joint Committee on Cancer (AJCC). We adopted the criteria for LABC reported by Haagensen and Stout (1943).
After preoperative chemotherapy was administered, breast tumours were classified according to clinical response. In the absence of clinical evidence of tumour in the breast without any new lesions occurring, the response to therapy was categorised as a clinically complete response (cCR). When the reduction in the sum of the products of the largest perpendicular diameters of the breast tumour was 50% or greater without the development of newer lesions prior to surgery, the response was judged to be partial (cPR). Clinical progressive disease (cPD) was defined as any increase greater than 25% of the sum of the products of the largest perpendicular diameters of any measurable lesions, or unequivocal appearance of new lesions after a minimum of two cycles of PC. Patients whose breast tumour response did not meet the definition of cCR, cPR, or cPD were considered to have stable disease (cSD). Thus, patients with cSD could have a tumour response of less than 50% or an increase in tumour size of <25%. This classification was also used to record the response of an axillary tumour to the neoadjuvant regimen in patients who had clinically positive nodes at diagnosis. The overall clinical response of both breast and axillary tumours was determined by combining the sum of the product of the tumour measurements in both the breast and axilla. The development of a clinically suspicious ipsilateral axillary tumour during chemotherapy was considered as evidence of cPD in patients whose axilla was clinically negative at diagnosis.
A median of 15 sections of the mastectomy or lumpectomy specimen was performed; these included sections from each quadrant, from the nipple–areola complex (if appropriate), from areas of suspicious or prior tumour involvement, and from the axillary contents (a median of seven sections). Pathologic response to neoadjuvant chemotherapy was assessed according to the criteria modified from that used by Feldman et al (1986). Complete pathological response of the primary (pCR) was defined as the absence of any macroscopic or microscopic evidence of invasive tumour in the surgical specimen. The same definition was applied to the axillary nodes. Partial pathological response was defined as 50% or greater reduction in the sum of products of the largest perpendicular diameters of residual lesions as compared with the size of the primary tumour regardless of the nodal status; pathologic stable disease (pSD) was defined as less than 50% reduction in the sum of products of largest perpendicular diameters of the residual disease as compared with the primary tumour size regardless of the nodal status; and pathological progressive disease (pPD) was defined as any increase greater than 25% of the sum of the products of largest perpendicular diameters of residual lesions as compared with the size of primary tumour regardless of the nodal status.
Overall survival (OS) was estimated from date of diagnosis to date of last follow-up or death from any cause. Disease-free survival (DFS) was calculated from the date of definitive surgery until last contact; recurrence ‘local, regional, or distant’; occurrence of contralateral breast cancer; occurrence of second primary cancer other than in the contralateral breast; or death without evidence of breast or second primary cancer.
Statistical methods
A two-sided Wilcoxon–Pratt test was used to compare tumour sizes before and after PC. Comparison of means and medians of continuous variables was performed using t-test statistics and nonparametric methods, respectively. Patient- and disease-related variables were first examined for their relation to response, while variables with a P-value of <0.10 were included in a stepwise multivariate logistic regression analysis to identify the independent predictors for pCR response (Cox and Snell, 1989). Age was entered both as continuous and as dichotomous values by using different cutoffs. The goodness-of-fit χ2, the Hosmer goodness-of-fit, and the Brown goodness-of-fit tests were used to test the hypotheses that the model at each step fits the data, that the predicted values fit the data, and that the logistic model is the best fitting model, respectively (Prentice, 1976; Hosmer and Lemeshow, 1980). Kappa's test of reliability was used to compare the agreement between clinical and pathologic response (Fleiss, 1981). Survival was estimated applying the method of Kaplan and Meier (1958), while the statistical procedure of Brookmeyer–Crowley was used to estimate the 95% confidence interval (CI) of median survival (Brookmeyer and Crowley, 1982). The log-rank test was used to assess the significance of unadjusted differences in survival (Mantel, 1966).
Exploring variables for their independent prognostic effect on survival was carried out using the multivariate stepwise Cox's proportional regression hazard model (Cox and Oakes, 1997). The relative importance of prognostic factors was measured by the chi-square values, based on the Wald test of the coefficient associated with each prognostic factor in the Cox model (Marubini and Valsecchi, 1995). Factors with larger chi-square values were more significant in each model. The chi-square value in the ranking of prognostic factors was used because its interpretation is unrelated to the coding of the covariate. The interpretation of the hazard ratio depends on the units or coding of the covariate.
The predictor with the highest level of statistical significance was used to introduce the model; other variables were then evaluated for further predictive information and added in turn, beginning with the variables with the highest level of statistical significance (i.e. the lowest P-values) and continuing until the P-value for the variable added exceeded 0.05. Continuous prognostic variables were also considered for inclusion in the model as dichotomous variables using various cutoff points only if they attained a P-value of ⩽0.1 in the univariate analysis. On the other hand, discrete variables with more than two categories were analysed by means of categories or indicator variables. Cases with unknown factors were excluded in the initial Cox regression analysis. If a factor did not predict for the event of interest, the cases with unknown values for that factor were again added, and the Cox regression was repeated with that particular factor dropped. Interactions between variables were not explored. In the derived model, plotting the log-minus-log survival function tested the proportionality assumption (Kalbfleisch and Prentice, 1980), and the goodness of fit was judged by plotting the cumulative baseline hazard function for residuals (Kay, 1977). We also compared the survival functions for variables after stratifying for baseline differences in additional variables (Kalbfleisch and Prentice, 1980). All reported P-values were two-tailed. We performed all data analyses using SAS System for Windows, release 8.02 and SPSS release 10.0.1 for Windows.
RESULTS
According to inclusion criteria, 126 patients were accrued and all were evaluable for response and toxicity analysis. Diagnosis was made by core biopsy or FNA in 89 (71%) and 37 (29%) of patients, respectively. Their median age was 41 years (95% CI, 40.1–43.7). Patient and disease characteristics are depicted in Table 1 . Most patients were premenopausal (79%) and approximately more than two-thirds (67%) had stage IIIA or IIIB.
Table 1. Patient and disease characteristics.
| Variables | No. (%) |
|---|---|
| Year of diagnosis | |
| 1995–1997 | 43 (34) |
| 1998–2000 | 83 (76) |
| Menopausal status | |
| Premenopausal | 100 (79) |
| Postmenopausal | 26 (21) |
| Age (years) | |
| <50 | 103 (82) |
| ⩾50 | 23 (18) |
| Primary tumour | |
| T2 | 15 (12) |
| T3 | 64 (51) |
| T4 | 47 (37) |
| Clinical nodal status | |
| N0 | 40 (32) |
| N1 | 65 (52) |
| N2–N3 | 21 (17) |
| Clinical nodal size | |
| Negative | 40 (32) |
| <2.5 cm | 60 (48) |
| More than 2.5 cm | 26 (20) |
| Stage | |
| IIA | 2 (2) |
| IIB | 40 (32) |
| IIIA | 35 (28) |
| IIIB | 49 (39) |
| Grade | |
| G1 | 4 (3) |
| G2 | 55 (44) |
| G3 | 51 (40) |
| Unknown | 16 (13) |
| Vascular invasion | |
| Negative | 83 (66) |
| Positive | 24 (19) |
| Unknown | 19 (15) |
| Oestrogen receptor | |
| Positive | 53 (42) |
| Negative | 43 (34) |
| Unknown | 30 (24) |
| Progesterone receptor | |
| Positive | 47 (37) |
| Negative | 49 (39) |
| Unknown | 30 (24) |
Neoadjuvant treatment
The median number of cycles of neoadjuvant chemotherapy was 3 (range, 5). Five patients received two additional cycles of doxorubicin and cyclophosphamide (AC) preoperatively after inadequate response to TP.
Clinical response
The mean primary baseline tumour size was 7 cm (95% CI, 7.3–8.5), while the mean size following chemotherapy was 2.2 cm (95% CI, 2.5–3.8). This difference was highly significant (P<0.0001). Clinical assessment of the primary tumour and the axillary nodal status after primary chemotherapy showed that 35 patients (28%) achieved cCR, while 80 (63%) demonstrated cPR to the primary chemotherapy. The remaining 11 patients (9%) had cSD (nine patients) and cPD (two patients). Of the five patients who achieved inadequate response to initial TP, four demonstrated cPR after the addition of AC.
Surgery
Modified radical mastectomy was performed in 86 patients (68%), while 36 (29%) had breast-conserving surgery. One patient had simple mastectomy and, in three patients, surgery was not performed due to disease progression and/or patient refusal. Axillary nodal dissection was performed in all except four patients. Of all patients with residual primary tumour undergoing surgery, resection margins were close or focally involved in 14 patients.
Pathological response
Pathological status of the primary tumour was known in 123 patients, while the axillary nodal status was pathologically known in 122 patients. The median number of dissected and positive axillary lymph nodes was 14 (range, 40) and 1 (range, 19), respectively. Table 2 shows that of the 123 patients, 29 patients (24%) had no invasive tumour (eight had noninvasive intraductal carcinoma in situ). Additionally, in nine patients (7%) only a microinvasive cancer was found and of these, five had negative axillary nodes. The table also shows that among those who had undergone surgery and had their axillary status known, the complete pathological response, where there were no invasive primary tumour and no axillary involvement, was 16% (20 of 122 patients). Additionally, five patients had negative axillary nodes and only a microinvasive cancer. The table also shows that patients with a pCR of the primary tumour were more likely to have a negative axillary lymph node status (20 of 29; 69% vs 22 of 93; 24%, P<0.0001).
Table 2. Pathological data of the 123 patients who underwent surgery.
|
No. of patients: pathological nodal status |
|||||
|---|---|---|---|---|---|
| Pathology of the primary tumour | N0 | N1–3 | N4–9 | ⩾N10 | Unknown |
| T0 | 20 | 8 | 1 | ||
| T1A | 5 | 4 | |||
| T1B | 3 | 4 | 2 | 2 | |
| T1C | 6 | 7 | 4 | 3 | |
| T2 | 6 | 12 | 15 | 9 | |
| T3 | 2 | 2 | 5 | 2 | 1 |
| Total | 42 | 37 | 27 | 16 | 1 |
Of the 37 patients who were initially diagnosed with FNA only, only two had no invasive primary tumour and no axillary involvement; however, during primary chemotherapy one demonstrated cCR and another cPR. The remaining patients were found to have either residual invasive disease (nine patients), axillary lymph node involvement (13 patients), or residual invasive tumour and axillary disease (13 patients).
Analysis of the clinical and pathological response according to the tumour receptor status was also carried out. Of the 96 patients with known ER, cCR was achieved in 10/51 (20%) ER− patients vs 17/45 (38%) in ER+ patients (P=0.048). Similarly, pCR was attained in 1/51 (2%) and in 13/45 (29%), respectively (P<0.0001). On the other hand, while PR status was not related to cCR, it was significantly associated with pCR (1/46 (2%) in PR− patients vs 13/50 (26%) in PR+ patients, P=0.0003). On the other hand, the stepwise multivariate logistic regression showed that failing to attain cCR was the only independent factor that predicted no pCR (odds ratio=3.02; 95% CI 1.65–5.52; P-value <0.0001).
Agreement between cCR and pCR of the primary tumour was evaluated. It was shown that concordance was demonstrated in 90 of the 123 patients (73%) with known pathologic data of the primary tumour. The κ-test was 0.313, which only suggests a moderate agreement between clinical and pathologic (Brookmeyer and Crowley, 1982).
Adjuvant therapy
Following surgery, adjuvant chemotherapy was offered to 117 patients. In all, 60 patients received FAC, 50 received AC, and seven received TP. The median number of cycles given was 4. Following adjuvant chemotherapy, radiotherapy was derived to 120 patients (95%) and adjuvant tamoxifen intended for 5 years was offered to 65 patients (52%) following radiotherapy.
Overall survival
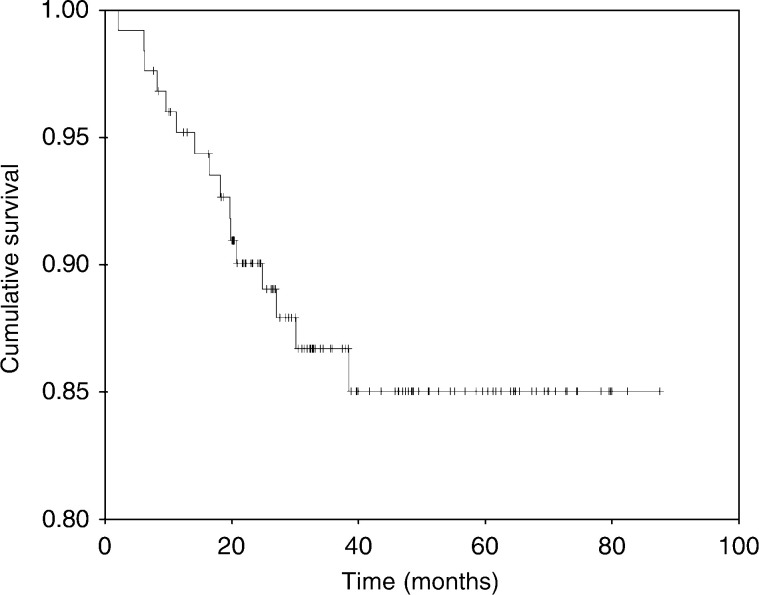
At a median follow-up of 37.5 months (95% CI, 31.5–43.3), 90 patients (71%) were alive and disease-free, 20 (16%) were alive with disease, and the remaining 16 (13%) were dead. The median OS was not reached; however, the projected 5-year OS (+SD) was 85+4% (Figure 1).
Figure 1.
Overall survival.
Relapse and DFS
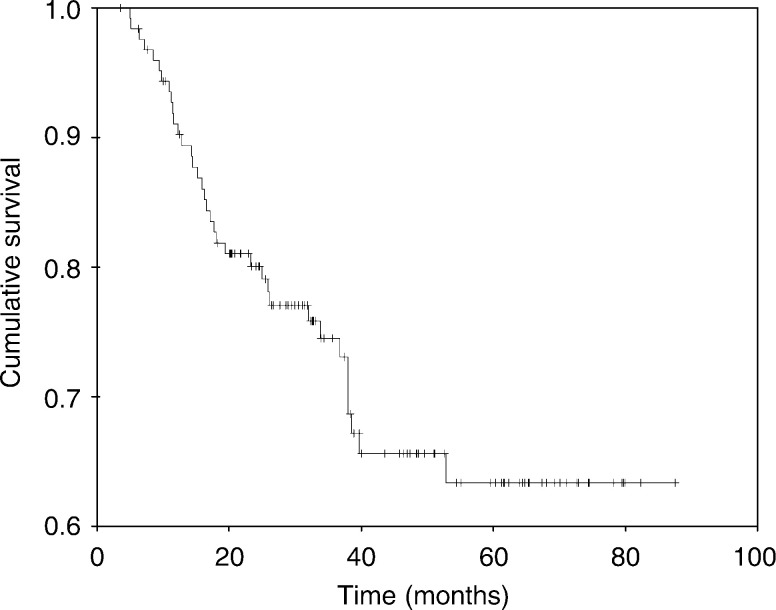
Of all 123 patients who had surgery, 35 (29%) experienced relapse. Distant relapse only or locoregional relapse only was demonstrated in 19 (15%), and six (5%) patients, respectively. Ten patients (8%) had distant relapse concurrently with local or regional relapse. Two events of contralateral breast recurrence were experienced, one in association with local and distant relapse and the other as a lone event. Of patients with close or focally involved margins (15 patients), six did not demonstrate relapse, while four, three, and two showed local or locoregional relapse, locoregional and distant relapse, and distant relapse only, respectively. The median DFS was not reached; nevertheless, the projected 5-year DFS (+SD) was 63+5% (Figure 2).
Figure 2.
Disease-free survival.
Univariate and multivariate analyses of prognostic factors of OS and DFS
Table 3 shows the univariate analysis of prognostic factors of OS and DFS. For OS, the following variables were statistically significant: clinical primary tumour stage, nodal status at baseline, clinical baseline stage, postneoadjuvant chemotherapy clinical primary tumour stage, pathological primary tumour, pathological nodal status, pathological stage, and adjuvant radiotherapy. On the other hand, the variables that were significant prognostically for DFS were: clinical primary tumour stage, clinical baseline stage, postneoadjuvant chemotherapy clinical primary tumour stage, pathological primary tumour, pathological nodal status, pathological stage, and adjuvant radiotherapy.
Table 3. Univariate analyses of the effect of variables on overall survival (OS) and disease-free survival (DFS).
| OS | DFS | |||
|---|---|---|---|---|
| Variable | No. (% survival) | P-value | % DFS | P-value |
| Age (years) | 0.36 | 0.22 | ||
| <50 | 103 (88) | 73 | ||
| >50 | 23 (83) | 55 | ||
| Menopausal status | 0.52 | 0.004 | ||
| Premenopausal | 100 (88) | |||
| Postmenopausal | 26 (85) | |||
| Clinical primary tumour (baseline) | 0.01 | 0.14 | ||
| T2 | 15 (93) | 80 | ||
| T3 | 64 (94) | 80 | ||
| T4 | 47 (77) | 57 | ||
| Clinical nodal status (baseline) | 0.01 | 0.02 | ||
| N0 | 40 (90) | 78 | ||
| N1 | 65 (92) | 72 | ||
| N2+N3 | 21 (67) | 57 | ||
| Clinical stage (baseline) | 0.023 | 0.41 | ||
| II | 42 (92) | 79 | ||
| IIIA | 35 (94) | 80 | ||
| IIIB | 49 (78) | 59 | ||
| Tumour grade (known in 110 patients) | 0.43 | 0.20 | ||
| G1+G2 | 59 (88) | 73 | ||
| G3 | 51 (82) | 63 | ||
| Oestrogen receptor | 0.35 | 0.84 | ||
| Positive | 53 (87) | 64 | ||
| Negative | 43 (93) | 81 | ||
| Unknown | 30 (80) | 70 | ||
| Progesterone receptor | 0.53 | 0.07 | ||
| Positive | 47 (89) | 74 | ||
| Negative | 49 (90) | 69 | ||
| Unknown | 30 (80) | 70 | ||
| Diagnosis to primary chemotherapy (days) | 0.57 | 0.04 | ||
| <21 | 94 (86) | 76 | ||
| >21 | 32 (90) | 59 | ||
| Clinical primary tumour (postneoadjuvant chemotherapy) | 0.004 | 0.64 | ||
| T0 | 37 (84) | 70 | ||
| T1 | 25 (100) | 80 | ||
| T2 | 40 (93) | 77 | ||
| T3+T4 | 24 (71) | 54 | ||
| Clinical nodal status (postadjuvant chemotherapy) | 0.28 | 0.41 | ||
| N0 | 97 (89) | 72 | ||
| N1+N2 | 29 (83) | 69 | ||
| Clinical response | 0.72 | |||
| cCR | 35 (89) | 77 | ||
| <cCR | 91 (87) | 69 | ||
| Tumour pathologic response (123 patients) | 0.026 | 0.0002 | ||
| T0 | 29 (97) | 93 | ||
| T1 | 40 (93) | 80 | ||
| T2 | 43 (84) | 56 | ||
| T3 | 11 (73) | 55 | ||
| Primary tumour pathologic response (123 patients) | 0.09 | 0.002 | ||
| pCR | 29 (97) | 93 | ||
| <pCR | 94 (86) | 66 | ||
| Pathological nodal status (122 patients) | 0.08 | 0.0001 | ||
| Negative | 42 (95) | 93 | ||
| Positive | 80 (86) | 62 | ||
| Pathological nodal status (122 patients) | 0.047 | <0.0001 | ||
| Negative | 42 (95) | 93 | ||
| 1–3 positive nodes | 37 (92) | 73 | ||
| 4–9 positive nodes | 27 (85) | 59 | ||
| More than 9 positive nodes | 16 (75) | 44 | ||
| Pathologic stage (postneoadjuvant chemotherapy) | 0.017 | 0.0006 | ||
| T0N0 | 20 (95) | 95 | ||
| I | 13 (100) | 100 | ||
| II (A+B) | 81 (86) | 64 | ||
| III (A+B) | 12 (67) | 50 | ||
| Adjuvant radiotherapy | 0.029 | 0.01 | ||
| Yes | 120 (88) | 73 | ||
| None | 6 (67) | 33 | ||
| Adjuvant tamoxifen (receptor-positive patients only, 57) | 0.46 | 0.51 | ||
| Yes (46 patients) | 42 (91) | 70 | ||
| No (11 patients) | 9 (82) | 63 | ||
The multivariate stepwise Cox's proportional regression hazard model failed to identify independent prognostic factors for OS. On the other hand, clinical response of the primary tumour (T0 vs >T0; odd ratio=0.60 (95% CI 0.39–0.94)), pathological response of the primary tumour (T0 vs >T0; odd ratio=0.39 (95% CI 0.25–059)), and the pathological nodal status (negative vs positive; odd ratio=0.18 (95% CI 0.05–0.60) were identified as independent prognostic variables for DFS.
Toxicity
The combination of PC was well tolerated with only rare events of grade III or IV toxicity. Hypersensitivity reaction to paclitaxel was reported in three patients and was severe in one with hypotension, bronchospasm, and seizure. After recovery, paclitaxel was not resumed. Myelosuppression was minimal; grade III neutropenia and anaemia developed in 11 and 2% with no grade III thrombocytopenia. No infection was observed concomitantly to grade III neutropenia. No treatment-related death occurred during chemotherapy. Nausea and vomiting were reported in 8%. Alopecia was universal. Other grade I–II toxicities included reversible renal impairment, electrolytes disturbance, ototoxicity, disorders, peripheral neuropathy, and extravasations. Dizziness, fatigue, and myalgia were commonly reported. Three patients had their third cycle of chemotherapy delayed by at least 1 week and all cycles but one were given at full dose.
DISCUSSION
Patients with LABC do very poorly when treated by locoregional therapy alone, with less than 10–20% of these patients surviving 5 years. Such therapy favourably affects locoregional control, but most relapses are due to the development of distant metastases (Zucali et al, 1976; Bruckman et al, 1979). Recently, however, neoadjuvant chemotherapy regimens have been shown to have a favourable influence on the outcome of patients with LABC (De Lena et al, 1981; Hortobagyi et al, 1988; Valagussa et al, 1990; Anderson et al, 1991; Smith et al, 1993; Scholl et al, 1994; Powles et al, 1995; Smith et al, 1995; Bonadonna et al, 1998; Fisher et al, 1998; Kuerer et al, 1999; Von Minckwitz et al, 1999; Wolmark et al, 2001).
Complete and partial clinical response to PC was demonstrated in 28 and 63% of patients, respectively. Moreover, the primary tumour showed no invasive component in 24% of patients and only microinvasive disease in an additional 7%; besides, among patients who had surgery and axillary dissection, 16% patients had no microscopic evidence of invasive cancer in their breast and axillary specimens. In the latter group, only one patient subsequently developed recurrence. While it may be possible that the two patients who had no pathological evidence of disease post neoadjuvant chemotherapy and were originally diagnosed using FNA only might have had in situ disease, it is rather unlikely considering the demonstrated clinical response during induction therapy.
The 16% rate of complete eradication of invasive disease is comparable to the 12% rate achieved using four cycles of FAC preoperatively in 372 patients in the MD Anderson study (Kuerer et al, 1999). Moreover, similar to the findings in the latter series, our data also showed that a pathologic complete primary tumour response was predictive of a complete axillary lymph node response (P<0.0001).
Considering that adverse prognostic features prevailed in patients in the current series, which includes a median tumour size of 7 cm, the current data provide further support to our earlier conclusion about the efficacy of primary PC for patients with LABC (Ezzat et al, 2000). At a median follow-up of 37 months, 71% were alive and disease-free, 16% were alive with disease, and the remaining 13% were dead. The projected 5-year OS and DFS was 85 and 63%, respectively. These survival data are comparable to those results reported in other series that included patients with more favourable prognostic characteristics (Bonadonna et al, 1998; Fisher et al, 1998; Kuerer et al, 1999; Wolmark et al, 2001). As a large number of patients received adjuvant chemotherapy (92%), radiation therapy (95%), and tamoxifen (52%), the independent additional contributions of these modalities to the obtained favourable results could not be ascertained.
While ER- or PR-disease was shown to be associated with higher clinical and/or pathological response, failure to attain cCR was the only variable identified that predicts failure to achieve pCR. Nonetheless, the overall agreement between cCR and pCR was only moderate. In contrast, in a recently published series from MD Anderson, negative ER status and higher nuclear grade were independently associated with pCR (Kuerer et al, 1999).
While no variable could be identified to prognosticate OS independently, clinical response of the primary tumour, pathological response of the primary tumour, and the pathological nodal status were identified as independent prognostic variables for DFS. In the NSABB B-18 study, the 9-year follow-up demonstrated significant associations between clinical response and achieving pCR and OS, DFS, and relapse-free survival (Wolmark et al, 2001). Also shown were the associations between pathologic lymph node status and survival. In the latter study, these significant associations persisted after adjustment for clinical tumour size at randomisation, clinical lymph node status, and age.
In conclusion, neoadjuvant PC as used in this phase II study in a multidisciplinary strategy was highly effective with an acceptable safety profile. Clinical and pathologic responses remain the most important variables that predict outcome.
References
- Anderson ED, Forrest AP, Hawkins RA, Anderson TJ, Leonard RC, Chetty U (1991) Primary systemic therapy for operable breast cancer. Br J Cancer 63: 561–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonadonna G, Valagussa P, Brambilla C, Ferrari L, Moliterni A, Tereniziani M, Zambetti M (1998) Primary chemotherapy in operable breast cancer. Eight-year experience at Milan Cancer Institute. J Clin Oncol 16: 93–100 [DOI] [PubMed] [Google Scholar]
- Brookmeyer R, Crowley J (1982) A confidence interval for the median survival time. Biometrics 38: 29–41 [Google Scholar]
- Bruckman J, Harris J, Levene M, Chaffey JT, Hellman S (1979) Results of treating stage III carcinoma of the breast by primary radiation therapy. Cancer 43: 985–993 [DOI] [PubMed] [Google Scholar]
- Comella G, Comella P, Scanni A, Apicella G, D’Aiuto G, Thomas R, Capasso I, DiBonito I, Piccolo S, Carteni G, Gravina A, Frasci G (1998) Cisplatin-epirubicin-paclitaxel weekly administration in advanced breast cancer. A phase I study. Proc Am Soc Clin Oncol 17: 510 (abstract) [Google Scholar]
- Cox DR, Oakes D (1997) Regression models and life tables (with discussion). J R Statist Soc B 34: 187–220 [Google Scholar]
- Cox DR, Snell EJ (1989) Analysis of Binary Data. London: Chapman & Hall [Google Scholar]
- De Lena M, Varini M, Zucali R, Viganotti G, Valaguss P, Veronesi U, Bonadonna G (1981) Multimodal treatment for locally advanced breast cancer. Result of chemotherapy-radiotherapy versus chemotherapy-surgery. Cancer Clin Trials 4: 229–236 [PubMed] [Google Scholar]
- Ezzat AA, Ibrahim EM, Ajarim DS, Rahal MM, Raja MA, Stuart RK, Tulbah AM, Kandil A, Al-Malik OA, Bazarbashi SM (2000) High complete pathological response in locally advanced breast cancer using paclitaxel and cisplatin. Breast Cancer Res Treat 62: 237–244 [DOI] [PubMed] [Google Scholar]
- Ezzat A, Raja MA, Berry J, Bazarbashi S, Zwann F, Rahal M, El-Warith A (1997) A phase II trial of circadian-timed paclitaxel and cisplatin therapy in metastatic breast cancer. Ann Oncol 8: 663–667 [DOI] [PubMed] [Google Scholar]
- Feldman LD, Hortobagyi GN, Buzdar AU, Ames FC, Blumenschein GR (1986) Pathological assessment to induction chemotherapy in breast cancer. Cancer Res 46: 2578–2581 [PubMed] [Google Scholar]
- Fisher B, Bryant J, Wolmark N, Mamounas E, Brown A, Fisher ER (1998) Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol 16: 2672–2685 [DOI] [PubMed] [Google Scholar]
- Fleiss JL (1981) Statistical Methods for Rates and Proportions, 2nd edn. New York: Macmillan [Google Scholar]
- Haagensen CD, Stout AP (1943) Carcinoma of the breast: criteria of inoperability. Ann Surg 118: 859–870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmanek P, Sobin LH (1987) TNM Classification of Malignant Tumors,. UICC International Union Against Cancer. Berlin,Germany: Springer-Verlag [Google Scholar]
- Henderson IC, Berry D, Demetri G, Cirrincione C, Goldstein L, Marino S, Ingle JN, Cooper MR, Canellos G, Borden E, Fleming G, Holland JF, Graziano S, Carpenter J, Muss H, Norton L (1998) Improved disease-free (DFS) and overall survival (OS) from the addition of sequential paclitaxel (T) but not from the escalation of doxorubicin (A) dose level in adjuvant chemotherapy of patients (pts) with node-positive primary breast cancer (BC). Proc Am Soc Clin Oncol 17: 390a (abstract) [Google Scholar]
- Hortobagyi GN (1997) Paclitaxel-based combination chemotherapy for breast cancer. Oncology 11(3 Supp 2): 29–37 [PubMed] [Google Scholar]
- Hortobagyi GN, Ames FC, Buzdar QU, Kau SW, McNeese MD, Paulus D, Hug V, Holmes FA, Romsdahl MM, Fraschini G (1988) Management of stage III primary breast cancer with primary chemotherapy, surgery and radiation therapy. Cancer 62: 2507–2516 [DOI] [PubMed] [Google Scholar]
- Hosmer DW, Lemeshow S (1980) Goodness-of-fit tests for the multiple logistic regression model. Commun Statist – Part A Theor Meth A9(10): 1043–1069 [Google Scholar]
- Kalbfleisch JD, Prentice RL (1980) The Statistical Analysis of Failure Time Data. New York: Wiley [Google Scholar]
- Kaplan EL, Meier P (1958) Nonparametric estimation from incomplete observations. J Am Statist Assoc 53: 457–481 [Google Scholar]
- Kay R (1977) Proportional hazard regression models and the analysis of censored survival data. Appl Statist 26: 227–237 [Google Scholar]
- Kourousis C, Kakolyris S, Androulakis N, Heras P, Vlchonicolis J, Vamvakas L, Vlata M, Hatzidaki D, Samonis G, Georgoulias V (1998) Salvage therapy with paclitaxel, vinorelbine, and cisplatin (PVC) in anthracycline-resistant advanced breast cancer. Am J Clin Oncol 21: 226–232 [DOI] [PubMed] [Google Scholar]
- Kuerer H, Newman LA, Smith TL, Ames FC, Hunt KK, Dhingra K, Theriault RL, Sing G, Binkley SM, Sneige N, Buchholz TA, Ross MI, McNeese MD, Buzdar AU, Hortobagyi GN, Singletary SE (1999) Clinical course of breast cancer patients with complete pathologic primary tumor and axillary lymph node response to doxorubicin-based neoadjuvant chemotherapy. J Clin Oncol 17: 460–469 [DOI] [PubMed] [Google Scholar]
- Lau DH, Young L, Xue L (1998) Paclitaxel: an angiogenesis antagonist in metastatic breast cancer model. Proc Am Soc Clin Oncol 17: 414 (abstract) [Google Scholar]
- Mantel N (1966) Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep 50: 163–170 [PubMed] [Google Scholar]
- Marubini E, Valsecchi MG (1995) Analysing Data from Clinical Trials and Observational Studies. New York: John Wiley and Sons [Google Scholar]
- Perez EA, Suman VJ, Krook JE, Stella PJ, Hartman LC, Hatfield AK, Fitch T, Ingle JN (1998) Phase II study of paclitaxel plus carboplatin as first-line chemotherapy for women with metastatic breast cancer (MBC): a north central cancer treatment group trial. Proc Am Soc Clin Oncol 17: 635 (abstract) [Google Scholar]
- Powles TJ, Hickish TF, Makris A, Ashley SE, O’Brien ME, Tidy VA, Casey S, Nash AG, Sacks N, Cosgrove D (1995) Randomized trial of chemoendocrine therapy started before or after surgery for treatment of primary breast cancer. J Clin Oncol 13: 547–552 [DOI] [PubMed] [Google Scholar]
- Prentice RL (1976) A generalization of the probit and logit methods for dose response curves. Biometrics 32: 761–867 [PubMed] [Google Scholar]
- Ray-Coquard I, Biron I, Bachelot T, Guastalla JP, Catimel G, Merrouche Y, Droz JP, Chauvin F, Blay JY (1998) Vinorelbine and cisplatin (CIVIC regimen) for the treatment of metastatic breast carcinoma after failure of anthracycline- and/or paclitaxel-containing regimens. Cancer 82: 134–140 [PubMed] [Google Scholar]
- Scholl SM, Foourquet A, Asselian B, Pierga JY, Vilcoq JR, Durand JC, Dorval T, Palangie T, Jouve M, Beuzeboc P (1994) Neoadjuvant versus adjuvant chemotherapy in premenopausal patients with tumors too large for breast conservative surgery: preliminary results of a randomized trial. Eur J Cancer 30A: 645–652 [DOI] [PubMed] [Google Scholar]
- Smith IE, Al-Moundhri M (1998) Primary chemotherapy in breast cancer. Biomed Pharmacother 52: 116–121 [DOI] [PubMed] [Google Scholar]
- Smith IE, Jones AL, O’Brien ME, McKinna JA, Sacks N, Baum M (1993) Primary medical (neo-adjuvant) chemotherapy for operable breast cancer. Eur J Cancer 29A: 1796–1799 [DOI] [PubMed] [Google Scholar]
- Smith IE, Walsh G, Jones A, Prendivilli J, Johnston PJ, Ramage F, Robertshaw H, Sacks N, Ebbs S (1995) High complete remissions with primary (neoadjuvant) infusional chemotherapy for large early breast cancer. J Clin Oncol 13: 424–429 [DOI] [PubMed] [Google Scholar]
- Valagussa P, Zambetti M, Bonadonna G, Zucali R, Mezzanotte G, Veronesi U (1990) Prognostic factors in locally advanced noninflammatory breast cancer. Long-term results following primary chemotherapy. Breast Cancer Res Treat 15: 137–147 [DOI] [PubMed] [Google Scholar]
- Volm M, Formenti S, Symmans F, Downey A, Muggia F (1998) Neo-adjuvant paclitaxel for locally advanced breast cancer (LABC): preliminary results. Proc Am Soc Clin Oncol 17: 695 (abstract) [Google Scholar]
- von Minckwitz G, Dan Costa S, Eiermann W, Blohmer J, Tulusan AH, Jackish C, Kaufmann M (1999) Maximized reduction of primary breast tumor size using preoperative chemotherapy with doxorubicin and docetaxel. J Clin Oncol 17: 1999–2005 [DOI] [PubMed] [Google Scholar]
- Wolmark N, Wang J, Mamounas E, Bryant J, Fisher B (2001) Preoperative chemotherapy in patients with operable breast cancer: nine-year results from the National Surgical Adjuvant Breast and Bowel Project B-18. J Nat Cancer Inst Monogr 30: 96–102 [DOI] [PubMed] [Google Scholar]
- Zucali R, Uslemghi C, Kenda R, Bonadonna G (1976) Natural history and survival of inoperable breast cancer treated with radiotherapy followed by radical mastectomy. Cancer 37: 1422–1431 [DOI] [PubMed] [Google Scholar]