Abstract
Contrast-enhanced (CE) MRI was used to monitor breast cancer response to neoadjuvant chemotherapy. Patients underwent CE MRI before and after therapy, together with conventional assessment methods (CAM). CE MRI was carried out at 1.5 T in the coronal plain with 3D sequences before and after bolus injection. An expert panel determined chemotherapy response using both CE MRI and CAM. Histopathological response in the surgical specimen was then used to determine the sensitivity and specificity of CE MRI and CAM. In total, 67 patients with 69 breast cancers were studied (mean age of 46 years). Tumour characteristics showed a high-risk tumour population: median size 49 mm: histopathological grade 3 (55%): oestrogen receptor (ER) negative (48%). Histopathological response was as follows: – complete pathological response (pCR) 17%; partial response (pPR) 68%; no response (NR) 15%. Sensitivity of CAM for pCR or pPR was 98% (CI 91–100%) and specificity was 50% (CI 19–81%). CE MRI sensitivity was 100% (CI 94–100%), and specificity was 80% (CI 44–97%). The absolute agreement between assessment methods and histopathology was marginally higher for CE MRI than CAM (81 vs 68%; P=0.09). In 71%, CE MRI increased diagnostic knowledge, although in 20% it was judged confusing or incorrect. The 2nd MRI study significantly increased diagnostic confidence, and in 19% could have changed the treatment plan. CE MRI persistently underestimated minimal residual disease. In conclusion, CE MRI of breast cancer proved more reliable for predicting histopathological response to neoadjuvant chemotherapy than conventional assessment methods.
Keywords: breast cancer, breast MRI, disease response, diagnostic impact, neoadjuvant chemotherapy
Breast cancer treatment has advanced over the past two decades, achieving a better outcome for women (Peto et al, 2000). One of several factors contributing to improved survival is chemotherapy, which is now widely used particularly for women under 50 years and those with high-grade ER negative or lymph node-positive tumours. When chemotherapy is given prior to definitive surgical treatment, there are various potential benefits (Valero et al, 2002):
Primary tumour response provides evidence of responsiveness to the chosen chemotherapeutic agent and predicts local control (Chollet et al, 2002)
A large tumour may become amenable to breast-conserving surgery (Makris et al, 1997; Fisher et al, 1998)
An ineffective therapeutic agent may be changed to one with clinical effect, or surgery may be advanced, thereby saving cost and patient suffering (Sotiriou et al, 2002; Pusztai et al, 2003)
There is no evidence of benefit in overall or disease-free survival as a result of the use of chemotherapy in the neoadjuvant setting (Fisher et al, 1998; Green and Hortobagyi, 2002). Marginally statistically significant treatment-by-age interactions appear to be emerging for survival and disease-free survival, suggesting that younger patients may benefit from preoperative therapy, whereas the reverse may be true for older patients (Wolmark et al, 2001). It is logical to initiate systemic treatment early in those patients with a high risk of micro-metastatic disease and to monitor the response. Conventional tools to monitor response include measurement of maximum tumour diameter with callipers; loss of signs of inflammation and reduction in lymph node size. Serial core biopsy has been used to show changes in tissue morphology by standard histological criteria and more recently with molecular markers (Sotiriou et al, 2002; Pusztai et al, 2003). Response of the primary tumour or regional nodes may be monitored radiologically with mammography or ultrasound (Helvie et al, 1996; Vinnicombe et al, 1996; Huber et al, 2000); and newer imaging modalities (Mankoff et al, 1999; Tiling et al, 2001), such as contrast-enhanced (CE) MRI are being increasingly used. The literature contains many single institution reports of the use of breast MRI for monitoring tumour response or predicting tumour extent prior to surgery. Table 1 summarises the literature to date on the use of breast MRI to monitor neoadjuvant chemotherapy. It can be seen from the right-hand column of this table that the findings have varied very substantially. Breast MRI appears to hold promise for this purpose, and provides measurements that are more accurate than clinical or ultrasound assessment (Davis et al, 1996; Esserman et al, 1999). It is important to know whether predictions of residual tumour are accurate. Breast MRI has good sensitivity for invasive tumour but relatively poor specificity in untreated disease (Mussurakis et al, 1996). The limitations are more apparent for invasive lobular carcinoma and in situ (Kinkel and Hylton, 2001). Contrast-uptake characteristics have been shown to be altered by chemotherapy, which may potentially diminish sensitivity and impair specificity (Rieber et al, 1997). Contrast uptake characteristics have been used to give an early indication of tumour response using innovative functional MR and computer methods (Padhani, 2002; Delille et al, 2003). It is therefore an open question whether CE MRI would be helpful in monitoring response, or whether limitations emerging from studies undertaken concurrent with this one would make it unreliable.
Table 1. Existing literature on the use of breast MRI to monitor response to chemotherapy.
| Author | Origin | Year | Number | Measurement | Pathology response |
|---|---|---|---|---|---|
| Knopp et al (1994) | Heidelberg, Germany | 1994 | Three case studies | Promising prediction | |
| Gilles et al (1994) | Villejuif, France | 1994 | 18 | (15 out of 18) 83% Highly predictive of residual tumour | |
| Abraham et al (1996) | Dallas & Little Rock, USA | 1996 | 39 | MRI correctly predicted residual disease in 30 out of 31 (97%) | |
| Rieber et al (1997) | Ulm, Germany | 1997 | 13 | Underestimates residual tumour | |
| Trecate et al (1998) | Milano, Italy | 1998 | 27 | Valid tool for monitoring response | |
| Tsuboi et al (1999) | Kochi, Japan | 1999 | 31 | Valuable for predicting response and margins | |
| Weatherall et al (2001) | Dallas, TX, USA | 2001 | 22 | Cc0.93MR, 0.63 mam | Accurate assessment of residual tumour |
| Nakamura et al (2001) | Tokyo, Japan | 2001 | 15 | Predict possibility of WLE. Mapping of dendritic tumours | |
| Drew et al (2001) | Hull, UK | 2001 | 17 | Sensitivity 100% | Accurate method of predicting residual tumour |
| Esserman et al (2001) | UCSF | 2001 | 33 | Patterns of tumours identified | |
| Partridge et al (2002) | UCSF | 2002 | 52 | R0.89, C0.60 | MRI detected all residual disease |
| Balu-Maestro et al (2002) | Nice, France | 2002 | 60 in 51 women | Valuable tool for size & multifocality prior to surgery | |
| Rieber et al (2002) | Ulm, Germany | 2002 | 58 | Accuracy NR 83.3%, PR 82.4% | Good for assessing response to chemo, unreliable in CR cases where 66.7% had residual tumour |
| Cheung et al (2003) | Kwei San, Taiwan | 2003 | 33 | Useful to assess early response and predict tumour size change | |
| Delille et al (2003) | Harvard, USA | 2003 | 14 | EFPcount accurate measure (P<.02) | Extraction flow product as marker of tumour response |
In this study, we have investigated the impact of breast MRI on decision-making. Almost all of our patients underwent mastectomy after neoadjuvant chemotherapy. It is therefore possible to correlate the extent of tumour detectable on breast MRI following chemotherapy with histopathology of the mastectomy specimen. The aims of our study were:
To determine whether CE MRI is an accurate indicator of tumour response to neoadjuvant chemotherapy in comparison to the histopathology gold standard.
To assess whether CE MRI increases the diagnostic confidence above that achieved by conventional assessment methods (CAM) (i.e. clinical examination, ultrasound and mammography)
To determine whether CE MRI can accurately identify a subset of patients who might be suitable for breast-conserving surgery following neoadjuvant chemotherapy.
PATIENTS AND METHODS
Patients and chemotherapy
The study had approval from the Local Research Ethics Committee for Cambridgeshire, and all women had given informed consent to participate. It is a retrospective review of 67 women with CE-MRI before and after neoadjuvant chemotherapy for breast cancer. The first 30 women were recruited between 1997 and 2001 to a prospective pilot study of CE MRI. The remaining 37 women were consecutively enroled once CE MRI monitoring became the standard of care at our centre in 2001. The decision to give neoadjuvant therapy was made by a multidisciplinary team, and in general included women less than 60 years with large, inflammatory or high-grade tumours. Chemotherapeutic regimens used in this series varied according to whether patients were treated in ongoing clinical trials (Appendix A). All cases had undergone mammography, ultrasound and core biopsy prior to the decision to treat with neoadjuvant chemotherapy, and the results of these tests were used by the team to determine which trials they should enter.
Table A1. Chemotherapy regimens in use.
| n | |
|---|---|
| Doxorubicin/cyclophosphamide six cycles | 41 |
| Doxorubicin/docetaxel six cycles | 21 |
| Epirubicin four cycles then CMF four cycles | 2 |
| Epirubicin four cycles | 2 |
| Docetaxel/Herceptin | 1 |
| Total | 67 |
Clinical trials (1997–2003): Anglo-Celtic 2 Study: doxorubicin/cyclophosphamide vs doxorubicin/docetaxel six cycles (Evans et al, 2002). _ Docetaxel/Herceptin expanded access phase 4 assessment (Howell et al, 2002). Standard off study treatment: doxorubicin/cyclophosphamide six cycles.
Contrast enhanced MRI (CE MRI)
The patients had two MRI studies, at the start of treatment and prior to surgery. The initial MRI study was usually undertaken prior to the first course of chemotherapy except in occasional cases where limitations of access to MRI resulted in imaging after the first treatment had been given. The 2nd MRI study was performed after all chemotherapy and prior to surgery. The studies were undertaken on a 1.5 T magnet from GE Signa Echospeed, which was upgraded in 2002 with Excite software. The MRI technique is similar to that described for the MARIBS study (Brown et al, 2000), with the exceptions that the dose of gadolinium was 0.16 mmol kg−1 body weight, and no proton density study was undertaken in our study (Scanning protocol – Appendix B). MRI interpretation used a synthesis of dynamic and morphological features, including the pattern of contrast uptake and washout characteristics, and the studies were examined at the console to allow dynamic contrast enhancement curves at any point in the 3D volume of the dynamic acquisition. The studies were reported by radiologists and presented to the multidisciplinary team for decisions on patient management.
Table B1. MR pulse sequence protocol for a breast MR examination in use for this study.
| Sequence type | Repetition time TR (ms) | Echo time TE (MS) 1.5 T | Flip angle (0) | Matrix size | Number of slices | Field of view (FOV) (MM) | Pixel size (mm x mm) | Number of averages | Acquisition time (min:s) |
|---|---|---|---|---|---|---|---|---|---|
| Precontrast, high-resolution 3D T1-weighted | 8.9 | 4.2 | 35 | 512 × 384 | 60 | 340 | 0.66 × 0.89 | 1 | 2 |
| Dynamic contrast enhanced, 3D T1-weighteda 2 sequences before contrast injection and 6 after | 8.8 | 4.2 | 35 | 256 × 256b | 480 | 340 | 1.33 × 1.33b | 1 | 8 × 1.10 |
| Postcontrast, high-resolution 3D T1-weighted with fat suppression | 18.9 | 4.2 | 35 | 512 × 384 | 60 | 340 | 0.66 × 0.89 | 1 | 5 |
Contrast injection was 0.16 mmol kg−1 body weight with an injection speed of 3 ml s−1, given after the second precontrast dynamic sequence.
The matrix size is optimised to ensure that the acquisition time of each 3D image is 70 s.
Conventional methods of assessment
All patients had a clinical assessment prior to each of six cycles of chemotherapy. This included calliper measurement of tumour size, degree of inflammation and size of palpable axillary nodes. All patients underwent mammography, ultrasound and core biopsy for diagnosis. Ultrasound was carried out after three and six cycles of chemotherapy. Conventional breast imaging comprised mammography, ultrasound and core biopsy according to the guidelines of the United Kingdom National Health Service Breast Screening Programme (NHSBSP) (Wilson et al, 2001).
Surgery
Surgery normally took place less than 4 weeks after the last course of chemotherapy. All patients underwent either mastectomy or wide local excision (WLE) with axillary lymph node dissection.
Histopathological response
Response to treatment was scored by standard UICC (Therasse et al, 2000) categories of Complete Response (CR), Partial Response (PR), Non-Response (NR), Disease Progression (DP). The reports were produced at the time of clinical care without particular knowledge of the MRI findings. It should be noted that after chemotherapy the criteria for grading cannot be applied, and so when grades are given here they are based on core biopsy (Harris et al, 2003).
There are no recognised standard criteria for grading of histological response following primary chemotherapy. In this study, mastectomy specimens were handled according to the NHSBSP guidelines (NHSBSP publication No. 3, 1995). In addition, in those cases where no macroscopic tumour could be identified, extensive sampling of the original tumour site (as identified by imaging methods and clinical examination prior to treatment) was performed.
On histological examination, where distinct in situ and invasive tumour was identified this was morphologically compared with tumour seen on the original core biopsy. The ratio of tumour cell mass to surrounding tissue was evaluated. If degenerative changes within tumour cells were present this was noted. The nature of the tissue surrounding tumour cells was noted, viz was it a cellular stroma similar to that seen in the original biopsy or was it inflamed, hypocellular scar tissue.
Thus:
No histological response. Tumour resembling that in the original core biopsy was present and it extended within the breast tissue for the same or a greater distance than originally estimated by imaging and clinical examination. The nature of the tissue surrounding the tumour was cellular and did not resemble bland scar tissue.
Complete histological response. Absence of invasive carcinoma cells/or <1single, degenerate carcinoma cell/10 high-power fields. Absence of in situ carcinoma.
Partial histological response. Tumour resembling that in the original core biopsy but which was considerably reduced in size (it is necessary to approximate size parameters used in clinical response staging). Presence of residual microscopic foci of invasive carcinoma but with reduced tumour cell/stroma ratio or residual pure in situ carcinoma.
Assessment of sensitivity, specificity of conventional assessment methods and CE MRI
Original records were used to score clinical response using CAM, CE MRI response and histopathological response, and from these sensitivity and specificity were calculated. Pathological response was regarded as the gold standard against which all evaluations of response were measured.
Assessment of diagnostic impact and confidence of knowledge
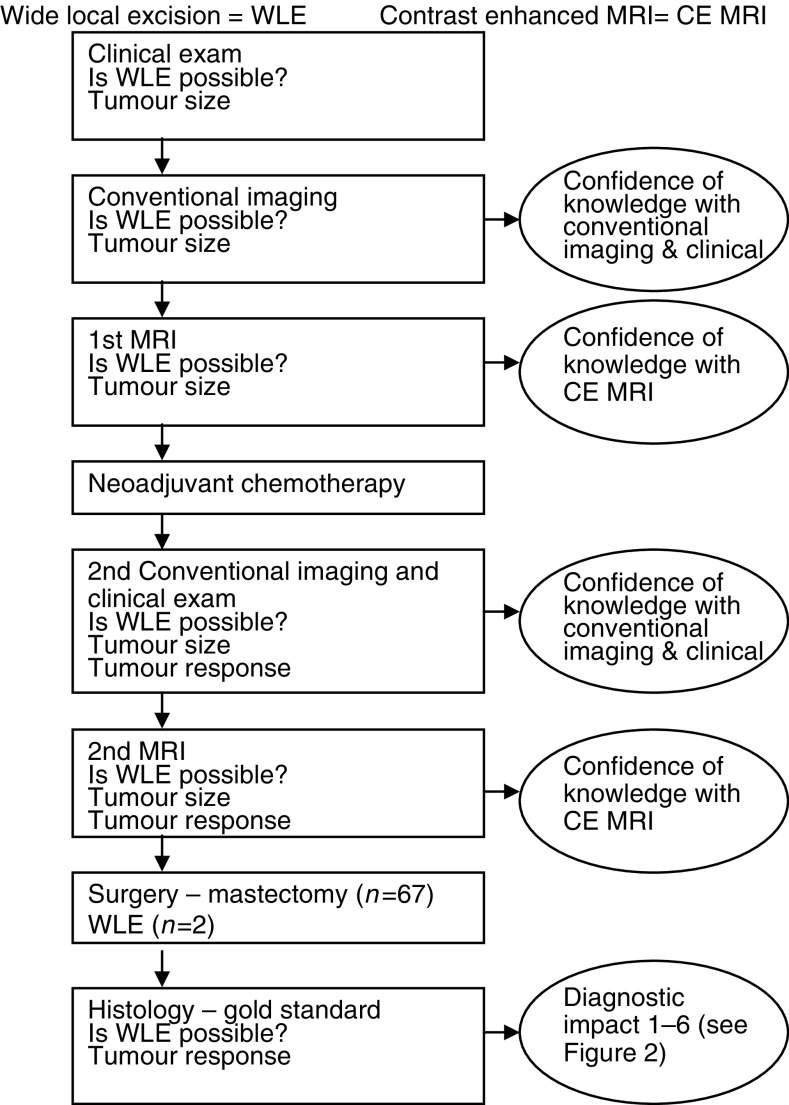
For the purposes of this study, all cases were reviewed by the multidisciplinary team and a research form completed to record the diagnostic impact. The review team consisted at minimum of a radiologist, a surgeon, an oncologist and a histopathologist, all expert in management of breast disease. The multidisciplinary review procedure is shown in Figure 1. The clinical notes were examined for the clinical findings recorded at the time of clinical care. The mammograms and ultrasound images with their original reports were presented as in a standard multidisciplinary team meeting. The MRI studies were presented to the clinical team using the images and reports taken at the time of clinical care of the patients. The multidisciplinary team were unaware of the histopathology findings when they interpreted the results of the MRI and other presurgical examinations. The pathology was presented from the histological report at the time of clinical care, and did not take particular account of MRI knowledge. The added value of MRI imaging was given a score 1…6 (Figure 2) by the team, taking into account the additional contribution made by the MRI study beyond that available with conventional breast imaging (Shuman et al, 1985a,1985b). A visual analogue scale was used to record confidence of knowledge with conventional breast imaging alone and with the addition of MRI (Blanchard et al, 1997). Confidence of knowledge included knowledge of the size, the contour, the extent and the suitability for any form of surgery. The two measures, added value and confidence of knowledge are complementary, since confidence of knowledge is an important aspect of value to a clinician. These are subjective clinical judgements.
Figure 1.
Algorithm for recording findings in the multidisciplinary meeting.
Figure 2.
Value of including MRI imaging.
Statistical analyses
Proportions in independent samples were compared using Fisher's exact test or linear by linear χ2 test as appropriate. Parametric and nonparametric paired comparisons of mean and median values were performed using the paired t-test and McNemar test, respectively. The relationship between initial tumour size and subsequent tumour response was assessed using one-way analysis of variance. Statistical significance was accepted at the conventional P<0.05 threshold.
RESULTS
The study evaluated 69 cancers in 67 women including two bilateral cases. In all, 67 cancers were treated with mastectomy and two with WLE. The mean age of the patients at the time of the 1st MRI was 46.2 (range 28 to 62 years). On initial radiological examination, the median size of the 69 tumours was 49.0 mm (range 9–100 mm). The core biopsy demonstrated that six (9%) tumours were grade 1, 25 (36%) grade 2 and 38 (55%) grade 3. These therefore represent relatively large, poor prognosis cancers in younger women. In total, 36 (52%) tumours were oestrogen receptor (ER) positive, and 33 (48%) ER negative, 21 out of 26 (81%) patients where the progesterone receptor (PR) status was recorded were PR negative. At definitive surgery, patients had a median of 13 nodes retrieved (range 0–42); 17 patients (25%) had more than four nodes involved, 23 (33%) had between one and four nodes involved, and 29 (42%) were node negative. In all, 12 (17%) patients presented with inflammatory cancers, 31 (45%) were known to be multifocal or diffuse lesions by their imaging or clinical findings, and a further 16 (23%) lesions were close to the nipple–areolar complex. After the initial clinical and radiological evaluation, a total of 52 (75%) tumours were considered not suitable for breast conservation within the protocols of our unit, where skin involvement by inflammation, very large size, multifocality and proximity to the nipple–areolar complex are all viewed as reasons for not using breast-conserving surgery. The median period between the 1st and 2nd MR imaging was 119 days (range 48–165 days). The median period between the 2nd MR and surgery was 24 days (range 2–77 days). The neoadjuvant chemotherapy included cases in trials, notably Anglo-Celtic 2, and cases off trial with a variety of regimens (Appendix A).
Estimation of response
Based on the gold standard histopathology findings, 12 lesions (17%) had complete response, 47 (68%) had partial response, 10 (15%) had no response following neoadjuvant chemotherapy (Table 2 ). There was no significant association between the grade of the tumour and the extent of disease response following chemotherapy. In total, 24% (nine out of 38) of grade 3 tumours had complete response to chemotherapy compared to 12% (three out of 25) of grade 2 tumours and 0% (zero out of six) of grade 1 tumours (P=0.10). Four out of 36 (11%) ER-positive tumours exhibited complete response at pathology compared to six out of 21 (29%) of tumours that were ER- and PR negative (P=0.15). Lesions that did not respond or progressed after neoadjuvant chemotherapy were slightly, but nonsignificantly, larger than lesions that exhibited partial or complete response (mean size 56 vs 49 vs 48 mm; P=0.56). Tumour response was clearly related to the extent of node involvement. In all, 97% (28 out of 29) of lesions with no node involvement had complete or partial response compared to 87% (20 out of 23) of lesions with between one and four positive nodes and 65% (11/17) lesions with more than four involved nodes (P<0.01).
Table 2. Comparison of clinical assessment with ultrasound and mammography and pathology rating of tumour response.
|
Conventional assessment methods (CAM) Clinical examination+mammography and ultrasound |
|||||
|---|---|---|---|---|---|
| Complete response | Partial response | No response | Disease progression | ||
| Pathology findings | Complete response | 9 | 3 | 0 | 0 |
| Partial response | 13 | 33 | 1 | 0 | |
| No response | 1 | 4 | 5 | 0 | |
| Disease progression | 0 | 0 | 0 | 0 | |
Conventional assessment methods and MRI in the estimation of tumour response
The clinical assessment of tumour response agreed with the pathology assessment in 47 out of 69 tumours (68%) (Table 2). CAM underestimated the degree of response in four cases, and overestimated the degree of response in 18 cases. In one case, a tumour which was categorised as completely responsive based on the clinical data had no response evident at pathological examination. CAM correctly identified 58 out of 59 lesions with complete or partial response based on pathology findings (sensitivity 98%; 95% confidence interval (CI) 91–100%). However, five out of 10 tumours with no response were incorrectly categorised as complete or partial response on the clinical assessment (specificity 50%; CI 19–81%).
There was agreement between MRI findings and the pathology findings in 56 out of 69 lesions (81%) (Table 3 ). The imaging findings underestimated the effect of chemotherapy in one case where disease appeared to have progressed on CE MRI, but where pathology results indicated no response. In a further 12 cases, MRI overestimated the response to therapy. The sensitivity (100%; CI 94–100%) and specificity (80%; CI 44–97%) of MRI for identifying complete or partially responsive tumours were higher than for the clinical assessment alone. The absolute agreement between MRI and pathology findings was marginally higher than the agreement between clinical assessment and pathology findings (81 vs 68%; P=0.09).
Table 3. Comparison of MRI and pathology rating of tumour response.
|
CE MRI findings |
|||||
|---|---|---|---|---|---|
| Complete response | Partial response | No response | Disease progression | ||
| Pathology findings | Complete response | 12 | 0 | 0 | 0 |
| Partial response | 10 | 37 | 0 | 0 | |
| No response | 1 | 1 | 7 | 1 | |
| Disease progression | 0 | 0 | 0 | 0 | |
Diagnostic confidence and therapeutic impact after MRI
In the majority of cases, the 2nd MRI study was judged to increase diagnostic knowledge (>70% see Table 4 ). However, in a substantial proportion of cases (17% see Table 4) the 2nd MRI was judged to have reduced diagnostic knowledge. These 12 cases have been examined in greater detail – see Table 5A .
Table 4. Potential diagnostic and therapeutic impact of 2nd MRI.
| Impact of MRI | N (%) |
|---|---|
| Diagnostic knowledge with 2nd MRI | |
| Better | 49 (71%) |
| Same | 8 (12%) |
| Worse | 12 (17%) |
| Diagnostic value of 2nd MRI | |
| Confusing or incorrect – extra studies required | 0 (0%) |
| Confusing or incorrect – no extra studies | 14 (20%) |
| No change in treatment plan | 6 (9%) |
| No change in treatment plan – increased diagnostic confidence | 36 (52%) |
| Could have caused change in treatment plan | 10 (15%) |
| Caused change in the treatment plan | 3 (4%) |
| Diagnostic confidence of 2nd MRI | |
| Without 2nd MRI (mean (s.d.)) | 5.8 (1.8) |
| With 2nd MRI (mean (s.d.)) | 7.9 (2.0)* |
| Therapeutic impact of 2nd MRI | |
| WLE possible based on surgery | 22/69 |
| MRI agrees with conventional assessment, WLE possible | 10 (45%) |
| MRI agrees with conventional assessment, WLE NOT possible | 5 (23%) |
| MRI disagrees with conventional assessment, suggests WLE possible | 5 (23%) |
| MRI disagrees with conventional assessment, suggests WLE NOT possible | 2 (9%) |
| WLE NOT possible based on surgery | 47/69 |
| MRI agrees with conventional assessment, WLE possible | 4 (9%) |
| MRI agrees with conventional assessment, WLE NOT possible | 35 (75%) |
| MRI disagrees with conventional assessment, suggests WLE possible | 4 (9%) |
| MRI disagrees with conventional assessment, suggests WLE NOT possible | 4 (9%) |
P<0.01, paired samples t-test for the difference in confidence pre- and post-2nd MRI.
Table 5. Cases in which the 2nd MRI study (A) reduced diagnostic knowledge n= 12 and (B) either did or had the potential to alter the treatment plan n=13.
| Case | Age, years | Clinical size, mm at start of treatment | Grade | ER | Quadrants involved | Spaced out cells present | DCIS present | cR | mR | pR | Comments |
|---|---|---|---|---|---|---|---|---|---|---|---|
| (A) | |||||||||||
| 1a,b | 46 | 50 | 1 | + | Multifoc | No | No | 1 | 1 | 2 | Underestimates residual tumour |
| 2a,b | 51 | Unmeasurable | 2 | + | Unifocal | No | No | 2 | 1 | 2 | Underestimates residual tumour |
| 6a,b | 47 | 60+ | 3 | + | Subareol | No | Yes | 2 | 1 | 2 | Underestimates residual tumour ILC |
| 8b | 41 | Unmeasurable | 3 | − | Diffuse | No | Yes | 1 | 2 | 2 | Underestimates residual tumour |
| 13b | 54 | 40 | 2 | + | Unifocal | No | Yes | 1 | 1 | 2 | Underestimates residual tumour |
| 25b | 39 | 60+ | 3 | − | Diffuse | No | Yes | 1 | 1 | 3 | Underestimates residual tumour |
| 29a,b | 34 | 60+ | 3 | − | Subareol | No | Yes | 1 | 1 | 1 | No contrast – failed injection CR report |
| 30a | 49 | 60+ | 2 | + | Unifocal | Yes | Yes | 2 | 1 | 2 | Underestimates residual tumour DCIS |
| 31a,b | 51 | 60+ | 2 | + | Multifoc | No | Yes | 2 | 2 | 2 | Underestimates residual tumour |
| 32b | 40 | 45 | 3 | − | Multifoc | No | No | 2 | 2 | 2 | Underestimates residual tumour |
| 34a | 43 | 30 | 3 | − | Unifocal | No | Yes | 2 | 1 | 1 | Residual DCIS |
| 41a,b | 34 | 60+ | 2 | + | Multifoc | No | Yes | 1 | 2 | 2 | Underestimates residual tumour ILC |
| 61a,b | 47 | 60+ | 1 | + | Multifoc | No | Yes | 2 | 1 | 2 | Underestimates residual tumour |
| 63a,b | 60 | Unmeasurable | 3 | + | Diffuse | No | Yes | 2 | 2 | 3 | Underestimates residual tumour |
| 66a,b | 47 | 45 | 3 | + | Subareol | No | Yes | 2 | 2 | 2 | Underestimates residual tumour |
| 78a,b | 28 | 60+ | 2 | − | Unifocal | Yes | No | 2 | 1 | 2 | Underestimates residual tumour |
| (B) | |||||||||||
| 7 | 52 | Unmeasurable | 2 | − | Unifocal | Yes | Yes | 1 | 1 | 2 | Could have endorsed WLE |
| 14 | 49 | 40 | 3 | − | Unifocal | No | Yes | 2 | 2 | 2 | Better estimate of residual tumour |
| 21 | 47 | 60+ | 2 | + | Subareol | No | Yes | 3 | 2 | 2 | Could have endorsed WLE |
| 34 | 43 | 30 | 2 | − | Unifocal | No | Yes | 2 | 1 | 1 | Could have endorsed WLE DCIS only |
| 37 | 57 | 54 | 1 | − | Multifoc | No | No | 1 | 1 | 1 | Could have endorsed WLE |
| 44 | 56 | 40 | 3 | − | Unifocal | No | No | 1 | 2 | 2 | Confirmed residual tumour |
| 52 | 43 | 50 | 2 | + | Subareol | No | Yes | 2 | 3 | 3 | Showed nonresponder. Skin thickening |
| 55 | 43 | 30 | 3 | + | Unifocal | No | Yes | 1 | 1 | 1 | Could have endorsed WLE |
| 59 | 52 | 40 | 3 | + | Unifocal | Yes | Yes | 1 | 1 | 1 | Could have endorsed WLE |
| 62bilat | 49 | 60+ | 2 | + | Diffuse | No | Yes | 2 | 2 | 2 | Contralateral tumour shown |
| 67 | 33 | 32 | 3 | + | Multifoc | No | No | 2 | 2 | 2 | Did not confirm multifocality suspected on ultrasound. Could have endorsed WLE |
| 71 | 37 | 55 | 2 | − | Subareol | No | No | 1 | 1 | 1 | Could have endorsed WLE |
Grade Bloom & Richardson grades 1, 2 & 3 (Elston and Ellis, 1991) oestrogen receptor=ER positive (+), negative (−).
Ductal carcinoma in situ=DCIS, invasive lobular carcinoma=ILC. Quadrants – unifocal, multifocal, diffuse, subareolar position.
cR=clinical response, mR=MRI response, pR=pathological response 1=complete response, 2=partial response, 3=static disease, 4=disease progression. Wide local excision WLE.
Cases in which the 2nd MRI study reduced diagnostic knowledge n=12.
Cases in which the MRI findings were thought to be confusing or incorrect n=14.
In the majority of patients the 2nd MRI study did not change the treatment plan but did increase diagnostic confidence (52%, Table 4). This confidence was clearly valued by clinicians. Again, in a substantial minority of cases (20%, Table 4) the MRI findings were thought to be confusing or incorrect. In the majority of these cases, the problem stemmed from the MRI study underestimating the amount of residual tumour by suggesting a complete response when, in fact, there were residual invasive tumour cells or DCIS. These cases have been examined more closely and the findings set out in Table 5A. In a further 19% of cases (Table 4) the 2nd MRI either did, or had the potential to alter the treatment plan. These cases have been summarised in Table 5b. The reasons included a contralateral nonpalpable tumour, complete response that could have allowed breast conservation, demonstration of nonresponse, demonstration of a single focus where multifocality was suspected. The mean diagnostic confidence for all patients significantly increased from 5.8 pre-2nd MRI to 7.9 post-2nd MRI (P<0.01; Table 4).
Surgical findings indicated that WLE would not have been possible following neoadjuvant chemotherapy in 47 out of 69 lesions (Table 4). In 35 out of these 47 cases, both conventional assessment and MRI correctly predicted the need for mastectomy. The remaining 12 cases were evenly split between cases where conventional assessment incorrectly predicted WLE was possible (4), cases where MRI incorrectly predicted WLE was possible (4), and cases were both presurgery evaluations incorrectly suggested that breast-conserving surgery was possible (4).
In all, 10 out of the 22 lesions (46%) where surgical findings demonstrated that WLE would have been feasible were correctly identified by both conventional assessment and MRI (Table 4). In five cases, MRI correctly contradicted the conventional assessment by suggesting that WLE was possible. However, in the remaining seven cases MRI suggested that WLE was possible when surgical findings indicated that it was not. The overall agreement with surgical findings regarding the feasibility of WLE was 74% (51 out of 69) for conventional assessment and 78% (54out of 69) for MRI (P=0.44). Cases where the 2nd MRI study disagreed with surgical findings regarding the possibility of WLE are summarised in more detail in Table 6 .
Table 6. Cases where the 2nd MRI study disagreed with surgical findings regarding the possibility of WLE.
| Case no. | Age in years | Radiological size, mm | Core biopsy grade | ER | Initial assessment allows WLE | MRI2 indicates WLE possible | Final pathology indicates WLE possible | DCIS present in final pathology | Spaced out cells present in final pathology | Comments |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 46 | 60 | 1 | + | No | No | Yes | No | No | 15 mm unifocal |
| 4 | 38 | 70 | 2 | + | No | No | Yes | Yes | No | 22 mm unifocal |
| 14 | 49 | 34 | 3 | − | Yes | No | Yes | Yes | No | 31 mm unifocal |
| 22 | 44 | 19 | 3 | − | No | No | Yes | No | No | No residual tumour |
| 27 | 45 | 35 | 3 | + | No | No | Yes | No | No | No residual tumour |
| 31 | 51 | 58 | 2 | + | No | No | Yes | Yes | No | 25 mm unifocal |
| 62 | 49 | 39 | 2 | + | No | No | Yes | Yes | No | 20 mm unifocal |
| 2 | 51 | 55 | 2 | + | Yes | Yes | No | No | No | Multifocal |
| 6 | 47 | 53 | 3 | + | No | Yes | No | Yes | No | Widespread residual |
| 10 | 41 | 51 | 2 | + | No | Yes | No | Yes | No | 41 mm residue |
| 11 | 60 | 38 | 3 | − | No | Yes | No | No | No | Mass+scattered foci |
| 13 | 54 | 34 | 2 | + | Yes | Yes | No | Yes | No | Multifocal |
| 29 | 34 | 100 | 3 | − | No | Yes | No | Yes | No | CR but inflammatory at outset |
| 38 | 43 | 60 | 2 | + | No | Yes | No | Yes | No | Widespread foci |
| 61 | 47 | 50 | 1 | + | No | Yes | No | Yes | No | Multifocal |
DISCUSSION
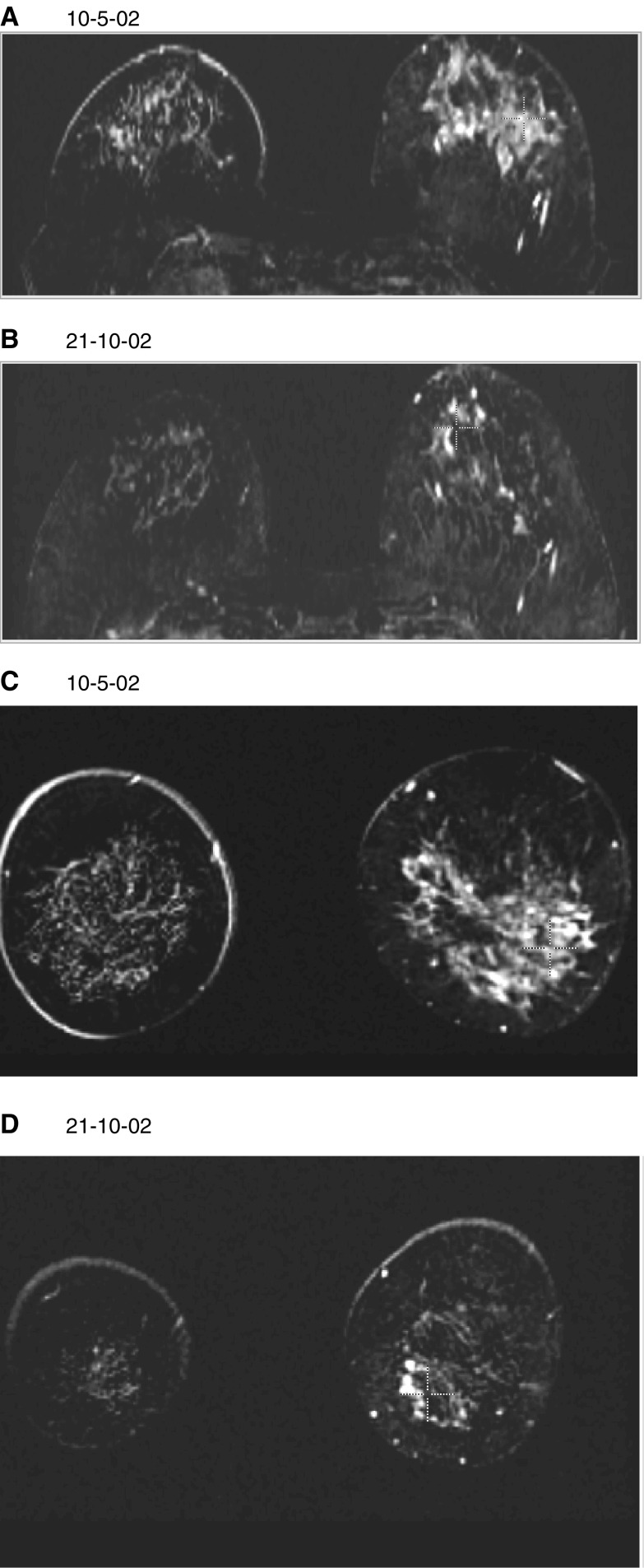
To achieve the objectives of neoadjuvant timing in the use of chemotherapy, it is necessary to understand the available preoperative methods of assessing response. Assessment of response to permit conservation surgery and to judge the effectiveness of the chemotherapy and the biological responsiveness of tumours are both important. Clinical methods have been unreliable (El-Didi et al, 2000), and there are clear limitations in the power of mammography (Huber et al, 2000) or ultrasound (for this purpose, ultrasound is widely used, but very poorly researched) to provide accurate information. CE breast MRI has attractions for this purpose, since it has high sensitivity for invasive tumour and is dependent on increase in vessel density and abnormal diffusion properties of tumour blood vessels for its mode of visualisation. It is essentially a tomographic technique, able to give attractive 3D images that can aid surgeons in planning their operative procedures (Figure 3).
Figure 3.
A subtracted image of a large tumour of the left breast before and after neoadjuvant chemotherapy. (A) and (B) reconstructed in the axial plane, (C) and (D) in the coronal plane. The patient was aged 52 years, had a clinically immeasurable tumour involving the nipple and so not suitable for WLE. It was grade 3, ER positive. She was treated with adriamycin and cyclophosphamide 6 cycles, off-study. She had a complete clinical response, but final histology showed residual grade 3 tumour with associated high-grade DCIS. The MRI studies show the considerable response, but correctly predicted residual tumour over a substantial volume, confirmed as over 60 mm on final histology.
There have been a number of studies testing CE breast MRI for this purpose, which have been summarised in Table 1. The conclusions of these studies have varied considerably and several of them have an up-beat approach that may give surgeons and oncologists false expectations of the capacity of the technique to be reliable in all the aspects needed for planning treatment. Our study is from a single multidisciplinary team in two hospitals, with clinical work of several experts in each discipline. We have used the contemporary reports and notes of the cases to assess the impact, real or potential, on clinical care provided by a CE MRI study at the start and end of neoadjuvant chemotherapy. We have used the histopathological response as the gold standard and since most of our patients had mastectomy, we have the potential to judge whether WLE could have been achieved. By using the full multidisciplinary team in evaluating each case, we hope to have avoided the bias that might ensue from radiologists alone assessing their value. Diagnostic and therapeutic impact methodology has been used for assessment of other new diagnostic procedures (Shuman et al, 1985b) including MRI (Hollingworth et al, 2000) for other purposes. We have moulded this methodology to apply to the present clinical scenario, for example by including an extra category – ‘MRI results could have caused a measurable change in the original plan’.
A few patients had their MRI study after the first cycle of chemotherapy was given, but these cases have not been excluded because the end points in this analysis relate to the presurgical MRI findings. The range of length between the 2nd MRI and surgery is determined by difficulties with scheduling of both MRI studies and reconstructive surgery in our hospital. The range of times between 1st and 2nd MRI studies is determined by the different regimens and patient episodes such as depression of white count which may have delayed cycles of chemotherapy. Whether these variations are important to the end points of this analysis is a matter of speculation, but they relate to pressures that would apply to real-life use of MRI out of the study setting. Our patients received a variety of chemotherapeutic regimens but this should make our CE MRI evaluation generalisable to actual clinical practice where chemotherapy choices vary. The analysis of the MRI study is not affected by the different chemotherapy regimens.
We have made the main comparison of sensitivity and specificity between clinical examination coupled with conventional breast imaging (mammography and breast ultrasound) and CE MRI. CE MRI had both better sensitivity and specificity than clinical measures in conjunction with conventional breast imaging for detecting complete or partial response. The sensitivity of conventional methods is high, but there are serious limitations in specificity. In all, 50% (five out of 10) of patients with no response on pathology were incorrectly classified as partial or complete responders on clinical assessment, mammography and ultrasound. The specificity of CE MRI was higher (eight out of 10), but, due to the small number of patients who did not respond to chemotherapy, this result did not reach statistical significance. Furthermore, CE MRI still gives erroneous results in a number of cases. This is demonstrated in Table 5a where it is seen that the commonest limitation of the value of CE MRI is to underestimate residual tumour when compared with final pathology. Several authors (Gilles et al, 1994; Drew et al, 2001; Weatherall et al, 2001; Partridge et al, 2002) have found very good prediction of residual tumour by breast MRI, whereas Rieber in two studies had findings similar to ours (Rieber et al, 1997, 2002). We wish to seek the explanation for this in greater detail, and will do so by a separate publication studying the dynamic analysis compared with detailed pathology. Many of the cases with small amounts of residual tumour have islands of invasive cells or of DCIS spread across the area previously occupied by the treated tumour. The contrast uptake of the CE MRI did not distinguish this minimal residual disease (MRD) from any background uptake within the glandular tissue of the breast. The chemotherapeutic agents attenuate the contrast uptake characteristics, rendering the curves on some occasions benign in appearance. Clinical assessment of response is based on the size of the palpable mass and the clinical evidence of reduction in lymphoedema or in lymph node size. CE MRI was shown here to be better than these clinical findings even when they are backed up by ultrasound.
The cases in which CE MRI gave added value are shown in Table 5b. These are demonstration of unsuspected contralateral tumour, endorsement of a decision for WLE, better demonstration of nonresponse by showing skin thickening, better estimate of residual tumour and proof of unifocal disease. In Table 6, we have scrutinised the performance of CE MRI in predicting the possibility of WLE after neoadjuvant chemotherapy. It was a surprise to us that so few cases were suitable for breast-conserving surgery from the start, and we do not have the power here to show whether MRI was helpful in a significant way when only the cases which from the outset could have been suitable are considered.
It has been shown that pathological response is predictive of survival (Kuerer et al, 1999; Amat et al, 2002; Chollet et al, 2002) and that outcome is predicted also by tumour grade and nodal involvement. It would be useful to know whether complete response on MRI is predictive of outcome, even when it fails to identify isolated foci of malignant cells or residual DCIS. If response on MRI were to be predictive of survival or disease-free survival, then this would be a test that gave predictive information prior to surgery and full pathology. Many more cases than the present study would be required to answer this question.
Within this series, the grade 3 and ER negative tumours tended to have a slightly better probability of complete response than the lower grade, ER-positive tumours, however neither of these differences reached statistical significance. A factor to consider here is the under staging that occurs with core biopsy, where 70% of cases have been shown to be upstaged between core biopsy and definitive surgical histology (Harris et al, 2003). After chemotherapy, we do not have satisfactory grading, and so the grades discussed here are all from core biopsy. The response rate that has been recorded cannot be appraised since it is dependent on the case selection for use of neoadjuvant chemotherapy and the different regimens in use, neither of which have been made standard in this study. We are looking at the effectiveness of CE MRI as a tool for monitoring, not at response rates in themselves. Our study shows that our MRI findings had very serious limitations in predicting the possibility of undertaking wide local excision after neoadjuvant chemotherapy, and that the shortfall was in both directions and not predictable from any particular characteristics of the cases. This study also shows how inaccurate clinical assessment of tumour response is, even when enhanced by ultrasound or mammography.
Even though our study has shown definite limitations to the reliability of MRI, the clinical team found that it provided valuable additional information. The problems with CE MRI are sufficient to continue to seek improved methods of assessing tumour response. Functional MRI, PET scanning and molecular imaging are emerging techniques that may offer more accurate information.
CONCLUSION
Our study suggests that CE MRI may have better sensitivity and specificity for predicting complete or partial response than clinical examination with conventional imaging. Moreover, the absolute agreement between MRI and pathology findings was marginally higher than the agreement between clinical assessment and pathology findings. CE breast MRI is robust in predicting nonresponse and disease progression. Our clinicians valued the visual images that are obtained which show tumour response. They feel that they give additional information – probably by giving a better measure of size and providing images that help them to understand the permeative properties of the tumour. In our series, MRI persistently underestimates residual malignancy where the tumour is not a single mass, and this may be a problem in some cases when it is used to predict the possibility of WLE.
Acknowledgments
The study was made possible by a grant from Addenbrooke's Charities. The patients were under the clinical care of Cambridge Breast Unit and Hinchingbrooke Hospital and MRI studies were undertaken by Chris Pittock, Anna Crawley, Ilse Joubert, Joanna Cook and Hayley Eales. Thanks are due to the following clinicians who have contributed cases, but are not cited as authors: Alan Freeman, Ruchi Sinnatamby, David Gilligan, Carlos Caldas, Oduru Ravisekar, Karen MacAdam, Charles Wilson, and to the following who have contributed their expertise: Adrian Dixon, David Lomas, Martin Graves, Richard Black, Clare Sims. Iqbal Warsi completed the data gathering and dataset cleaning and Jackie Green gave administrative support throughout.
Appendix A
Table A2. Cases included in the study.
| Case | Age | Size | Infl | Gr | ER | PgR | Hist | Trial | cR | mR | pR |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 01 | 46 | 60 | n | 1 | + | IDC | AC2 | cCR | mPR | pPR | |
| 02 | 51 | 55 | n | 2 | + | IDC | AC2 | cPR | mPR | pPR | |
| 03 | 51 | 65 | n | 3 | + | IDC | cCR | mPR | pPR | ||
| 50 | 40 | 55 | n | 3 | − | − | IDC | cCR | mPR | pPR | |
| 46 | 44 | 95 | n | 3 | − | − | IDC | cPR | mPR | pPR | |
| 04 | 38 | 70 | n | 2 | + | IDC | AC2 | cPR | mPR | pPR | |
| 05 | 55 | 45 | n | 1 | + | IDC | cPR | mPR | pPR | ||
| 06 | 47 | 53 | n | 3 | + | ILC | AC2 | cPR | mCR | pPR | |
| 07 | 52 | 70 | n | 2 | − | 0 | IDC | AC2 | cCR | mCR | pPR |
| 51 | 31 | 48 | n | 2+ | − | − | IDC | cPR | mCR | pCR | |
| 08 | 41 | 70 | y | 3 | − | − | IDC | AC2 | cCR | mPR | pPR |
| 10 | 41 | 51 | n | 2 | + | ILC | cPR | mPR | pPR | ||
| 11 | 60 | 38 | n | 3 | − | + | IDC | cPR | mPR | pPR | |
| 12 | 56 | 88 | y | 1 | + | IDC | cNR | mNR | pNR | ||
| 42 | 61 | 65 | n | 3 | − | − | IDC | cNR | mNR | pNR | |
| 13 | 54 | 34 | n | 2 | + | IDC | AC2 | cCR | mCR | pPR | |
| 41 | 34 | 69 | y | 2 | + | ILC | AC2 | cCR | mPR | pPR | |
| 14 | 49 | 34 | n | 3 | − | − | IDC | cPR | mPR | pPR | |
| 40 | 61 | 34 | n | 3 | + | IDC | AC2 | cPR | mCR | pPR | |
| 15 | 54 | 32 | n | 3 | + | IDC | AC2 | cCR | mPR | pPR | |
| 16 | 39 | 53 | y | 3 | − | 0 | IDC | cNR | mDP | pNR | |
| 59 | 52 | 55 | n | 3 | + | IDC | AC2 | cCR | mCR | pCR | |
| 17 | 49 | 70 | y | 2 | − | 0 | IDC | cPR | mPR | pPR | |
| 18 | 47 | 49 | n | 3 | − | − | IDC | AC2 | cPR | mPR | pPR |
| 54 | 49 | 69 | y | 3 | − | − | IDC | AC2 | cCR | mCR | pCR |
| 55 | 43 | 35 | n | 3 | + | IDC | AC2 | cCR | mCR | pCR | |
| 19 | 35 | 50 | n | 3 | − | + | IDC | AC2 | cPR | mPR | pPR |
| 20 | 45 | 90 | y | 2 | + | IDC | cPR | mPR | pPR | ||
| 21 | 47 | 45 | n | 2 | + | IDC | AC2 | cNR | mPR | pPR | |
| 47 | 30 | n | 3 | − | + | IDC | cPR | mNR | pNR | ||
| 22 | 45 | 19 | y | 3 | − | − | IDC | AC2 | cPR | mCR | pCR |
| 23 | 49 | 40 | n | 3 | − | − | IDC | AC2 | cPR | mPR | pPR |
| 43 | 50 | 46 | n | 3 | − | − | IDC | AC2 | cPR | mPR | pPR |
| 39 | 44 | 39 | n | 3 | + | IDC | AC2 | cCR | mCR | pCR | |
| 25 | 39 | 45 | n | 3 | − | − | IDC | AC2 | cCR | mCR | pNR |
| 26 | 33 | 26 | n | 3 | − | − | IDC | AC2 | cPR | mPR | pCR |
| 27 | 45 | 35 | n | 3 | + | IDC | cCR | mCR | pCR | ||
| 57 | 47 | 36 | n | 3 | − | − | IDC | AC2 | cPR | mNR | pNR |
| 29 | 34 | 100 | y | 3 | − | − | IDC/ILC | cCR | mCR | pCR | |
| 30 | 49 | 54 | n | 2 | + | IDC | AC2 | cPR | mCR | pPR | |
| 31 | 51 | 58 | n | 2 | + | IDC | AC2 | cPR | mPR | pPR | |
| 32 | 40 | 44 | n | 3 | − | − | IDC | AC2 | cPR | mPR | pPR |
| 33 | 41 | 90 | y | 3 | + | IDC | cCR | mPR | pPR | ||
| 34 | 43 | 30 | n | 3 | − | 0 | IDC | AC2 | cPR | mCR | pCR |
| 58 | 59 | 66 | n | 3 | − | − | IDC | cPR | mPR | pPR | |
| 35 | 49 | 66 | n | 2 | + | IDC | cNR | mNR | pNR | ||
| 36 | 45 | 49 | n | 2 | + | ILC | AC2 | cPR | mPR | pPR | |
| 61 | 45 | 50 | n | 3 | + | IDC | AC2 | cPR | mCR | pPR | |
| 37 | 57 | 64 | n | 2 | − | − | ILC | AC2 | cCR | mCR | pCR |
| 38 | 43 | 60 | y | 2 | + | IDC | AC2 | cCR | mPR | pPR |
Key to column headings: Size=radiological (mammography and ultrasound) size at diagnosis, inflamm=inflammatory changes present, grade= Bloom and Richardson nuclear grade (Elston and Ellis, 1991) on core biopsy, ER=oestrogen receptor status on core biopsy, PgR=progesterone receptor status, Histology=core biopsy (CB) histology, IDC Invasive ductal carcinoma, ILC=invasive lobular carcinoma, Chemo=neoadjuvant chemotherapy regimen, cR=clinical response, mR=MRI response, pR=pathological response, CR=complete response, PR=partial response, NR=nonresponder, DP=disease progression. AC2=Anglo-Celtic 2 trial.
Appendix B
See Table B1.
References
- Abraham DC, Jones RC, Jones SE, Cheek JH, Peters GN, Knox SM, Grant MD, Hampe DW, Savino DA, Harms SE (1996) Evaluation of neoadjuvant chemotherapeutic response of locally advanced breast cancer by magnetic resonance imaging. Cancer 78: 91–100 [DOI] [PubMed] [Google Scholar]
- Amat S, Penault-Llorca F, Cure H, Le Bouedec G, Achard JL, Van Praagh I, Feillel V, Mouret-Reynier MA, Dauplat J, Chollet P (2002) Scarff–Bloom–Richardson (SBR) grading: a pleiotropic marker of chemosensitivity in invasive ductal breast carcinomas treated by neoadjuvant chemotherapy. Int J Oncol 20: 791–796 [PubMed] [Google Scholar]
- Balu-Maestro C, Chapellier C, Bleuse A, Chanalet I, Chauvel C, Largillier R (2002) Imaging in evaluation of response to neoadjuvant breast cancer treatment benefits of MRI. Breast Cancer Res Treat 72: 145–152 [DOI] [PubMed] [Google Scholar]
- Blanchard TK, Bearcroft PW, Dixon AK, Lomas DJ, Teale A, Constant CR, Hazleman BL (1997) Magnetic resonance imaging or arthrography of the shoulder: which do patients prefer? Br J Radiol 70: 786–790 [DOI] [PubMed] [Google Scholar]
- Brown J, Buckley D, Coulthard A, Dixon AK, Dixon JM, Easton DF, Eeles RA, Evans DG, Gilbert FG, Graves M, Hayes C, Jenkins JP, Jones AP, Keevil SF, Leach MO, Liney GP, Moss SM, Padhani AR, Parker GJ, Pointon LJ, Ponder BA, Redpath TW, Sloane JP, Turnbull LW, Walker LG, Warren RM (2000) Magnetic resonance imaging screening in women at genetic risk of breast cancer: imaging and analysis protocol for the UK multicentre study [In Process Citation]. Magn Reson Imaging 18: 765–776 [DOI] [PubMed] [Google Scholar]
- Cheung YC, Chen SC, Su MY, See LC, Hsueh S, Chang HK, Lin YC, Tsai CS (2003) Monitoring the size and response of locally advanced breast cancers to neoadjuvant chemotherapy (weekly paclitaxel and epirubicin) with serial enhanced MRI. Breast Cancer Res Treat 78: 51–58 [DOI] [PubMed] [Google Scholar]
- Chollet P, Amat S, Cure H, de Latour M, Le Bouedec G, Mouret-Reynier MA, Ferriere JP, Achard JL, Dauplat J, Penault-Llorca F (2002) Prognostic significance of a complete pathological response after induction chemotherapy in operable breast cancer. Br J Cancer 86: 1041–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis PL, Staiger MJ, Harris KB, Ganott MA, Klementaviciene J, McCarty Jr KS, Tobon H (1996) Breast cancer measurements with magnetic resonance imaging, ultrasonography, and mammography. Breast Cancer Res Treat 37: 1–9 [DOI] [PubMed] [Google Scholar]
- Delille J, Stanetz P, Yeh E, Halpern E, Kopans D, Garrido L (2003) Invasive ductal breast carcinoma response to neoadjuvant chemotherapy: noninvasive monitoring with functional MR imaging – pilot study. Radiology 228: 63–69 [DOI] [PubMed] [Google Scholar]
- Drew PJ, Kerin MJ, Mahapatra T, Malone C, Monson JR, Turnbull LW, Fox JN (2001) Evaluation of response to neoadjuvant chemoradiotherapy for locally advanced breast cancer with dynamic contrast-enhanced MRI of the breast. Eur J Surg Oncol 27: 617–620 [DOI] [PubMed] [Google Scholar]
- El-Didi MH, Moneer MM, Khaled HM, Makarem S (2000) Pathological assessment of the response of locally advanced breast cancer to neoadjuvant chemotherapy and its implications for surgical management. Surg Today 30: 249–254 [DOI] [PubMed] [Google Scholar]
- Elston CW, Ellis IO (1991) Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology 19: 403–410 [DOI] [PubMed] [Google Scholar]
- Esserman L, Hylton N, Yassa L, Barclay J, Frankel S, Sickles E (1999) Utility of magnetic resonance imaging in the management of breast cancer: evidence for improved preoperative staging [In Process Citation]. J Clin Oncol 17: 110–119 [DOI] [PubMed] [Google Scholar]
- Esserman L, Kaplan E, Partridge S, Tripathy D, Rugo H, Park J, Hwang S, Kuerer H, Sudilovsky D, Lu Y, Hylton N (2001) MRI phenotype is associated with response to doxorubicin and cyclophosphamide neoadjuvant chemotherapy in stage III breast cancer. Ann Surg Oncol 8: 549–559 [DOI] [PubMed] [Google Scholar]
- Evans T, Gould A, Foster L, Crown J, Leonard R, Mansi J, Anglo-Celtic Collaborators (2002) Phase III randomised trial of adriamycin (A) and docetaxel (D) versus A and cyclophosphamide (C) as primary medical therapy (PMT) in women with breast cancer: an ACCOG study. J Clin Oncol 21, Abs 136 [Google Scholar]
- Fisher B, Bryant J, Wolmark N, Mamounas E, Brown A, Fisher ER, Wickerham DL, Begovic M, DeCillis A, Robidoux A, Margolese RG, Cruz Jr AB, Hoehn JL, Lees AW, Dimitrov NV, Bear HD (1998) Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol 16: 2672–2685 [DOI] [PubMed] [Google Scholar]
- Gilles R, Guinebretiere JM, Toussaint C, Spielman M, Rietjens M, Petit JY, Contesso G, Masselot J, Vanel D (1994) Locally advanced breast cancer: contrast-enhanced subtraction MR imaging of response to preoperative chemotherapy. Radiology 191: 633–638 [DOI] [PubMed] [Google Scholar]
- Green M, Hortobagyi GN (2002) Neoadjuvant chemotherapy for operable breast cancer. Oncology (Huntingt) 16: 871–884 889; discussion 889–90, 892–4, 897–8 [PubMed] [Google Scholar]
- Harris GC, Denley HE, Pinder SE, Lee AH, Ellis IO, Elston CW, Evans A (2003) Correlation of histologic prognostic factors in core biopsies and therapeutic excisions of invasive breast carcinoma. Am J Surg Pathol 27: 11–15 [DOI] [PubMed] [Google Scholar]
- Helvie MA, Joynt LK, Cody RL, Pierce LJ, Adler DD, Merajver SD (1996) Locally advanced breast carcinoma: accuracy of mammography versus clinical examination in the prediction of residual disease after chemotherapy. Radiology 198: 327–332 [DOI] [PubMed] [Google Scholar]
- Hollingworth W, Todd CJ, Bell MI, Arafat Q, Girling S, Karia KR, Dixon AK (2000) The diagnostic and therapeutic impact of MRI: an observational multi-centre study. Clin Radiol 55: 825–831 [DOI] [PubMed] [Google Scholar]
- Howell A, Houston S, Coleman R, Earl H, Wroath A, Miles D (2002) Safety and efficacy of Herceptin (H) alone or in combination with a taxoid (T) in HER2-positive metastatic breast cancer (MBC): the UK Expanded Access Programme (EAP). J Clin Oncol 21, Abs 1855 [Google Scholar]
- Huber S, Wagner M, Zuna I, Medl M, Czembirek H, Delorme S (2000) Locally advanced breast carcinoma: evaluation of mammography in the prediction of residual disease after induction chemotherapy. Anticancer Res 20: 553–558 [PubMed] [Google Scholar]
- Kinkel K, Hylton NM (2001) Challenges to interpretation of breast MRI. J Magn Reson Imaging 13: 821–829 [DOI] [PubMed] [Google Scholar]
- Knopp MV, Brix G, Junkermann HJ, Sinn HP (1994) MR mammography with pharmacokinetic mapping for monitoring of breast cancer treatment during neoadjuvant therapy. Magn Reson Imaging Clin N Am 2: 633–658 [PubMed] [Google Scholar]
- Kuerer HM, Newman LA, Smith TL, Ames FC, Hunt KK, Dhingra K, Theriault RL, Singh G, Binkley SM, Sneige N, Buchholz TA, Ross MI, McNeese MD, Buzdar AU, Hortobagyi GN, Singletary SE (1999) Clinical course of breast cancer patients with complete pathologic primary tumor and axillary lymph node response to doxorubicin-based neoadjuvant chemotherapy. J Clin Oncol 17: 460–469 [DOI] [PubMed] [Google Scholar]
- Makris A, Powles TJ, Dowsett M, Osborne CK, Trott PA, Fernando IN, Ashley SE, Ormerod MG, Titley JC, Gregory RK, Allred DC (1997) Prediction of response to neoadjuvant chemoendocrine therapy in primary breast carcinomas. Clin Cancer Res 3: 593–600 [PubMed] [Google Scholar]
- Mankoff DA, Dunnwald LK, Gralow JR, Ellis GK, Drucker MJ, Livingston RB (1999) Monitoring the response of patients with locally advanced breast carcinoma to neoadjuvant chemotherapy using [technetium 99 m]-sestamibi scintimammography. Cancer 85: 2410–2423 [PubMed] [Google Scholar]
- Mussurakis S, Buckley DL, Coady AM, Turnbull LW, Horsman A (1996) Observer variability in the interpretation of contrast enhanced MRI of the breast. Br J Radiol 69: 1009–1016 [DOI] [PubMed] [Google Scholar]
- Nakamura S, Kenjo H, Nishio T, Kazama T, Do O, Suzuki K (2001) 3D-MR mammography-guided breast conserving surgery after neoadjuvant chemotherapy: clinical results and future perspectives with reference to FDG-PET. Breast Cancer 8: 351–354 [DOI] [PubMed] [Google Scholar]
- NHSBSP publication No. 3 (1995) Pathology Reporting in Breast Cancer Screening. Sheffield: National Coordinating Group for Breast Screening Pathology [Google Scholar]
- Padhani A (2002) Functional MRI for anticancer therapy assessment. Eur H Cancer 38: 2116–2127 [DOI] [PubMed] [Google Scholar]
- Partridge SC, Gibbs JE, Lu Y, Esserman LJ, Sudilovsky D, Hylton NM (2002) Accuracy of MR imaging for revealing residual breast cancer in patients who have undergone neoadjuvant chemotherapy. AJR Am J Roentgenol 179: 1193–1199 [DOI] [PubMed] [Google Scholar]
- Peto R, Boreham J, Clarke M, Davies C, Beral V (2000) UK and USA breast cancer deaths down 25% in year 2000 at ages 20–69 years. Lancet 355: 1822. [DOI] [PubMed] [Google Scholar]
- Pusztai L, Atyers M, Simmons F, Damokosh A, Hess K, Valero V, Clark E, Ross J, Hortobagyi GN, Stec J (2003) Emerging science: prospective validation of gene expression profiling based on prediction of complete pathologic response to neoadjuvant paclitaxel/FAC chemotherapy in breast cancer. J Clin Oncol 22, Proc. Asco. [DOI] [PubMed] [Google Scholar]
- Rieber A, Brambs HJ, Gabelmann A, Heilmann V, Kreienberg R, Kuhn T (2002) Breast MRI for monitoring response of primary breast cancer to neo-adjuvant chemotherapy. Eur Radiol 12: 1711–1719 [DOI] [PubMed] [Google Scholar]
- Rieber A, Zeitler H, Rosenthal H, Gorich J, Kreienberg R, Brambs HJ, Tomczak R (1997) MRI of breast cancer: influence of chemotherapy on sensitivity. Br J Radiol 70: 452–458 [DOI] [PubMed] [Google Scholar]
- Shuman WP, Griffin BR, Haynor DR, Johnson JS, Jones DC, Cromwell LD, Moss AA (1985a) MR imaging in radiation therapy planning. Work in progress. Radiology 156: 143–147 [DOI] [PubMed] [Google Scholar]
- Shuman WP, Griffin BR, Yoshy CS, Listerud JA, Mack LA, Rowberg AH, Moss AA (1985b) The impact of CT CORRELATE ScoutView images on radiation therapy planning. AJR Am J Roentgenol 145: 633–638 [DOI] [PubMed] [Google Scholar]
- Sotiriou C, Powles TJ, Dowsett M, Jazaeri AA, Feldman AL, Assersohn L, Gadisetti C, Libutti SK, Liu ET (2002) Gene expression profiles derived from fine needle aspiration correlate with response to systemic chemotherapy in breast cancer. Breast Cancer Res 4: R3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Therasse P, Arbuck S, Eisenhauer E, Wanders J, Kaplan R, Rubinstein L, Verweij J, Glabbeke M, van Oosterom A, Christina M, Gwyther S (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92: 205–216 [DOI] [PubMed] [Google Scholar]
- Tiling R, Linke R, Untch M, Richter A, Fieber S, Brinkbaumer K, Tatsch K, Hahn K (2001) 18F-FDG PET and 99mTc-sestamibi scintimammography for monitoring breast cancer response to neoadjuvant chemotherapy: a comparative study. Eur J Nucl Med 28: 711–720 [DOI] [PubMed] [Google Scholar]
- Trecate G, Ceglia E, Stabile F, Tesoro-Tess JD, Mariani G, Zambetti M, Musumeci R (1998) Locally advanced breast tumors. Role of magnetic resonance in the assessment of response to preoperative therapy and of neoplastic residue before the operation. Radiol Med (Torino) 95: 449–455 [PubMed] [Google Scholar]
- Tsuboi N, Ogawa Y, Inomata T, Yoshida D, Yoshida S, Moriki T, Kumon M (1999) Changes in the findings of dynamic MRI by preoperative CAF chemotherapy for patients with breast cancer of stage II and III: pathologic correlation. Oncol Rep 6: 727–732 [DOI] [PubMed] [Google Scholar]
- Valero V, Buzdar AU, McNeese M, Singletary E, Hortobagyi GN (2002) Primary chemotherapy in the treatment of breast cancer: the University of Texas M D Anderson Cancer Center experience. Clin Breast Cancer 3(Suppl 2): S63–S68 [DOI] [PubMed] [Google Scholar]
- Vinnicombe SJ, MacVicar AD, Guy RL, Sloane JP, Powles TJ, Knee G, Husband JE (1996) Primary breast cancer: mammographic changes after neoadjuvant chemotherapy, with pathologic correlation. Radiology 198: 333–340 [DOI] [PubMed] [Google Scholar]
- Weatherall PT, Evans GF, Metzger GJ, Saborrian MH, Leitch AM (2001) MRI vs histologic measurement of breast cancer following chemotherapy: comparison with X-ray mammography and palpation. J Magn Reson Imaging 13: 868–875 [DOI] [PubMed] [Google Scholar]
- Wilson R, Asbury D, Cooke J, Michell M, Patnick J (2001) Clinical Guidelines for Breast Cancer Screening Assessment. Vol. 49. pp. 32 Sheffield: NHSBSP [Google Scholar]
- Wolmark N, Wang J, Mamounas E, Bryant J, Fisher B (2001) Preoperative chemotherapy in patients with operable breast cancer: nine-year results from National Surgical Adjuvant Breast and Bowel Project B-18. J Natl Cancer Inst Monogr 30: 96–102 [DOI] [PubMed] [Google Scholar]