Abstract
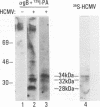
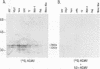
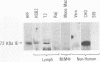
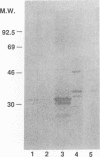
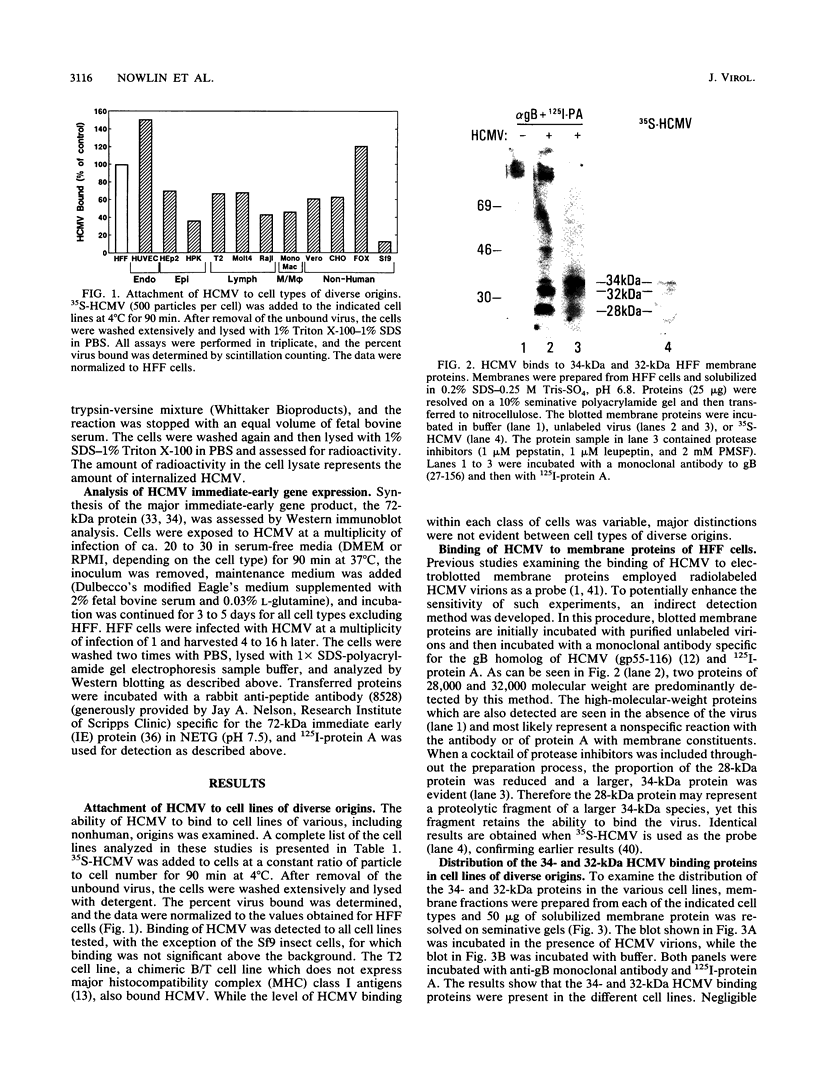
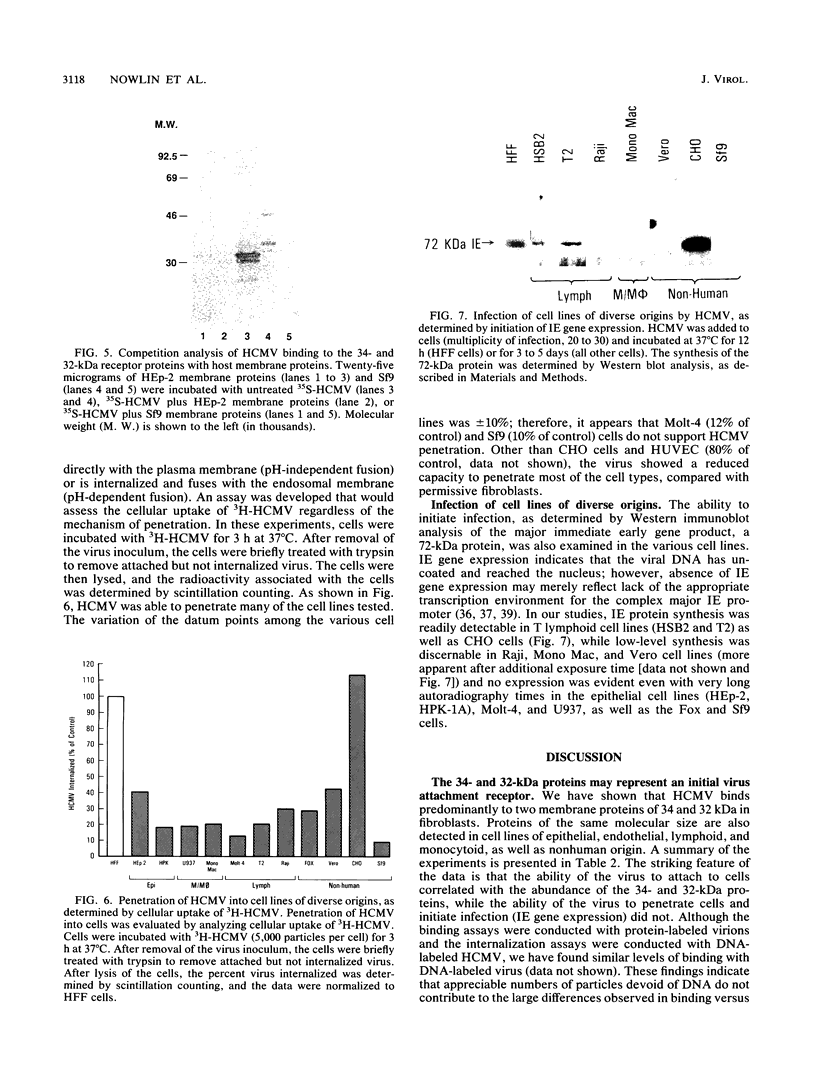
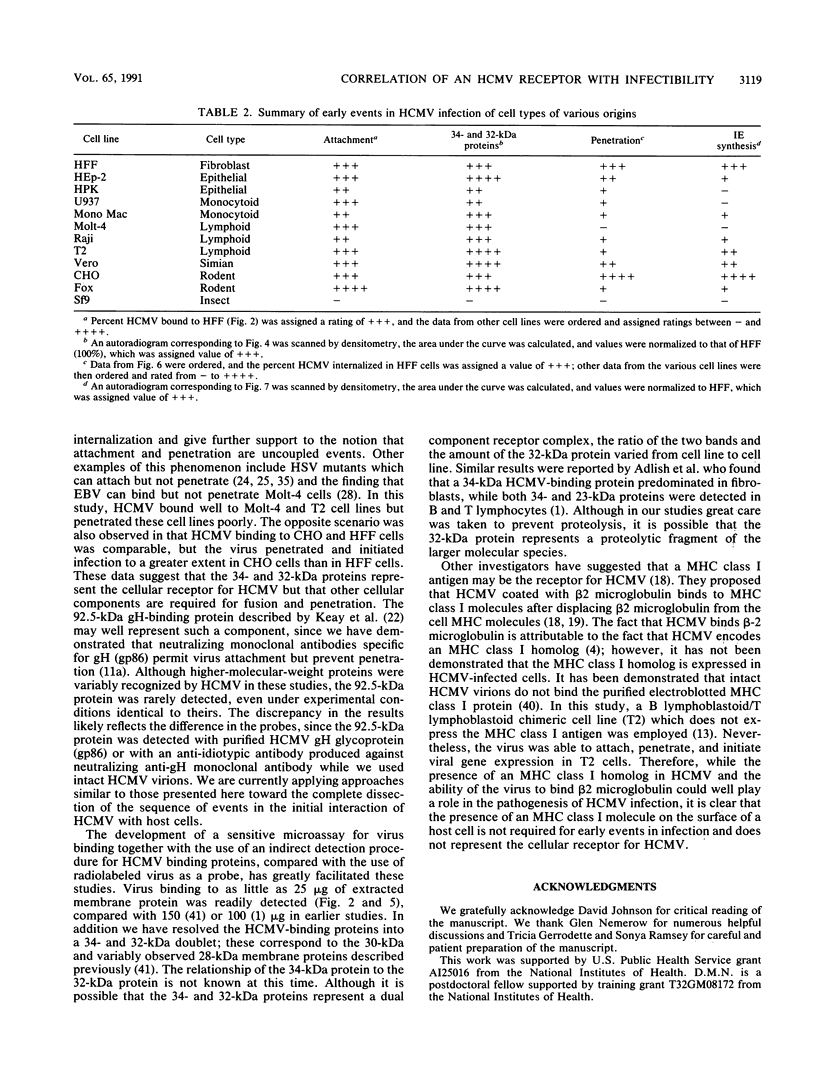
Previous studies have demonstrated that human cytomegalovirus (HCMV) specifically binds to a fibroblast membrane glycoprotein(s) with a molecular mass from 30 to 34 kDa. In this study, the distribution of the putative receptor proteins was analyzed in a variety of cell types, including cell types representative of those that are infected in vivo. Using a sensitive microbinding assay (to score virus attachment) and an indirect detection method (to score HCMV-binding proteins), we found that the 34- and 32-kDa HCMV binding proteins are ubiquitous molecules, broadly distributed among diverse cell types. In addition, the level of virus attachment was found to correlate with the abundance of the 34- and 32-kDa cellular proteins, while the ability of the virus to penetrate cells and initiate infection did not. The results support the hypothesis that the 34- and 32-kDa cellular proteins represent the HCMV (attachment) receptor. The data also support the notion that additional cellular components are required for virus entry and fusion.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adlish J. D., Lahijani R. S., St Jeor S. C. Identification of a putative cell receptor for human cytomegalovirus. Virology. 1990 Jun;176(2):337–345. doi: 10.1016/0042-6822(90)90003-a. [DOI] [PubMed] [Google Scholar]
- Ahearn J. M., Hayward S. D., Hickey J. C., Fearon D. T. Epstein-Barr virus (EBV) infection of murine L cells expressing recombinant human EBV/C3d receptor. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9307–9311. doi: 10.1073/pnas.85.23.9307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck S., Barrell B. G. Human cytomegalovirus encodes a glycoprotein homologous to MHC class-I antigens. Nature. 1988 Jan 21;331(6153):269–272. doi: 10.1038/331269a0. [DOI] [PubMed] [Google Scholar]
- Betts R. F., Freeman R. B., Douglas R. G., Jr, Talley T. E. Clinical manifestations of renal allograft derived primary cytomegalovirus infection. Am J Dis Child. 1977 Jul;131(7):759–763. doi: 10.1001/archpedi.1977.02120200041010. [DOI] [PubMed] [Google Scholar]
- Byrn R. A., Sekigawa I., Chamow S. M., Johnson J. S., Gregory T. J., Capon D. J., Groopman J. E. Characterization of in vitro inhibition of human immunodeficiency virus by purified recombinant CD4. J Virol. 1989 Oct;63(10):4370–4375. doi: 10.1128/jvi.63.10.4370-4375.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capon D. J., Chamow S. M., Mordenti J., Marsters S. A., Gregory T., Mitsuya H., Byrn R. A., Lucas C., Wurm F. M., Groopman J. E. Designing CD4 immunoadhesins for AIDS therapy. Nature. 1989 Feb 9;337(6207):525–531. doi: 10.1038/337525a0. [DOI] [PubMed] [Google Scholar]
- Carel J. C., Frazier B., Ley T. J., Holers V. M. Analysis of epitope expression and the functional repertoire of recombinant complement receptor 2 (CR2/CD21) in mouse and human cells. J Immunol. 1989 Aug 1;143(3):923–930. [PubMed] [Google Scholar]
- Cohen G. H., Isola V. J., Kuhns J., Berman P. W., Eisenberg R. J. Localization of discontinuous epitopes of herpes simplex virus glycoprotein D: use of a nondenaturing ("native" gel) system of polyacrylamide gel electrophoresis coupled with Western blotting. J Virol. 1986 Oct;60(1):157–166. doi: 10.1128/jvi.60.1.157-166.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton T., Courtney R. J. Evidence for post-translational glycosylation of a nonglycosylated precursor protein of herpes simplex virus type 1. J Virol. 1984 Nov;52(2):630–637. doi: 10.1128/jvi.52.2.630-637.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Compton T., Courtney R. J. Virus-specific glycoproteins associated with the nuclear fraction of herpes simplex virus type 1-infected cells. J Virol. 1984 Feb;49(2):594–597. doi: 10.1128/jvi.49.2.594-597.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cranage M. P., Kouzarides T., Bankier A. T., Satchwell S., Weston K., Tomlinson P., Barrell B., Hart H., Bell S. E., Minson A. C. Identification of the human cytomegalovirus glycoprotein B gene and induction of neutralizing antibodies via its expression in recombinant vaccinia virus. EMBO J. 1986 Nov;5(11):3057–3063. doi: 10.1002/j.1460-2075.1986.tb04606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeMars R., Rudersdorf R., Chang C., Petersen J., Strandtmann J., Korn N., Sidwell B., Orr H. T. Mutations that impair a posttranscriptional step in expression of HLA-A and -B antigens. Proc Natl Acad Sci U S A. 1985 Dec;82(23):8183–8187. doi: 10.1073/pnas.82.23.8183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fingeroth J. D., Weis J. J., Tedder T. F., Strominger J. L., Biro P. A., Fearon D. T. Epstein-Barr virus receptor of human B lymphocytes is the C3d receptor CR2. Proc Natl Acad Sci U S A. 1984 Jul;81(14):4510–4514. doi: 10.1073/pnas.81.14.4510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher R. A., Bertonis J. M., Meier W., Johnson V. A., Costopoulos D. S., Liu T., Tizard R., Walker B. D., Hirsch M. S., Schooley R. T. HIV infection is blocked in vitro by recombinant soluble CD4. Nature. 1988 Jan 7;331(6151):76–78. doi: 10.1038/331076a0. [DOI] [PubMed] [Google Scholar]
- Frade R., Barel M., Ehlin-Henriksson B., Klein G. gp140, the C3d receptor of human B lymphocytes, is also the Epstein-Barr virus receptor. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1490–1493. doi: 10.1073/pnas.82.5.1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller A. O., Spear P. G. Specificities of monoclonal and polyclonal antibodies that inhibit adsorption of herpes simplex virus to cells and lack of inhibition by potent neutralizing antibodies. J Virol. 1985 Aug;55(2):475–482. doi: 10.1128/jvi.55.2.475-482.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy J. E., McKeating J. A., Griffiths P. D. Cytomegalovirus strain AD169 binds beta 2 microglobulin in vitro after release from cells. J Gen Virol. 1987 Mar;68(Pt 3):777–784. doi: 10.1099/0022-1317-68-3-777. [DOI] [PubMed] [Google Scholar]
- Grundy J. E., McKeating J. A., Ward P. J., Sanderson A. R., Griffiths P. D. Beta 2 microglobulin enhances the infectivity of cytomegalovirus and when bound to the virus enables class I HLA molecules to be used as a virus receptor. J Gen Virol. 1987 Mar;68(Pt 3):793–803. doi: 10.1099/0022-1317-68-3-793. [DOI] [PubMed] [Google Scholar]
- Iida K., Nadler L., Nussenzweig V. Identification of the membrane receptor for the complement fragment C3d by means of a monoclonal antibody. J Exp Med. 1983 Oct 1;158(4):1021–1033. doi: 10.1084/jem.158.4.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson D. C., Burke R. L., Gregory T. Soluble forms of herpes simplex virus glycoprotein D bind to a limited number of cell surface receptors and inhibit virus entry into cells. J Virol. 1990 Jun;64(6):2569–2576. doi: 10.1128/jvi.64.6.2569-2576.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keay S., Merigan T. C., Rasmussen L. Identification of cell surface receptors for the 86-kilodalton glycoprotein of human cytomegalovirus. Proc Natl Acad Sci U S A. 1989 Dec;86(24):10100–10103. doi: 10.1073/pnas.86.24.10100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klatzmann D., Champagne E., Chamaret S., Gruest J., Guetard D., Hercend T., Gluckman J. C., Montagnier L. T-lymphocyte T4 molecule behaves as the receptor for human retrovirus LAV. Nature. 1984 Dec 20;312(5996):767–768. doi: 10.1038/312767a0. [DOI] [PubMed] [Google Scholar]
- Ligas M. W., Johnson D. C. A herpes simplex virus mutant in which glycoprotein D sequences are replaced by beta-galactosidase sequences binds to but is unable to penetrate into cells. J Virol. 1988 May;62(5):1486–1494. doi: 10.1128/jvi.62.5.1486-1494.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little S. P., Jofre J. T., Courtney R. J., Schaffer P. A. A virion-associated glycoprotein essential for infectivity of herpes simplex virus type 1. Virology. 1981 Nov;115(1):149–160. doi: 10.1016/0042-6822(81)90097-0. [DOI] [PubMed] [Google Scholar]
- Macher A. M., Reichert C. M., Straus S. E., Longo D. L., Parrillo J., Lane H. C., Fauci A. S., Rook A. H., Manischewitz J. F., Quinnan G. V., Jr Death in the AIDS patient: role of cytomegalovirus. N Engl J Med. 1983 Dec 8;309(23):1454–1454. doi: 10.1056/NEJM198312083092312. [DOI] [PubMed] [Google Scholar]
- Marsh M., Helenius A. Virus entry into animal cells. Adv Virus Res. 1989;36:107–151. doi: 10.1016/S0065-3527(08)60583-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menezes J., Seigneurin J. M., Patel P., Bourkas A., Lenoir G. Presence of Epstein-Barr virus receptors, but absence of virus penetration, in cells of an Epstein-Barr virus genome-negative human lymphoblastoid T line (Molt 4). J Virol. 1977 Jun;22(3):816–821. doi: 10.1128/jvi.22.3.816-821.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mettenleiter T. C., Zsak L., Zuckermann F., Sugg N., Kern H., Ben-Porat T. Interaction of glycoprotein gIII with a cellular heparinlike substance mediates adsorption of pseudorabies virus. J Virol. 1990 Jan;64(1):278–286. doi: 10.1128/jvi.64.1.278-286.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muilerman H. G., ter Hart H. G., Van Dijk W. Specific detection of inactive enzyme protein after polyacrylamide gel electrophoresis by a new enzyme-immunoassay method using unspecific antiserum and partially purified active enzyme: application to rat liver phosphodiesterase I. Anal Biochem. 1982 Feb;120(1):46–51. doi: 10.1016/0003-2697(82)90315-3. [DOI] [PubMed] [Google Scholar]
- Nemerow G. R., Mullen J. J., 3rd, Dickson P. W., Cooper N. R. Soluble recombinant CR2 (CD21) inhibits Epstein-Barr virus infection. J Virol. 1990 Mar;64(3):1348–1352. doi: 10.1128/jvi.64.3.1348-1352.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemerow G. R., Wolfert R., McNaughton M. E., Cooper N. R. Identification and characterization of the Epstein-Barr virus receptor on human B lymphocytes and its relationship to the C3d complement receptor (CR2). J Virol. 1985 Aug;55(2):347–351. doi: 10.1128/jvi.55.2.347-351.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinnan G. V., Jr, Masur H., Rook A. H., Armstrong G., Frederick W. R., Epstein J., Manischewitz J. F., Macher A. M., Jackson L., Ames J. Herpesvirus infections in the acquired immune deficiency syndrome. JAMA. 1984 Jul 6;252(1):72–77. [PubMed] [Google Scholar]
- Rand K. H., Pollard R. B., Merigan T. C. Increased pulmonary superinfections in cardiac-transplant patients undergoing primary cytomegalovirus infection. N Engl J Med. 1978 Apr 27;298(17):951–953. doi: 10.1056/NEJM197804272981705. [DOI] [PubMed] [Google Scholar]
- Sarmiento M., Haffey M., Spear P. G. Membrane proteins specified by herpes simplex viruses. III. Role of glycoprotein VP7(B2) in virion infectivity. J Virol. 1979 Mar;29(3):1149–1158. doi: 10.1128/jvi.29.3.1149-1158.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenberg R. M., Depto A. S., Fortney J., Nelson J. A. Regulated expression of early and late RNAs and proteins from the human cytomegalovirus immediate-early gene region. J Virol. 1989 Jun;63(6):2699–2708. doi: 10.1128/jvi.63.6.2699-2708.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenberg R. M., Thomsen D. R., Stinski M. F. Structural analysis of the major immediate early gene of human cytomegalovirus. J Virol. 1984 Jan;49(1):190–199. doi: 10.1128/jvi.49.1.190-199.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinski M. F. Human cytomegalovirus: glycoproteins associated with virions and dense bodies. J Virol. 1976 Aug;19(2):594–609. doi: 10.1128/jvi.19.2.594-609.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinski M. F., Thomsen D. R., Stenberg R. M., Goldstein L. C. Organization and expression of the immediate early genes of human cytomegalovirus. J Virol. 1983 Apr;46(1):1–14. doi: 10.1128/jvi.46.1.1-14.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor H. P., Cooper N. R. Human cytomegalovirus binding to fibroblasts is receptor mediated. J Virol. 1989 Sep;63(9):3991–3998. doi: 10.1128/jvi.63.9.3991-3998.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor H. P., Cooper N. R. The human cytomegalovirus receptor on fibroblasts is a 30-kilodalton membrane protein. J Virol. 1990 Jun;64(6):2484–2490. doi: 10.1128/jvi.64.6.2484-2490.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M., Reimann K. A., DeLong P. A., Liu T., Fisher R. A., Letvin N. L. Effect of recombinant soluble CD4 in rhesus monkeys infected with simian immunodeficiency virus of macaques. Nature. 1989 Jan 19;337(6204):267–270. doi: 10.1038/337267a0. [DOI] [PubMed] [Google Scholar]
- Weis J. J., Tedder T. F., Fearon D. T. Identification of a 145,000 Mr membrane protein as the C3d receptor (CR2) of human B lymphocytes. Proc Natl Acad Sci U S A. 1984 Feb;81(3):881–885. doi: 10.1073/pnas.81.3.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wentworth B. B., French L. Plaque assay of cytomegalovirus strains of human origin. Proc Soc Exp Biol Med. 1970 Nov;135(2):253–258. doi: 10.3181/00379727-135-35031. [DOI] [PubMed] [Google Scholar]
- White J. M., Littman D. R. Viral receptors of the immunoglobulin superfamily. Cell. 1989 Mar 10;56(5):725–728. doi: 10.1016/0092-8674(89)90674-0. [DOI] [PubMed] [Google Scholar]
- WuDunn D., Spear P. G. Initial interaction of herpes simplex virus with cells is binding to heparan sulfate. J Virol. 1989 Jan;63(1):52–58. doi: 10.1128/jvi.63.1.52-58.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler-Heitbrock H. W., Thiel E., Fütterer A., Herzog V., Wirtz A., Riethmüller G. Establishment of a human cell line (Mono Mac 6) with characteristics of mature monocytes. Int J Cancer. 1988 Mar 15;41(3):456–461. doi: 10.1002/ijc.2910410324. [DOI] [PubMed] [Google Scholar]