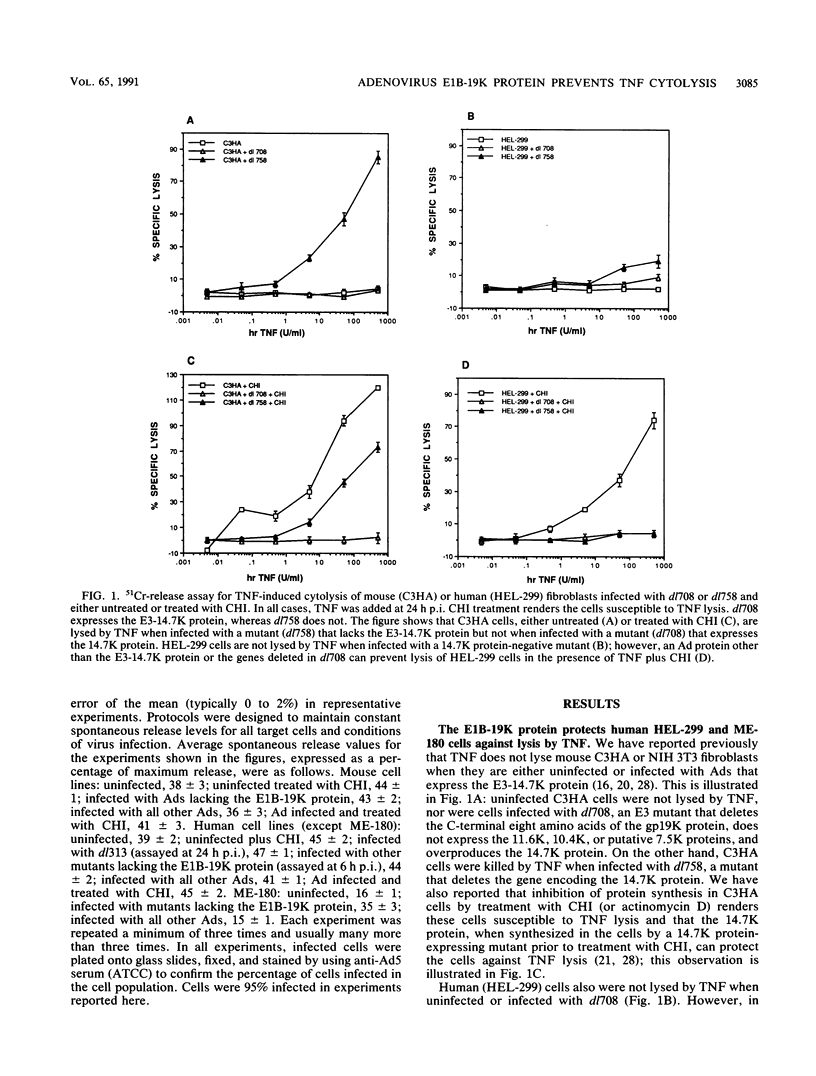
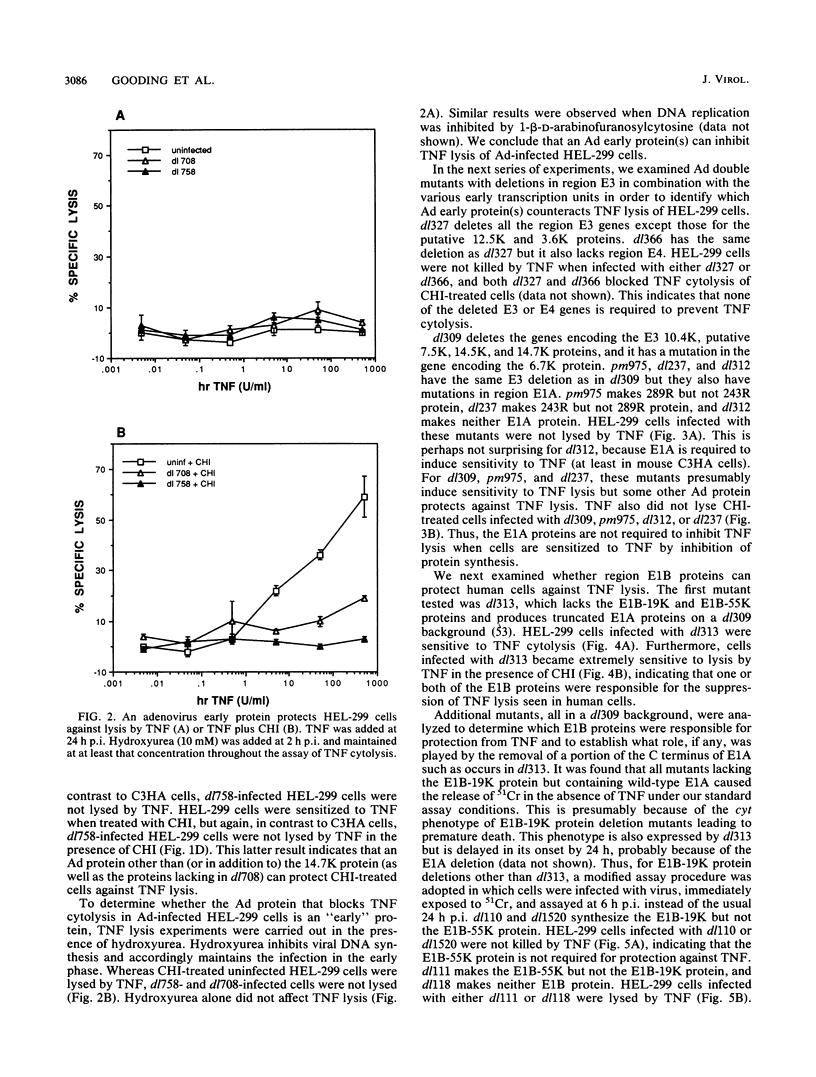
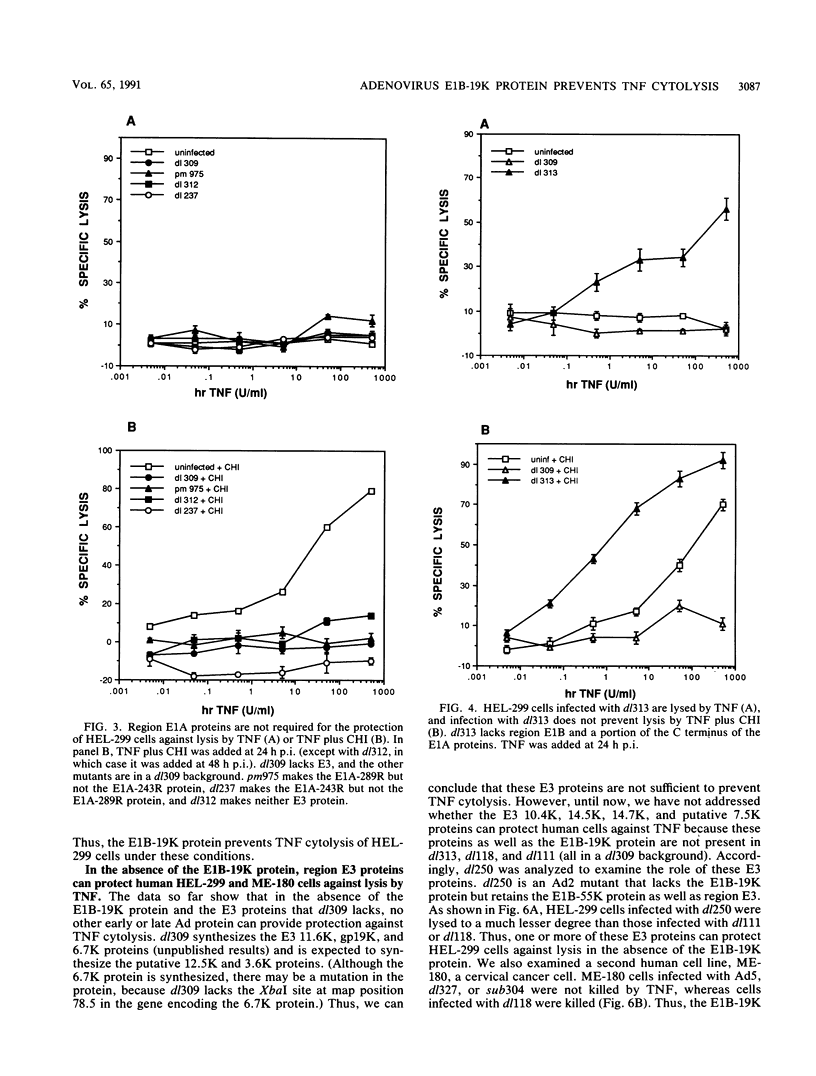
Abstract
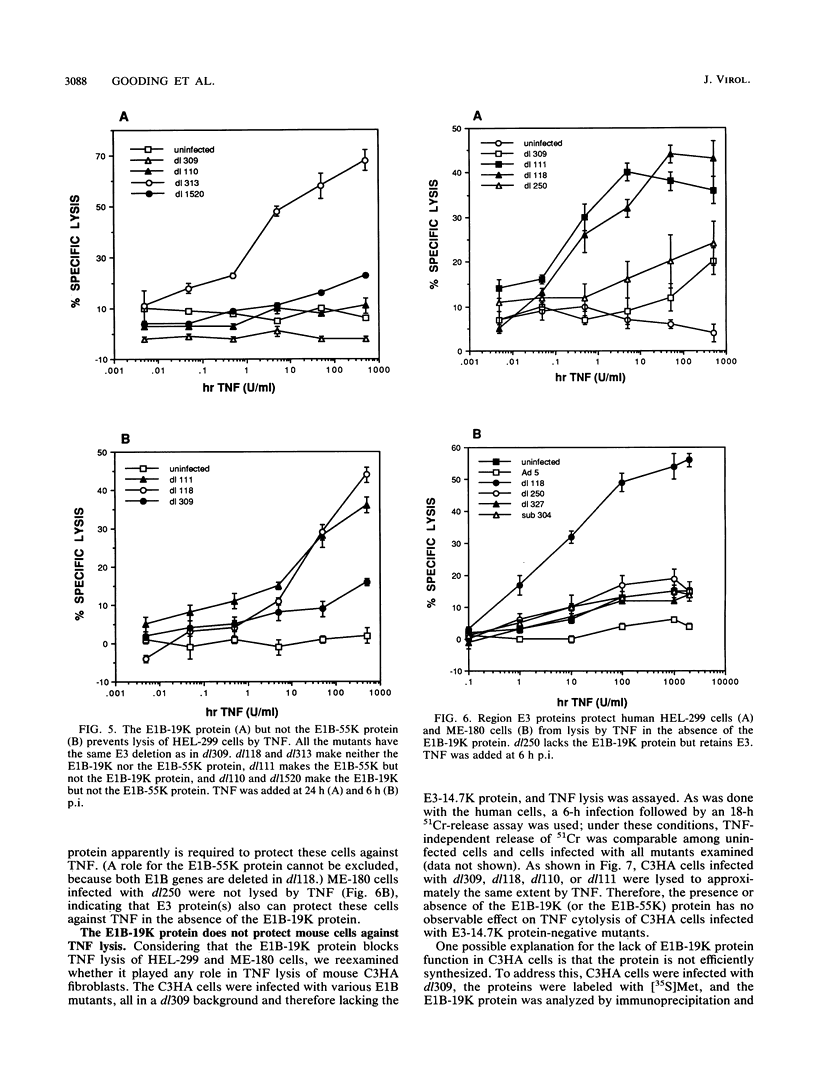
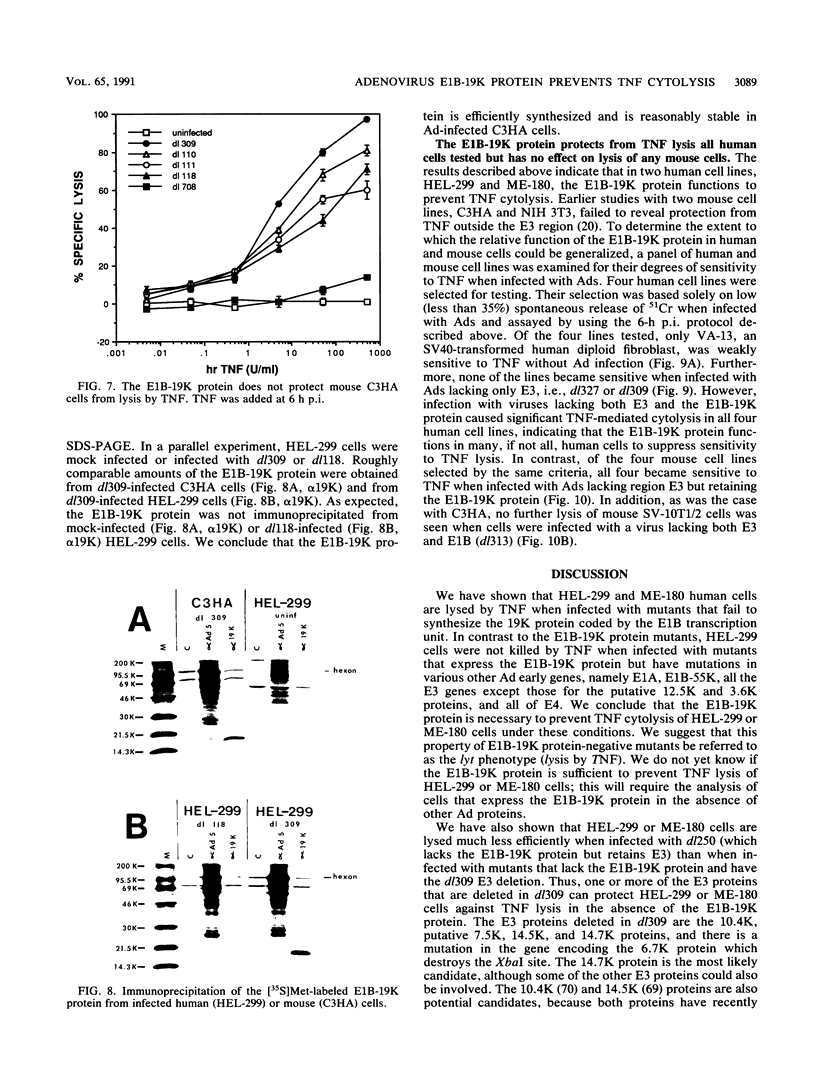
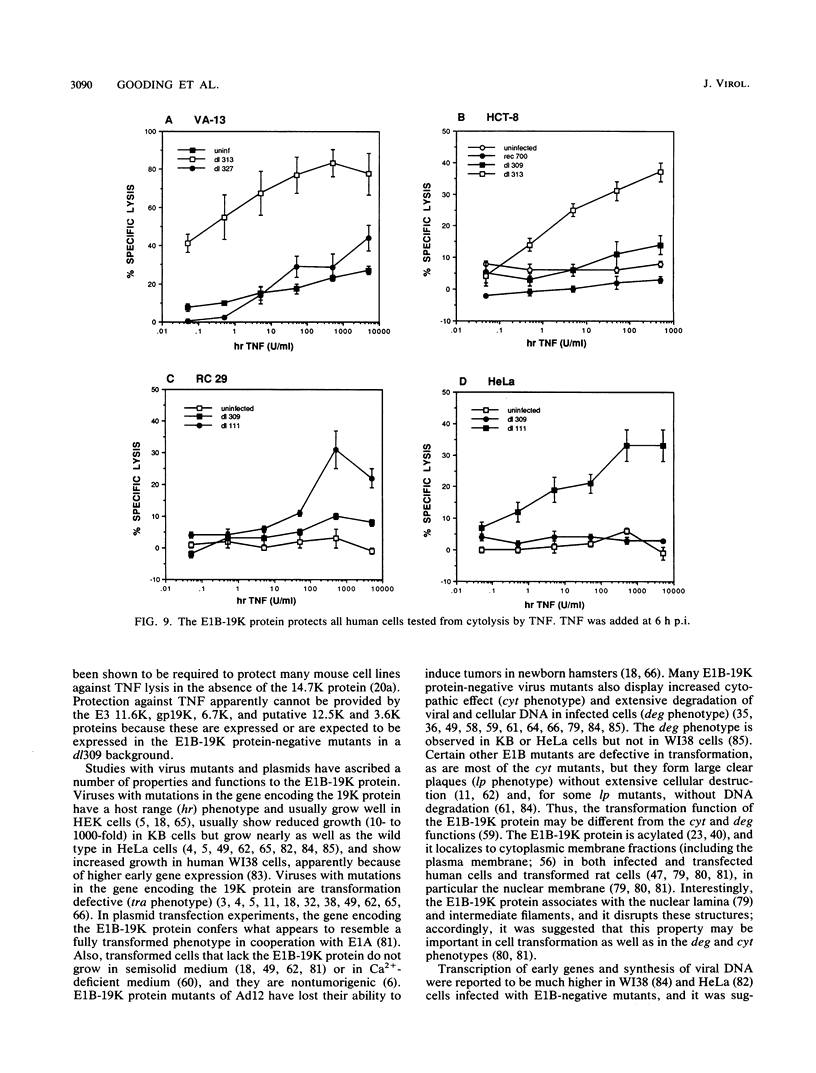
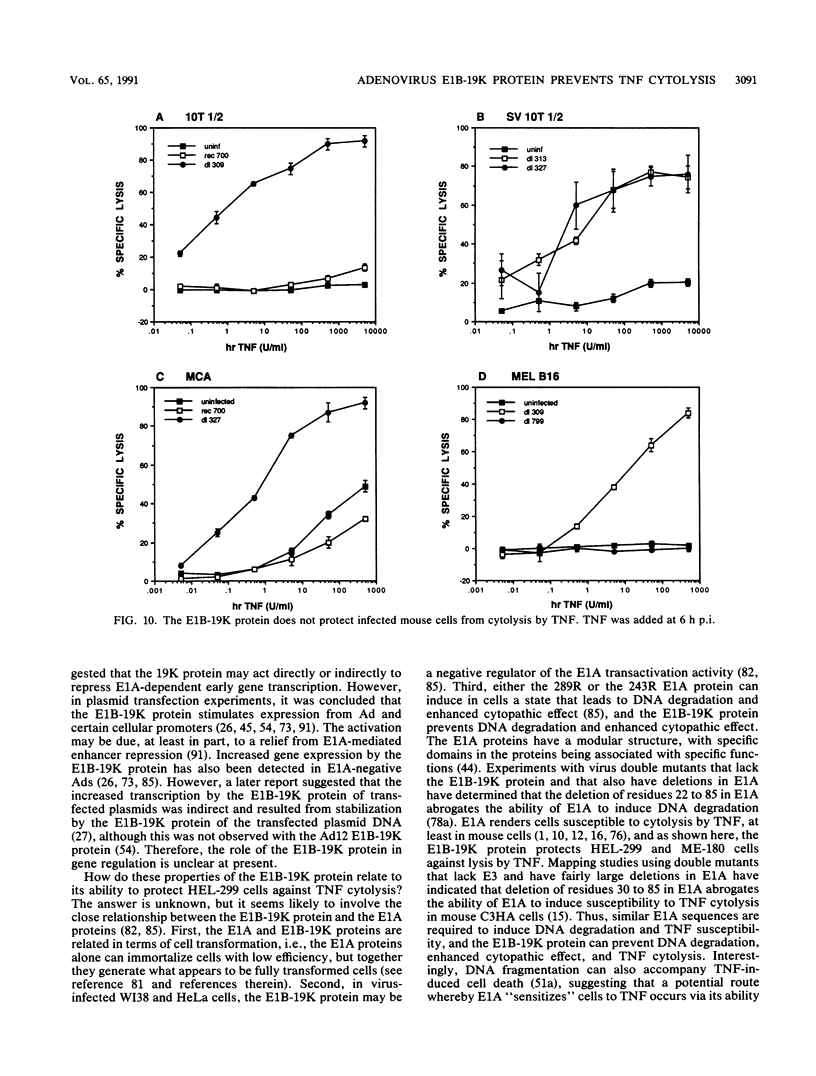
Tumor necrosis factor (TNF) is a multifunctional immunoregulatory protein that is secreted by activated macrophages and is believed to have antiviral activities. We reported earlier that when mouse C3HA fibroblasts are infected with human adenoviruses, the 289R and 243R proteins encoded by region E1A render the cells susceptible to lysis by TNF, and a 14,700-molecular-weight protein (14.7K protein) encoded by region E3 protects the cells against lysis by TNF. We now report that the 19,000-molecular-weight (19K) (176R) protein encoded by the E1B transcription unit can protect human HEL-299 fibroblasts and human ME-180 cervical carcinoma cells against lysis by TNF. This was determined by infecting cells with adenovirus double mutants that lack region E3 and do or do not express the E1B-19K protein and by measuring cytolysis by using a short-term (18-h) 51Cr-release assay. Under these assay conditions, the 51Cr release was specific to TNF and was not a consequence of the cyt phenotype associated with E1B-19K protein-negative mutants. Also, by using virus double mutants that lack E3 in combination with other early regions, we found that E1A, the E1B-55K protein-encoding gene, E3, and E4 are not required to protect HEL-299 cells against TNF cytolysis. Three additional human cancer cell lines (HeLa, HCT8, and RC29) and a simian virus 40-transformed WI38 cell line (VA-13) also required E1B for protection against TNF cytolysis, indicating that the E1B-19K protein is required to protect many if not all human cell types against lysis by TNF when infected by adenovirus. The E1B-19K protein was not able to protect six different adenovirus-infected mouse cell lines against TNF lysis, even though the protein was shown to be efficiently expressed in one of the cell lines. HEL-299 or ME-180 cells infected by a mutant that lacks the E1B-19K protein but retains region E3 were not lysed by TNF, indicating that one or more of the E3 proteins can protect these cells against TNF lysis in the absence of the E1B-19K protein. Thus, the E3-14.7K but not the E1B-19K protein can protect adenovirus-infected mouse cells against TNF cytolysis, whereas the E1B-19K protein as well as one or more of the E3 proteins can protect adenovirus-infected human cells against TNF cytolysis.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames R. S., Holskin B., Mitcho M., Shalloway D., Chen M. J. Induction of sensitivity to the cytotoxic action of tumor necrosis factor alpha by adenovirus E1A is independent of transformation and transcriptional activation. J Virol. 1990 Sep;64(9):4115–4122. doi: 10.1128/jvi.64.9.4115-4122.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arakawa T., Hsu Y. R., Toth E., Stebbing N. The antiviral activity of recombinant human tumor necrosis factor-alpha. J Interferon Res. 1987 Feb;7(1):103–105. doi: 10.1089/jir.1987.7.103. [DOI] [PubMed] [Google Scholar]
- Babiss L. E., Fisher P. B., Ginsberg H. S. Effect on transformation of mutations in the early region 1b-encoded 21- and 55-kilodalton proteins of adenovirus 5. J Virol. 1984 Nov;52(2):389–395. doi: 10.1128/jvi.52.2.389-395.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker D. D., Berk A. J. Adenovirus proteins from both E1B reading frames are required for transformation of rodent cells by viral infection and DNA transfection. Virology. 1987 Jan;156(1):107–121. doi: 10.1016/0042-6822(87)90441-7. [DOI] [PubMed] [Google Scholar]
- Bernards R., Schrier P. I., Bos J. L., Van der Eb A. J. Role of adenovirus types 5 and 12 early region 1b tumor antigens in oncogenic transformation. Virology. 1983 May;127(1):45–53. doi: 10.1016/0042-6822(83)90369-0. [DOI] [PubMed] [Google Scholar]
- Bernards R., de Leeuw M. G., Houweling A., van der Eb A. J. Role of the adenovirus early region 1B tumor antigens in transformation and lytic infection. Virology. 1986 Apr 15;150(1):126–139. doi: 10.1016/0042-6822(86)90272-2. [DOI] [PubMed] [Google Scholar]
- Beutler B., Cerami A. The biology of cachectin/TNF--a primary mediator of the host response. Annu Rev Immunol. 1989;7:625–655. doi: 10.1146/annurev.iy.07.040189.003205. [DOI] [PubMed] [Google Scholar]
- Brady H. A., Wold W. S. Identification of a novel sequence that governs both polyadenylation and alternative splicing in region E3 of adenovirus. Nucleic Acids Res. 1987 Nov 25;15(22):9397–9416. doi: 10.1093/nar/15.22.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlin C. R., Tollefson A. E., Brady H. A., Hoffman B. L., Wold W. S. Epidermal growth factor receptor is down-regulated by a 10,400 MW protein encoded by the E3 region of adenovirus. Cell. 1989 Apr 7;57(1):135–144. doi: 10.1016/0092-8674(89)90179-7. [DOI] [PubMed] [Google Scholar]
- Chen M. J., Holskin B., Strickler J., Gorniak J., Clark M. A., Johnson P. J., Mitcho M., Shalloway D. Induction by E1A oncogene expression of cellular susceptibility to lysis by TNF. Nature. 1987 Dec 10;330(6148):581–583. doi: 10.1038/330581a0. [DOI] [PubMed] [Google Scholar]
- Chinnadurai G. Adenovirus 2 Ip+ locus codes for a 19 kd tumor antigen that plays an essential role in cell transformation. Cell. 1983 Jul;33(3):759–766. doi: 10.1016/0092-8674(83)90018-1. [DOI] [PubMed] [Google Scholar]
- Cook J. L., May D. L., Wilson B. A., Holskin B., Chen M. J., Shalloway D., Walker T. A. Role of tumor necrosis factor-alpha in E1A oncogene-induced susceptibility of neoplastic cells to lysis by natural killer cells and activated macrophages. J Immunol. 1989 Jun 15;142(12):4527–4534. [PubMed] [Google Scholar]
- Creasey A. A., Doyle L. V., Reynolds M. T., Jung T., Lin L. S., Vitt C. R. Biological effects of recombinant human tumor necrosis factor and its novel muteins on tumor and normal cell lines. Cancer Res. 1987 Jan 1;47(1):145–149. [PubMed] [Google Scholar]
- Deutscher S. L., Bhat B. M., Pursley M. H., Cladaras C., Wold W. S. Novel deletion mutants that enhance a distant upstream 5' splice in the E3 transcription unit of adenovirus 2. Nucleic Acids Res. 1985 Aug 26;13(16):5771–5788. doi: 10.1093/nar/13.16.5771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duerksen-Hughes P. J., Hermiston T. W., Wold W. S., Gooding L. R. The amino-terminal portion of CD1 of the adenovirus E1A proteins is required to induce susceptibility to tumor necrosis factor cytolysis in adenovirus-infected mouse cells. J Virol. 1991 Mar;65(3):1236–1244. doi: 10.1128/jvi.65.3.1236-1244.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duerksen-Hughes P., Wold W. S., Gooding L. R. Adenovirus E1A renders infected cells sensitive to cytolysis by tumor necrosis factor. J Immunol. 1989 Dec 15;143(12):4193–4200. [PubMed] [Google Scholar]
- Feduchi E., Alonso M. A., Carrasco L. Human gamma interferon and tumor necrosis factor exert a synergistic blockade on the replication of herpes simplex virus. J Virol. 1989 Mar;63(3):1354–1359. doi: 10.1128/jvi.63.3.1354-1359.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui Y., Saito I., Shiroki K., Shimojo H. Isolation of transformation-defective, replication-nondefective early region 1B mutants of adenovirus 12. J Virol. 1984 Jan;49(1):154–161. doi: 10.1128/jvi.49.1.154-161.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gooding L. R., Elmore L. W., Tollefson A. E., Brady H. A., Wold W. S. A 14,700 MW protein from the E3 region of adenovirus inhibits cytolysis by tumor necrosis factor. Cell. 1988 May 6;53(3):341–346. doi: 10.1016/0092-8674(88)90154-7. [DOI] [PubMed] [Google Scholar]
- Gooding L. R., Sofola I. O., Tollefson A. E., Duerksen-Hughes P., Wold W. S. The adenovirus E3-14.7K protein is a general inhibitor of tumor necrosis factor-mediated cytolysis. J Immunol. 1990 Nov 1;145(9):3080–3086. [PubMed] [Google Scholar]
- Gooding L. R. Specificities of killing by T lymphocytes generated against syngeneic SV40 transformants: studies employing recombinants within the H-2 complex. J Immunol. 1979 Mar;122(3):1002–1008. [PubMed] [Google Scholar]
- Graham F. L., Smiley J., Russell W. C., Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977 Jul;36(1):59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- Grand R. J., Roberts C., Gallimore P. H. Acylation of adenovirus type 12 early region 1b 18-kDa protein. Further evidence for its localisation in the cell membrane. FEBS Lett. 1985 Feb 25;181(2):229–235. doi: 10.1016/0014-5793(85)80265-9. [DOI] [PubMed] [Google Scholar]
- Green M., Wold W. S. Human adenoviruses: growth, purification, and transfection assay. Methods Enzymol. 1979;58:425–435. doi: 10.1016/s0076-6879(79)58157-9. [DOI] [PubMed] [Google Scholar]
- Halbert D. N., Cutt J. R., Shenk T. Adenovirus early region 4 encodes functions required for efficient DNA replication, late gene expression, and host cell shutoff. J Virol. 1985 Oct;56(1):250–257. doi: 10.1128/jvi.56.1.250-257.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrmann C. H., Dery C. V., Mathews M. B. Transactivation of host and viral genes by the adenovirus E1B 19K tumor antigen. Oncogene. 1987;2(1):25–35. [PubMed] [Google Scholar]
- Herrmann C. H., Mathews M. B. The adenovirus E1B 19-kilodalton protein stimulates gene expression by increasing DNA levels. Mol Cell Biol. 1989 Dec;9(12):5412–5423. doi: 10.1128/mcb.9.12.5412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton T. M., Ranheim T. S., Aquino L., Kusher D. I., Saha S. K., Ware C. F., Wold W. S., Gooding L. R. Adenovirus E3 14.7K protein functions in the absence of other adenovirus proteins to protect transfected cells from tumor necrosis factor cytolysis. J Virol. 1991 May;65(5):2629–2639. doi: 10.1128/jvi.65.5.2629-2639.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton T. M., Tollefson A. E., Wold W. S., Gooding L. R. A protein serologically and functionally related to the group C E3 14,700-kilodalton protein is found in multiple adenovirus serotypes. J Virol. 1990 Mar;64(3):1250–1255. doi: 10.1128/jvi.64.3.1250-1255.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howe J. A., Mymryk J. S., Egan C., Branton P. E., Bayley S. T. Retinoblastoma growth suppressor and a 300-kDa protein appear to regulate cellular DNA synthesis. Proc Natl Acad Sci U S A. 1990 Aug;87(15):5883–5887. doi: 10.1073/pnas.87.15.5883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen O., Kabelitz D. Tumor necrosis factor selectively inhibits activation of human B cells by Epstein-Barr virus. J Immunol. 1988 Jan 1;140(1):125–130. [PubMed] [Google Scholar]
- Jones N., Shenk T. Isolation of adenovirus type 5 host range deletion mutants defective for transformation of rat embryo cells. Cell. 1979 Jul;17(3):683–689. doi: 10.1016/0092-8674(79)90275-7. [DOI] [PubMed] [Google Scholar]
- Jones N., Shenk T. Isolation of deletion and substitution mutants of adenovirus type 5. Cell. 1978 Jan;13(1):181–188. doi: 10.1016/0092-8674(78)90148-4. [DOI] [PubMed] [Google Scholar]
- Kaddurah-Daouk R., Lillie J. W., Daouk G. H., Green M. R., Kingston R., Schimmel P. Induction of a cellular enzyme for energy metabolism by transforming domains of adenovirus E1a. Mol Cell Biol. 1990 Apr;10(4):1476–1483. doi: 10.1128/mcb.10.4.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohase M., Henriksen-DeStefano D., May L. T., Vilcek J., Sehgal P. B. Induction of beta 2-interferon by tumor necrosis factor: a homeostatic mechanism in the control of cell proliferation. Cell. 1986 Jun 6;45(5):659–666. doi: 10.1016/0092-8674(86)90780-4. [DOI] [PubMed] [Google Scholar]
- Kumai H., Takemori N., Hashimoto S. Role of adenovirus type 2 early region 1B 19K protein stability in expression of the cyt and deg phenotypes. J Gen Virol. 1989 Aug;70(Pt 8):1975–1986. doi: 10.1099/0022-1317-70-8-1975. [DOI] [PubMed] [Google Scholar]
- Lai Fatt R. B., Mak S. Mapping of an adenovirus function involved in the inhibition of DNA degradation. J Virol. 1982 Jun;42(3):969–977. doi: 10.1128/jvi.42.3.969-977.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lillie J. W., Loewenstein P. M., Green M. R., Green M. Functional domains of adenovirus type 5 E1a proteins. Cell. 1987 Sep 25;50(7):1091–1100. doi: 10.1016/0092-8674(87)90175-9. [DOI] [PubMed] [Google Scholar]
- Mak I., Mak S. Transformation of rat cells by cyt mutants of adenovirus type 12 and mutants of adenovirus type 5. J Virol. 1983 Mar;45(3):1107–1117. doi: 10.1128/jvi.45.3.1107-1117.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyama T., Hamamoto Y., Soma G., Mizuno D., Yamamoto N., Kobayashi N. Cytocidal effect of tumor necrosis factor on cells chronically infected with human immunodeficiency virus (HIV): enhancement of HIV replication. J Virol. 1989 Jun;63(6):2504–2509. doi: 10.1128/jvi.63.6.2504-2509.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlade C. J., Tremblay M. L., Yee S. P., Ross R., Branton P. E. Acylation of the 176R (19-kilodalton) early region 1B protein of human adenovirus type 5. J Virol. 1987 Oct;61(10):3227–3234. doi: 10.1128/jvi.61.10.3227-3234.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mestan J., Brockhaus M., Kirchner H., Jacobsen H. Antiviral activity of tumour necrosis factor. Synergism with interferons and induction of oligo-2',5'-adenylate synthetase. J Gen Virol. 1988 Dec;69(Pt 12):3113–3120. doi: 10.1099/0022-1317-69-12-3113. [DOI] [PubMed] [Google Scholar]
- Mestan J., Digel W., Mittnacht S., Hillen H., Blohm D., Möller A., Jacobsen H., Kirchner H. Antiviral effects of recombinant tumour necrosis factor in vitro. 1986 Oct 30-Nov 5Nature. 323(6091):816–819. doi: 10.1038/323816a0. [DOI] [PubMed] [Google Scholar]
- Montell C., Fisher E. F., Caruthers M. H., Berk A. J. Resolving the functions of overlapping viral genes by site-specific mutagenesis at a mRNA splice site. Nature. 1982 Feb 4;295(5848):380–384. doi: 10.1038/295380a0. [DOI] [PubMed] [Google Scholar]
- Moran E., Mathews M. B. Multiple functional domains in the adenovirus E1A gene. Cell. 1987 Jan 30;48(2):177–178. doi: 10.1016/0092-8674(87)90418-1. [DOI] [PubMed] [Google Scholar]
- Natarajan V. Adenovirus-2 E1a and E1b gene products regulate enhancer mediated transcription. Nucleic Acids Res. 1986 Dec 9;14(23):9445–9456. doi: 10.1093/nar/14.23.9445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paya C. V., Kenmotsu N., Schoon R. A., Leibson P. J. Tumor necrosis factor and lymphotoxin secretion by human natural killer cells leads to antiviral cytotoxicity. J Immunol. 1988 Sep 15;141(6):1989–1995. [PubMed] [Google Scholar]
- Persson H., Katze M. G., Philipson L. Purification of a native membrane-associated adenovirus tumor antigen. J Virol. 1982 Jun;42(3):905–917. doi: 10.1128/jvi.42.3.905-917.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persson H., Oberg B., Philipson L. Purification and characterization of an early protein (E14K) from adenovirus type 2-infected cells. J Virol. 1978 Oct;28(1):119–139. doi: 10.1128/jvi.28.1.119-139.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pilder S., Logan J., Shenk T. Deletion of the gene encoding the adenovirus 5 early region 1b 21,000-molecular-weight polypeptide leads to degradation of viral and host cell DNA. J Virol. 1984 Nov;52(2):664–671. doi: 10.1128/jvi.52.2.664-671.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reis L. F., Le J. M., Hirano T., Kishimoto T., Vilcek J. Antiviral action of tumor necrosis factor in human fibroblasts is not mediated by B cell stimulatory factor 2/IFN-beta 2, and is inhibited by specific antibodies to IFN-beta. J Immunol. 1988 Mar 1;140(5):1566–1570. [PubMed] [Google Scholar]
- Scanlon M., Laster S. M., Wood J. G., Gooding L. R. Cytolysis by tumor necrosis factor is preceded by a rapid and specific dissolution of microfilaments. Proc Natl Acad Sci U S A. 1989 Jan;86(1):182–186. doi: 10.1073/pnas.86.1.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmid D. S., Hornung R., McGrath K. M., Paul N., Ruddle N. H. Target cell DNA fragmentation is mediated by lymphotoxin and tumor necrosis factor. Lymphokine Res. 1987 Summer;6(3):195–202. [PubMed] [Google Scholar]
- Schneider J. F., Fisher F., Goding C. R., Jones N. C. Mutational analysis of the adenovirus E1a gene: the role of transcriptional regulation in transformation. EMBO J. 1987 Jul;6(7):2053–2060. doi: 10.1002/j.1460-2075.1987.tb02470.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shenk T., Jones N., Colby W., Fowlkes D. Functional analysis of adenovirus-5 host-range deletion mutants defective for transformation of rat embryo cells. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):367–375. doi: 10.1101/sqb.1980.044.01.041. [DOI] [PubMed] [Google Scholar]
- Shiroki K., Kato H., Kawai S. Tandemly repeated hexamer sequences within the beta interferon promoter can function as an inducible regulatory element in activation by the adenovirus E1B 19-kilodalton protein. J Virol. 1990 Jun;64(6):3063–3068. doi: 10.1128/jvi.64.6.3063-3068.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. H., Ziff E. B. The amino-terminal region of the adenovirus serotype 5 E1a protein performs two separate functions when expressed in primary baby rat kidney cells. Mol Cell Biol. 1988 Sep;8(9):3882–3890. doi: 10.1128/mcb.8.9.3882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K. J., Gallimore P. H., Grand R. J. The expression of Ad12 E1B 19K protein on the surface of adenovirus transformed and infected human cells. Oncogene. 1989 Apr;4(4):489–497. [PubMed] [Google Scholar]
- Stein R. W., Corrigan M., Yaciuk P., Whelan J., Moran E. Analysis of E1A-mediated growth regulation functions: binding of the 300-kilodalton cellular product correlates with E1A enhancer repression function and DNA synthesis-inducing activity. J Virol. 1990 Sep;64(9):4421–4427. doi: 10.1128/jvi.64.9.4421-4427.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stillman B. W., White E., Grodzicker T. Independent mutations in Ad2ts111 cause degradation of cellular DNA and defective viral DNA replication. J Virol. 1984 May;50(2):598–605. doi: 10.1128/jvi.50.2.598-605.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian T., Kuppuswamy M., Chinnadurai G. An adenovirus 2-coded tumor antigen located on the endoplasmic reticulum and nuclear envelope is required for growth of transformed cells in Ca2+-deficient media. Mol Cell Biol. 1985 Nov;5(11):3297–3300. doi: 10.1128/mcb.5.11.3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian T., Kuppuswamy M., Gysbers J., Mak S., Chinnadurai G. 19-kDa tumor antigen coded by early region E1b of adenovirus 2 is required for efficient synthesis and for protection of viral DNA. J Biol Chem. 1984 Oct 10;259(19):11777–11783. [PubMed] [Google Scholar]
- Subramanian T., Kuppuswamy M., Mak S., Chinnadurai G. Adenovirus cyt+ locus, which controls cell transformation and tumorigenicity, is an allele of lp+ locus, which codes for a 19-kilodalton tumor antigen. J Virol. 1984 Nov;52(2):336–343. doi: 10.1128/jvi.52.2.336-343.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian T., Kuppuswamy M., Nasr R. J., Chinnadurai G. An N-terminal region of adenovirus E1a essential for cell transformation and induction of an epithelial cell growth factor. Oncogene. 1988 Feb;2(2):105–112. [PubMed] [Google Scholar]
- Takemori N., Cladaras C., Bhat B., Conley A. J., Wold W. S. cyt gene of adenoviruses 2 and 5 is an oncogene for transforming function in early region E1B and encodes the E1B 19,000-molecular-weight polypeptide. J Virol. 1984 Dec;52(3):793–805. doi: 10.1128/jvi.52.3.793-805.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takemori N. Genetic studies with tumorigenic adenoviruses. 3. Recombination in adenovirus type 12. Virology. 1972 Jan;47(1):157–167. doi: 10.1016/0042-6822(72)90249-8. [DOI] [PubMed] [Google Scholar]
- Takemori N., Riggs J. L., Aldrich C. D. Genetic studies with tumorigenic adenoviruses. II. Heterogeneity of cyt mutants of adenovirus type 12. Virology. 1969 May;38(1):8–15. doi: 10.1016/0042-6822(69)90122-6. [DOI] [PubMed] [Google Scholar]
- Takemori N., Riggs J. L., Aldrich C. Genetic studies with tumorigenic adenoviruses. I. Isolation of cytocidal (cyt) mutants of adenovirus type 12. Virology. 1968 Dec;36(4):575–586. doi: 10.1016/0042-6822(68)90189-x. [DOI] [PubMed] [Google Scholar]
- Thimmappaya B., Weinberger C., Schneider R. J., Shenk T. Adenovirus VAI RNA is required for efficient translation of viral mRNAs at late times after infection. Cell. 1982 Dec;31(3 Pt 2):543–551. doi: 10.1016/0092-8674(82)90310-5. [DOI] [PubMed] [Google Scholar]
- Tollefson A. E., Krajcsi P., Pursley M. H., Gooding L. R., Wold W. S. A 14,500 MW protein is coded by region E3 of group C human adenoviruses. Virology. 1990 Mar;175(1):19–29. doi: 10.1016/0042-6822(90)90182-q. [DOI] [PubMed] [Google Scholar]
- Tollefson A. E., Krajcsi P., Yei S. P., Carlin C. R., Wold W. S. A 10,400-molecular-weight membrane protein is coded by region E3 of adenovirus. J Virol. 1990 Feb;64(2):794–801. doi: 10.1128/jvi.64.2.794-801.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tollefson A. E., Wold W. S. Identification and gene mapping of a 14,700-molecular-weight protein encoded by region E3 of group C adenoviruses. J Virol. 1988 Jan;62(1):33–39. doi: 10.1128/jvi.62.1.33-39.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vacheron F., Rudent A., Perin S., Labarre C., Quero A. M., Guenounou M. Production of interleukin 1 and tumour necrosis factor activities in bronchoalveolar washings following infection of mice by influenza virus. J Gen Virol. 1990 Feb;71(Pt 2):477–479. doi: 10.1099/0022-1317-71-2-477. [DOI] [PubMed] [Google Scholar]
- Vales L. D., Darnell J. E., Jr Promoter occlusion prevents transcription of adenovirus polypeptide IX mRNA until after DNA replication. Genes Dev. 1989 Jan;3(1):49–59. doi: 10.1101/gad.3.1.49. [DOI] [PubMed] [Google Scholar]
- Van Damme J., De Ley M., Van Snick J., Dinarello C. A., Billiau A. The role of interferon-beta 1 and the 26-kDa protein (interferon-beta 2) as mediators of the antiviral effect of interleukin 1 and tumor necrosis factor. J Immunol. 1987 Sep 15;139(6):1867–1872. [PubMed] [Google Scholar]
- Vanhaesebroeck B., Timmers H. T., Pronk G. J., van Roy F., Van der Eb A. J., Fiers W. Modulation of cellular susceptibility to the cytotoxic/cytostatic action of tumor necrosis factor by adenovirus E1 gene expression is cell type-dependent. Virology. 1990 Jun;176(2):362–368. doi: 10.1016/0042-6822(90)90006-d. [DOI] [PubMed] [Google Scholar]
- Wang E. W., Scott M. O., Ricciardi R. P. An adenovirus mRNA which encodes a 14,700-Mr protein that maps to the last open reading frame of region E3 is expressed during infection. J Virol. 1988 Apr;62(4):1456–1459. doi: 10.1128/jvi.62.4.1456-1459.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinberg D. H., Ketner G. A cell line that supports the growth of a defective early region 4 deletion mutant of human adenovirus type 2. Proc Natl Acad Sci U S A. 1983 Sep;80(17):5383–5386. doi: 10.1073/pnas.80.17.5383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White E., Blose S. H., Stillman B. W. Nuclear envelope localization of an adenovirus tumor antigen maintains the integrity of cellular DNA. Mol Cell Biol. 1984 Dec;4(12):2865–2875. doi: 10.1128/mcb.4.12.2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White E., Cipriani R. Role of adenovirus E1B proteins in transformation: altered organization of intermediate filaments in transformed cells that express the 19-kilodalton protein. Mol Cell Biol. 1990 Jan;10(1):120–130. doi: 10.1128/mcb.10.1.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White E., Cipriani R., Sabbatini P., Denton A. Adenovirus E1B 19-kilodalton protein overcomes the cytotoxicity of E1A proteins. J Virol. 1991 Jun;65(6):2968–2978. doi: 10.1128/jvi.65.6.2968-2978.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White E., Cipriani R. Specific disruption of intermediate filaments and the nuclear lamina by the 19-kDa product of the adenovirus E1B oncogene. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9886–9890. doi: 10.1073/pnas.86.24.9886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White E., Denton A., Stillman B. Role of the adenovirus E1B 19,000-dalton tumor antigen in regulating early gene expression. J Virol. 1988 Sep;62(9):3445–3454. doi: 10.1128/jvi.62.9.3445-3454.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White E., Faha B., Stillman B. Regulation of adenovirus gene expression in human WI38 cells by an E1B-encoded tumor antigen. Mol Cell Biol. 1986 Nov;6(11):3763–3773. doi: 10.1128/mcb.6.11.3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White E., Grodzicker T., Stillman B. W. Mutations in the gene encoding the adenovirus early region 1B 19,000-molecular-weight tumor antigen cause the degradation of chromosomal DNA. J Virol. 1984 Nov;52(2):410–419. doi: 10.1128/jvi.52.2.410-419.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White E., Stillman B. Expression of adenovirus E1B mutant phenotypes is dependent on the host cell and on synthesis of E1A proteins. J Virol. 1987 Feb;61(2):426–435. doi: 10.1128/jvi.61.2.426-435.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyte P., Williamson N. M., Harlow E. Cellular targets for transformation by the adenovirus E1A proteins. Cell. 1989 Jan 13;56(1):67–75. doi: 10.1016/0092-8674(89)90984-7. [DOI] [PubMed] [Google Scholar]
- Wilson-Rawls J., Saha S. K., Krajcsi P., Tollefson A. E., Gooding L. R., Wold W. S. A 6700 MW membrane protein is encoded by region E3 of adenovirus type 2. Virology. 1990 Sep;178(1):204–212. doi: 10.1016/0042-6822(90)90395-8. [DOI] [PubMed] [Google Scholar]
- Wold W. S., Deutscher S. L., Takemori N., Bhat B. M., Magie S. C. Evidence that AGUAUAUGA and CCAAGAUGA initiate translation in the same mRNA region E3 of adenovirus. Virology. 1986 Jan 15;148(1):168–180. doi: 10.1016/0042-6822(86)90412-5. [DOI] [PubMed] [Google Scholar]
- Wong G. H., Goeddel D. V. Tumour necrosis factors alpha and beta inhibit virus replication and synergize with interferons. 1986 Oct 30-Nov 5Nature. 323(6091):819–822. doi: 10.1038/323819a0. [DOI] [PubMed] [Google Scholar]
- Wong G. H., Krowka J. F., Stites D. P., Goeddel D. V. In vitro anti-human immunodeficiency virus activities of tumor necrosis factor-alpha and interferon-gamma. J Immunol. 1988 Jan 1;140(1):120–124. [PubMed] [Google Scholar]
- Yoshida K., Venkatesh L., Kuppuswamy M., Chinnadurai G. Adenovirus transforming 19-kD T antigen has an enhancer-dependent trans-activation function and relieves enhancer repression mediated by viral and cellular genes. Genes Dev. 1987 Sep;1(7):645–658. doi: 10.1101/gad.1.7.645. [DOI] [PubMed] [Google Scholar]
- van Dam H., Offringa R., Smits A. M., Bos J. L., Jones N. C., van der Eb A. J. The repression of the growth factor-inducible genes JE, c-myc and stromelysin by adenovirus E1A is mediated by conserved region 1. Oncogene. 1989 Oct;4(10):1207–1212. [PubMed] [Google Scholar]