Abstract
Eph receptor tyrosine kinases (RTKs) and their membrane-bound ligands, the ephrins, are essential for embryonic vascular development. Recently, it has been demonstrated that overexpression of specific Ephs and ephrins is associated with a poor prognosis in human tumours. Our group has shown that EphB and the ephrin-B subfamilies are coexpressed in human colorectal cancer, and ephrin-B2 is expressed at higher levels in human colorectal cancer than in adjacent normal mucosa. As the Eph/ephrin system is involved in embryologic vasculogenesis and ephrin-B2 is expressed ubiquitously in all colon cancers studied in our laboratory, we hypothesised that overexpression of ephrin-B2 in colon cancer cells may induce tumour angiogenesis and increase tumour growth. To investigate this hypothesis, we stably transfected KM12L4 human colon cancer cells with ephrin-B2 to study its effect on tumour growth in vivo. We found that overexpression of ephrin-B2 markedly decreased tumour growth in a mouse xenograft model. Immunohistochemical staining showed that ephrin-B2 transfectants produced higher tumour microvessel density and lower tumour cell proliferation than did parental or vector-transfected control cells. Using 51Cr-labelled red blood cells (RBCs) to determine the functional blood volume in tumours, we demonstrated that tumours from ephrin-B2-transfected cells had significantly decreased blood volume compared with tumours from parental or vector-transfected control cells. Evaluation of in vitro parameters of cell cycle mediators demonstrated no alteration in the cell cycle. Although ephrin-B2 transfection increased tumour vessel density, the decrease in blood perfusion suggests that these vessels may be ‘dysfunctional’. We conclude that overexpression of ephrin-B2 suppresses tumour cell growth and vascular function in this in vivo colon cancer model.
Keywords: ephrin-B2, colorectal cancer, angiogenesis, tyrosine kinase, receptor, ligand
The largest subfamily of receptor tyrosine kinases (RTKs), the Eph family, and their ligands (ephrins), play important roles in physiologic and pathologic vascular processes. Both the Ephs (receptors) and ephrins (ligands) are divided into two groups, A and B subfamilies (Eph Nomenclature Committee, 1997). The binding of ephrin ligands to Eph receptors is dependent on cell–cell interactions. The interactions of ephrins and Ephs have recently been shown to function via bi-directional signalling. The ephrins binding to Eph receptors initiate phosphorylation at specific tyrosine residues in the cytoplasmic domain of the Eph receptors. In response to Eph receptor binding, the ephrin ligand also undergoes tyrosine phosphorylation, so-called ‘reverse’ signalling (Holland et al, 1996; Bruckner et al, 1997).
Eph RTKs and their ephrin ligands play important roles in embryonic vascular development. Disruption of specific Eph receptors and ligands (including ephrin-B2) leads to defective vessel remodelling, organisation, and sprouting resulting in embryonic death (Wang et al, 1998; Adams et al, 1999; Gale and Yancopoulos, 1999; Helbling et al, 2000). Coordinated expression of the Eph/ephrin system determines the phenotype of embryonic vascular structures: ephrin-B2 is present on arterial endothelial cells (ECs), whereas EphB4 is present on venous ECs (Gale and Yancopoulos, 1999; Shin et al, 2001). Recently, specific Ephs and ephrins have been implicated in tumour growth and angiogenesis. The Ephs and ephrins have been found to be overexpressed in many human tumours (Kiyokawa et al, 1994; Vogt et al, 1998; Bruce et al, 1999; Tang et al, 1999a, 1999b; Walker-Daniels et al, 1999; Ogawa et al, 2000; Varelias et al, 2002), and higher expression levels of Ephs and ephrins have been found to correlate with more aggressive and metastatic tumours (Vogt et al, 1998; Nakamoto and Bergemann, 2002).
Our group has shown that EphB and the ephrin-B subfamilies are co-expressed in human colorectal cancer, and ephrin-B2 is expressed at higher levels in human colorectal cancer than in adjacent normal mucosa (Liu et al, 2002). As the Eph/ephrin system is involved in embryologic vasculogenesis and ephrin-B2 is expressed ubiquitously in all colon cancers studied in our laboratory, we hypothesised that overexpression of ephrin-B2 in colon cancer cells may induce tumour angiogenesis and increase tumour growth. We found that overexpression of ephrin-B2 in colon cancer cells did lead to an increase in tumour angiogenesis, but that these vessels were not functional. Paradoxically, overexpression of ephrin-B2 was associated with a marked decrease in tumour growth that was likely secondary to the development of an inefficient vascular network.
MATERIALS AND METHODS
Cells and cell cultures
The KM12L4, KM20, KM23, KM12C, and KM12SM human colon cancer cell lines were provided by Dr IJ Fidler (The University of Texas M.D. Anderson Cancer Center, Houston, TX, USA) (Morikawa et al, 1988). HT-29 (human colorectal cancer cells), MRC-5 (human lung fibroblasts), 10T1/2 (murine fibroblasts), CCD-33Co and CCD-112Co (human colorectal fibroblasts), and human umbilical vein endothelial cells (HUVECs) were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). MRC-5, CCD-33Co, CCD-112Co, and all colon cancer cell lines were cultured in minimal essential medium (MEM) supplemented with 10% foetal bovine serum (FBS), penicillin–streptomycin, vitamins, sodium pyruvate, L-glutamine, and nonessential amino acids (Life Technologies, Inc., Grand Island, NY, USA). Murine 10T1/2 cells were grown in basal Eagle's medium supplemented with 10% FBS, and 2 U ml−1 penicillin–streptomycin. HUVECs were cultured on 0.5% gelatin (Sigma Chemical Co. St Louis, MO, USA) in modified MEM supplemented with 15% FBS, 10 ng ml−1 recombinant human basic fibroblast growth factor, penicillin–streptomycin, vitamins, sodium pyruvate, L-glutamine, and nonessential amino acids. All cells were grown at 37°C in 5% CO2 and 95% air.
Antibodies for immunohistochemistry
Antibodies for immunohistochemical analysis were obtained as follows: rabbit anti-human ephrin-B2 antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA), rat anti-mouse CD31/PECAM-1 antibody and peroxidase-conjugated rat anti-mouse immunoglobulin G1 (IgG1) from Pharmingen (San Diego, CA, USA); mouse antiproliferating cell nuclear antigen (PCNA) clone PC10 DAKO A/S from DAKO Corp. (Carpinteria, CA, USA); and peroxidase-conjugated goat anti-rat IgG (H+L) and peroxidase-conjugated rat anti-mouse IgG2a from Serotec Harlan Bioproducts for Science, Inc. (Indianapolis, IN, USA).
Isolation of mRNA and Northern blot analysis
Polyadenylated mRNA was extracted from 107–108 cells from colon cancer cell lines or nonmalignant cell lines growing in culture by using a FastTrack mRNA isolation kit (Invitrogen Corp., San Diego, CA, USA). Northern blot analysis was performed as previously described (Ellis et al, 1996). After prehybridisation, the membranes were probed for ephrin-B2 full-length cDNA (the probe was a generous gift from Dr Renping Zhou, Laboratory for Cancer Research, College of Pharmacy, Rutgers University, Piscataway, NJ, USA; Yue et al, 1999), EphB4 (the probe was a purified polymerase chain reaction product with the primer sequences as follows: forward, 5′-GTCTGACTTTGGCCTTTCCC-3′; reverse, 5′-TGACATCACCTCCCACATCA-3′), and glyceraldehyde-phosphate dehydrogenase (internal control; ATCC). Each cDNA probe was purified by agarose gel electrophoresis, recovered by using a QIAEX gel extraction kit (QIAGEN Inc., Chatsworth, CA, USA), and radiolabelled by a random primer technique with a commercially available kit (Amersham Life Science Inc., Arlington Heights, IL, USA). Nylon filters were washed at 65°C with 30 mmol l−1 NaCl, 3 mmol l−1 sodium citrate (pH 7.2), and 0.1% sodium dodecyl sulphate. Autoradiography was then performed.
Subcloning of ephrin-B2 into pcDNA3 and DNA transfection
Ephrin-B2 was subcloned into the Kpn I/Not I sites of pcDNA3 (Invitrogen), a eukaryotic expression vector driven by the human cytomegalovirus promoter and containing a neomycin-resistance gene. Completeness and orientation of the insert were determined by means of restriction enzyme analyses and DNA sequencing (Core Sequencing Facilities, M.D. Anderson Cancer Center).
The ephrin-B2 sense plasmid as well as vector plasmid alone (pcDNA3) were transfected into KM12L4 cells utilising the FuGENE 6 transfection system, according to the manufacturer's protocol (Boehringer Mannheim Co., Indianapolis, IN, USA). At 48 h after transfection, medium containing G418 (400 μg ml−1) was used to select for clones. Individual colonies were expanded and examined for ephrin-B2 expression by Northern blot analysis. Clones with a high expression of exogenous ephrin-B2 were pooled to establish an additional experimental cell line, the ephrin-B2 S pool.
Animals and tumour cell inoculation
Male nude mice (8 weeks old) were obtained from the National Cancer Institute's Animal Production Area (Frederick, MD, USA), acclimated for 1 week, caged in groups of five, and fed a diet of animal chow and water ad libitum throughout the experiment. Mice were randomised to one of the four groups, with similar mean body weights among the four groups. With a 30-gauge needle and a 1-ml syringe, 200 μl (containing 1 × 106 cells) of KM12L4, pcDNA3-transfected KM12L4, or ephrin-B2-sense transfected KM12L4 (S2 or S pool) cells were injected subcutaneously in the right upper flank of 10 mice each. Prior to injection, a trypan blue exclusion test was carried out to assure that cell viability was greater than 80%. Tumour growth was measured every other day. Tumour volume was calculated using the formula: volume=(diameter2 × length)/2. All animal studies were conducted according to a protocol approved by the Institutional Animal Care and Use Committee (IACUC) of M.D. Anderson Cancer Center and were performed in accordance with the UKCCCR guidelines for the welfare of animals in experimental neoplasia (Workman et al, 1998). All animals were euthanised 15 days after tumour cell inoculation because of the large volume of the KM12L4 tumours (maximum size permitted by the IACUC), and the tumours were harvested for further analysis. Harvested tumours were placed in 10% formalin for paraffin fixation or placed in optimum cutting temperature (OCT; Miles Inc., Elkhart, IN, USA) solution and snap frozen for frozen sectioning. This experiment was performed three times with similar results.
Immunohistochemistry of paraffin-embedded and frozen tissues and immunofluorescent staining of cells
Paraffin-embedded tumours were sectioned (4–6 μm thick), mounted on positively charged Superfrost slides (Fisher Scientific Co., Houston, TX, USA), and allowed to dry overnight at room temperature. Sections were deparaffinised in xylene followed by 100, 95, and 80% ethanol and rehydrated in phosphate-buffered saline (PBS; pH 7.5). These sections were used for haematoxylin and eosin (H&E) staining and detection of PCNA. Sections analysed for PCNA were microwaved for 5 min to increase antigen retrieval before staining, and stained as described below.
Tumours frozen in OCT solution were sectioned (8–10 μm thick), mounted on positively charged Superfrost slides, and air-dried for 30 min. Tissues were fixed in cold acetone (5 min), 1 : 1 acetone/chloroform (5 min), and acetone (5 min) and then washed with PBS three times for 3 min each. These sections were used for CD31 staining. Specimens were then incubated with 3% hydrogen peroxide in PBS (v v−1) for 12 min at room temperature to block endogenous peroxidase. Sections were washed three times for 3 min each with PBS (pH 7.5) and incubated for 20 min at room temperature in a protein-blocking solution consisting of PBS supplemented with 1% normal goat serum and 5% normal horse serum.
The primary antibodies directed against CD31 and PCNA were diluted 1 : 100 and 1 : 50, respectively, in protein-blocking solution and applied to the sections, which were then incubated overnight at 4°C. Sections were then rinsed three times for 3 min each in PBS and incubated for 10 min in protein-blocking solution before the addition of peroxidase-conjugated secondary antibody. The secondary antibodies used to stain for CD31 [peroxidase-conjugated goat anti-rat IgG (H+L)] and PCNA (peroxidase-conjugated rat anti-mouse IgG2a) were diluted 1 : 200 and 1 : 100, respectively, in protein-blocking solution. After incubation with the secondary antibody for 1 h at room temperature, the samples were washed and incubated with stable diaminobenzidine (Research Genetics, Huntsville, AL, USA) substrate. Staining was monitored under a bright field microscope, and the reaction was stopped by washing with distilled water. For the CD31 staining, sections were counterstained with Gill's No. 3 haematoxylin (Sigma Chemical Co.) for 15 s and mounted with Universal Mount (Research Genetics). Control specimens were treated with a similar procedure except that the primary antibody was omitted.
Ephrin-B2-sense-transfected cells (S2) and vector-transfected cells (KM12L4/pcDNA3) were seeded in the wells of four-chamber slides (Fisher Scientific Co.) and cells were grown until 80% confluent. The cells were fixed with cold acetone (Sigma Chemical Co.) for 10 min and washed with PBS twice for 5 min each. Immunofluorescent staining was performed as previously described (Liu et al, 2002) with the following modifications. After the cells were incubated overnight at 4°C with the primary antibody (ephrin-B2, 1 : 100 dilution), washed, and incubated with protein-blocking solution, they were incubated with the Alexa 594-conjugated secondary antibody (1 : 400 dilution of Alexa 594 goat anti-rabbit IgG (H+L); (Molecular Probes, Eugene, OR, USA)) for 60 min at room temperature. The slides were then washed with PBS and the coverslips were mounted on glass slides using mounting solution consisting of Hoechst 33342 fluorescent dye (Sigma Chemical Co.) and PBS.
Quantification of tumour microvessel density and tumour cell proliferation
To quantify tumour microvessel density and tumour cell proliferation, tumour microvessels (stained for CD31) and PCNA-positive cells were evaluated by light microscopy and counted in three random 0.159 mm2 fields at × 100 magnification using a Sony 3-chip camera (Sony, Montvale, NJ, USA) mounted on a Zeiss universal microscope (Carl Zeiss, Thornwood, NY, USA) and Optimas Image Analysis software (Bioscan, Edmond, WA, USA) installed on a Compaq computer with Pentium chip, a frame grabber, an optical disk storage system, and a Sony Mavigraph UP-D7000 digital color printer (Tokyo, Japan). Microvessels were quantified according to the method described by Ahmad et al (2001).
Determination of functional blood volume/flow in tumours
Five nude mice were killed and their blood was harvested in heparinised syringes (approximately 1 ml per mouse) for radiolabelling. Red blood cells (RBCs) were centrifuged at 1000 rpm for 5 min and resuspended in 1 ml of PBS containing Hank's balanced salt solution (HBSS) (Life Technologies). In all, 1 mCi of radiochromium (51Cr) (Amersham) was added to label the RBCs. The labelling reaction was carried out in a 37°C water bath with shaking for 2 h. The 51Cr-labelled RBCs were then washed twice with PBS for 15 min each time and resuspended in HBSS. A 0.2-ml suspension of 51Cr-labelled RBCs was injected intravenously into the tail vein of tumour-bearing mice ((n=10/group), ∼0.05 mCi per mouse). At 10 min after injection of 51Cr-labelled RBCs, 10 μl of blood was collected from the tail vein of each mouse for radioactive count reading. The mice were then killed, the tumours were removed, and radioactive counts of the tumours were recorded with the Packard Auto-Gamma counter (Downers Grove, IL, USA). The blood volume in the tumours was calculated using the formula: blood volume (μl g−1)=tumour radioactive count (cpm g−1)/blood radioactive count (cpm μl−1).
Statistical analysis
Tumour weights, tumour volumes, tumour microvessel densities, and numbers of PCNA-positive cells were compared between groups by using one-way analysis of variance (ANOVA). Functional blood volume in tumours (51Cr labelling) was compared using the Mann–Whitney U test for nonparametric data. All analyses were carried out using InStat Statistical Software (GraphPad Software, San Diego, CA, USA) with P⩽0.05 considered to be significant.
RESULTS
Ephrin-B2 and EphB4 mRNA expression in normal and colon cancer cell lines
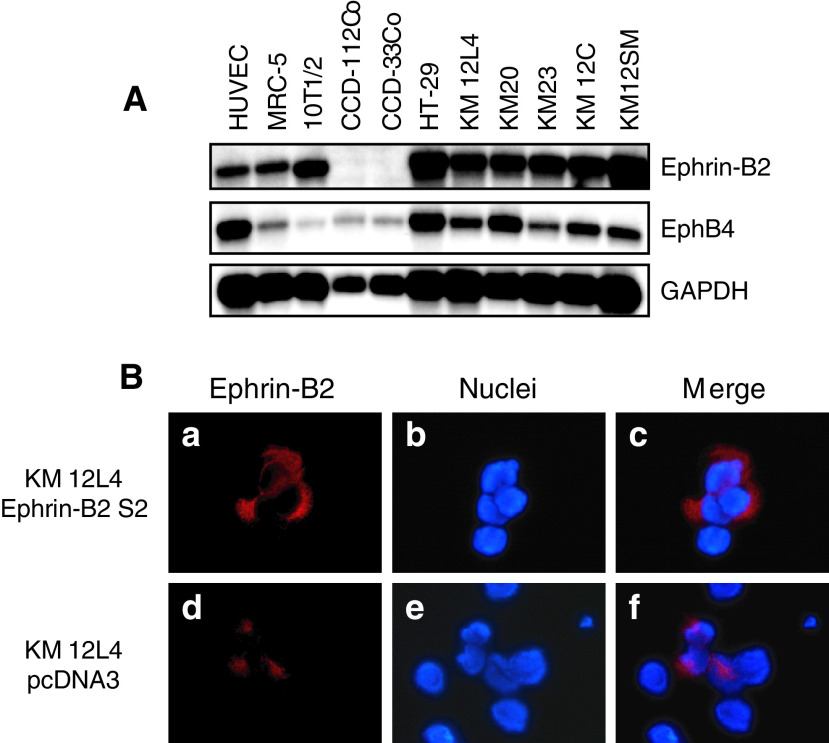
Northern blotting was performed to determine the frequency of expression of ephrin-B2 and EphB4 in human nonmalignant cell lines and colon cancer cell lines. All the nonmalignant cell lines and colon cancer cell lines examined expressed ephrin-B2 and EphB4 (Figure 1A). However, the expression of EphB4 mRNA was lower in the nonmalignant cell lines, with the exception of HUVECs, than in the colon cancer cell lines.
Figure 1.
Expression of ephrin-B2 and EphB4 in cell lines and immunofluorescent staining of Ephrin-B2 in transfected colon cancer cell lines. (A) Coexpression of ephrin-B2 and EphB4 mRNA in the various normal and colon cancer cell lines. GAPDH, glyceraldehyde-phosphate dehydrogenase. (B) Immunofluorescent staining with ephrin-B2 was performed on ephrin-B2 transfected (S2) and vector transfected cells. Representative pictures showed that the staining of ephrin-B2 was much stronger (a, red) on the cell surface (c, merged image) in ephrin-B2-sense-transfected KM12L4 cells compared to the pcDNA3-transfected cells (d, red and f, merged image).
Transfection of Ephrin-B2 in to colon cancer cells and its effect on tumour growth
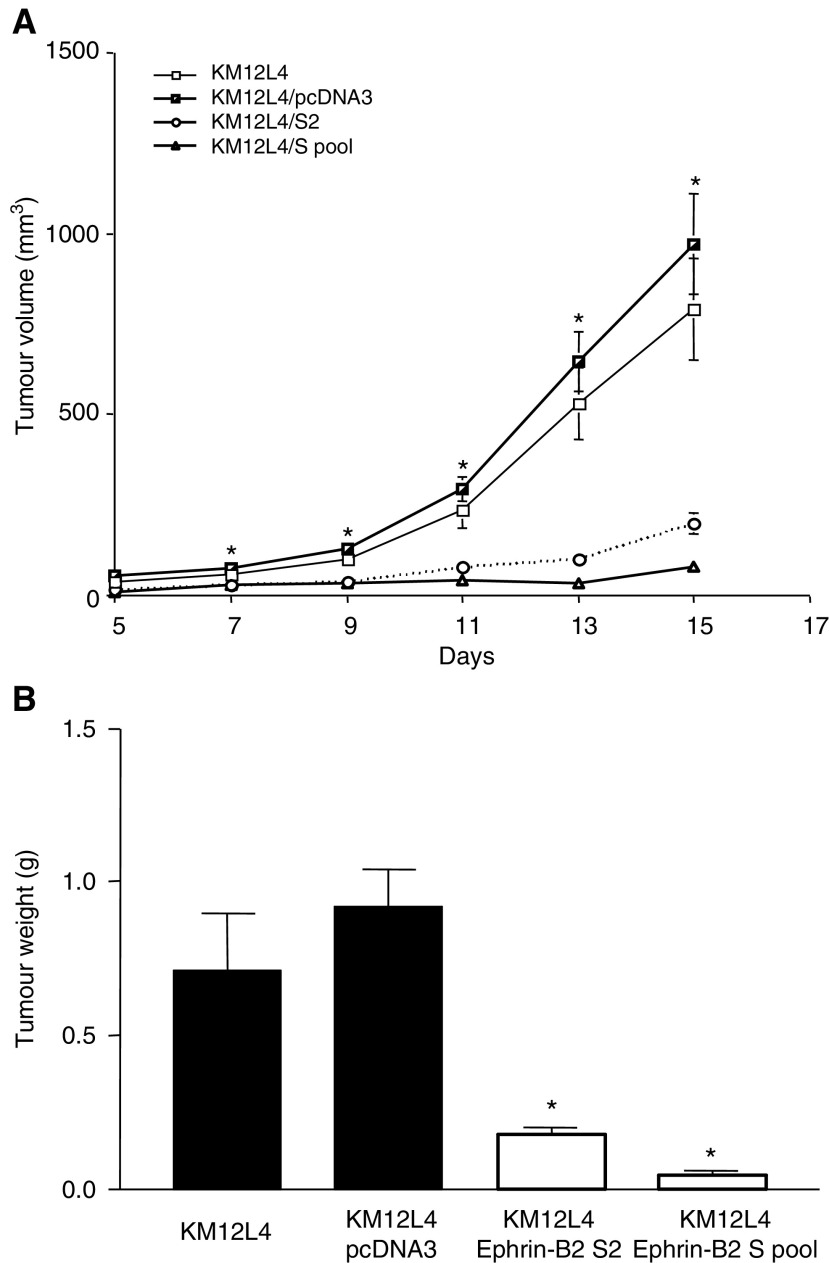
Northern blot analysis demonstrated higher levels of ephrin-B2 in transfected cells (S2 and S pool), as expected (data not shown). In addition, to confirm that the transfected ephrin-B2 protein product localised to the cell surface, we performed immunofluorescent staining on cells in vitro. In ephrin-B2 transfected cells, we noted localisation of the protein to the cell surface and its expression was greater than that in control cells (Figure 1B). As expected, the ephrin-B2 receptor, EphB4, was unchanged in the ephrin-B2-sense transfected cells (data not shown). To determine whether overexpression of ephrin-B2 affects the growth of KM12L4 tumours, we injected KM12L4 tumour cells transfected with a sense construct for ephrin-B2 subcutaneously in nude mice. Tumours from ephrin-B2-sense-transfected cells (S2 and S pool) had significantly smaller volumes (Figure 2A) and weights (Figure 2B) than did tumours from parental KM12L4 or pcDNA3-transfected cells (controls). All mice were killed on day 15 since the tumours in the control groups were of maximal size as outlined by the IACUC. These findings were confirmed in two subsequent studies.
Figure 2.
Effect of ephrin-B2 overexpression on the growth of tumours from ephrin-B2-sense-transfected KM12L4 cells in nude mice. (A) Mean tumour volumes were determined every other day following subcutaneous injection (n=10). On days 7–15, the mean volumes of tumours from ephrin-B2-sense-transfected cells (S2 and S pool) were significantly smaller than those of control tumours from parental KM12L4 cells or vector-transfected cells (*P<0.0001, ANOVA). Error bars indicate the standard error (s.e.m.) of the mean. (B) After 15 days, the mice injected with ephrin-B2-sense-transfected KM12L4 cells (white bars) had significantly less heavy tumours than did control mice (black bars) (*P<0.0001, ANOVA). Error bars indicate s.e.m. of the mean.
Effect of ephrin-B2 overexpression on vessel density and tumour cell proliferation
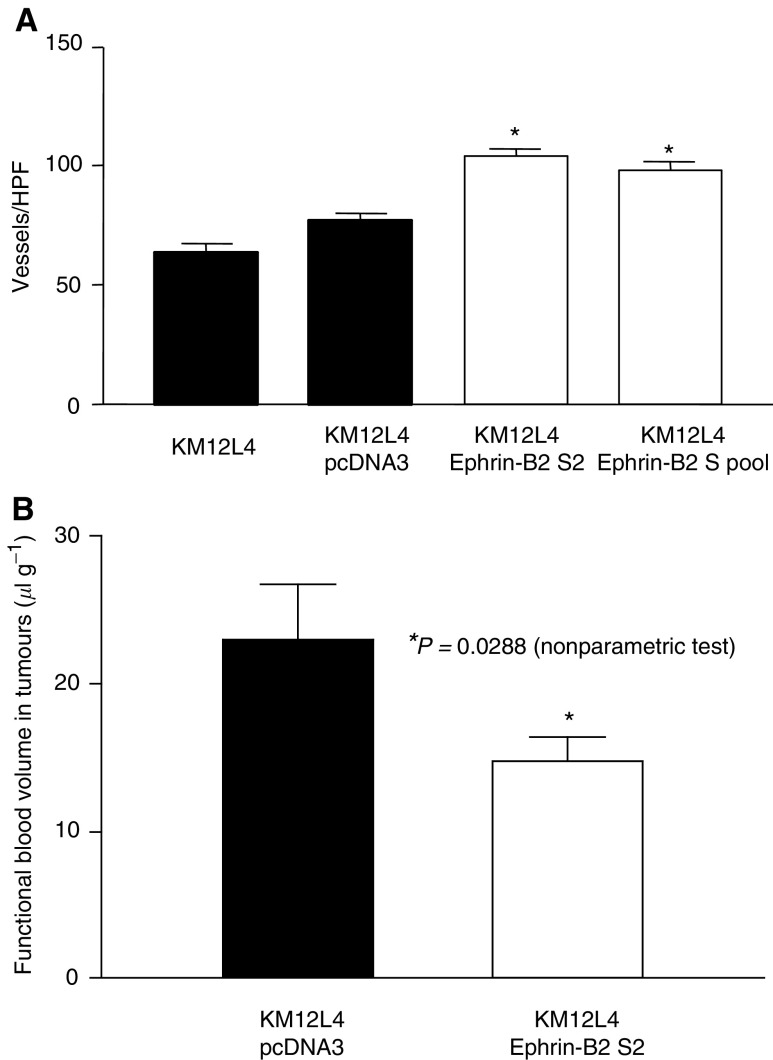
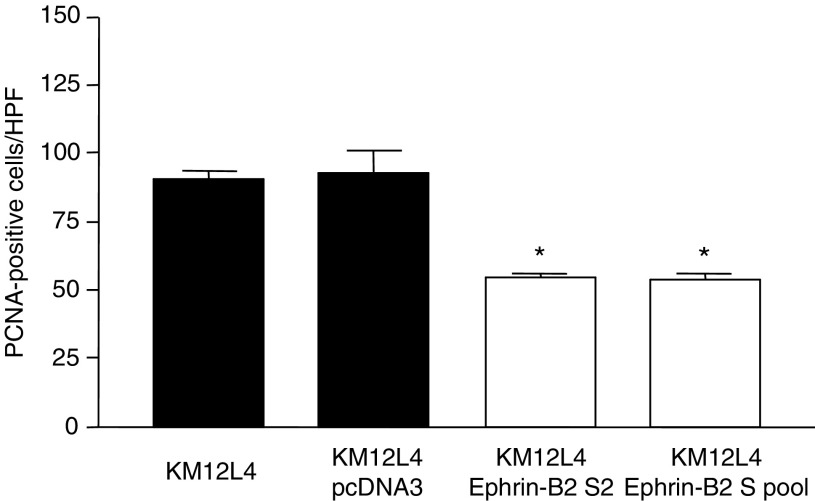
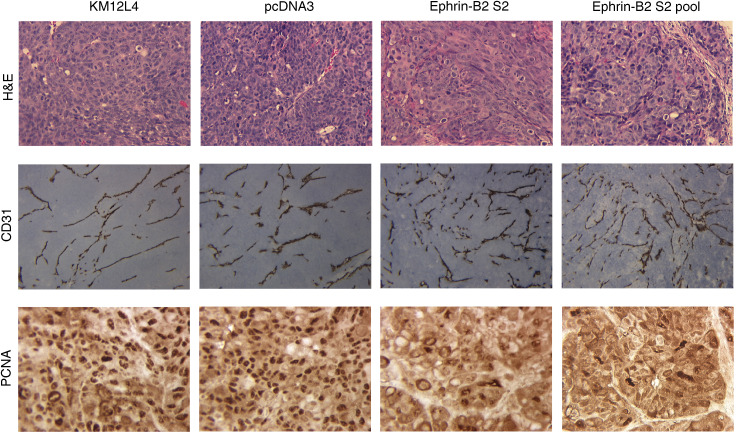
Immunohistochemical staining for CD31 in tumour sections demonstrated that tumours from ephrin-B2-sense-transfected KM12L4 cells (overexpressing ephrin-B2) had significantly higher tumour vessel counts compared to controls (P<0.0001, Figure 3A). Immunohistochemical staining for PCNA to determine tumour cell proliferation rates revealed that ephrin-B2 overexpression led to significantly lower numbers of PCNA-positive cells than in controls (P<0.0001) (Figure 4). However, careful inspection of the vessel morphology demonstrated small, thin-walled vessels (Figure 5). Representative results of immunostaining are shown in Figure 5.
Figure 3.
Effect of ephrin-B2 overexpression on tumour vessel counts and blood volume in tumours. (A) Immunohistochemical staining for CD31 in tumour sections was performed. The mean vessel count in tumours from mice injected with ephrin-B2-sense-transfected KM12L4 cells (white bars) was significantly greater than in control tumours (black bars) (*P<0.0001, ANOVA). Error bars indicate s.e.m. of the mean. HPF, high-power field. (B) 51Cr-labelled RBCs suspended in HBSS were injected into the tail vein of tumour-bearing nude mice. The mean blood volume in tumours from ephrin-B2-sense-transfected KM12L4 cells (S2) was significantly less than in control tumours from vector-transfected cells (*P=0.0288, nonparametric test). Error bars indicate s.e.m. of the mean.
Figure 4.
Effect of ephrin-B2 sense transfection on tumour cell proliferation. Immunohistochemical staining for PCNA in tumour sections was performed. The mean number of PCNA-positive cells in tumours from mice injected with ephrin-B2-sense-transfected KM12L4 cells (white bars) were significantly lower than in control tumours (black bars) (*P<0.0001, ANOVA). Error bars indicate s.e.m. of the mean. HPF, high-power field.
Figure 5.
Immunohistochemical staining H&E, CD31, and PCNA. Immunohistochemical staining with H&E (top row; × 100), CD31 (middle row; × 100), and PCNA (bottom row; × 100) was performed on tumour sections. Representative sections demonstrate more vessels and fewer PCNA positive cells in tumours from ephrin-B2-sense-transfected KM12L4 cells (columns 3 and 4). The vessels in the experimental tumours were morphologically distinct from normal vessels in that they were small and less prominent that those in control tumours (columns 1 and 2).
Effect of ephrin-B2 overexpression on functional blood volume/flow in tumours
Overexpression of ephrin-B2 led to an increase in tumour angiogenesis (vessel count by CD31 staining) but a decrease in tumour size and tumour cell proliferation (PCNA staining). This apparent paradox could be explained by the development of an inefficient blood supply in the ephrin-B2-overexpressing tumours. Therefore, we hypothesised that despite more vessels being present in the ephrin-B2-overexpressing tumours, these tumours have a decreased functional vascular volume/flow due to inefficient blood supply. To investigate this hypothesis, we used 51Cr-labelled RBCs to determine blood volume in the tumours. 51Cr-labelled RBCs were injected intravenously via the tail vein into tumour-bearing mice (pcDNA3 and ephrin-B2 S2 transfectant, 10 mice/each group). Tumours from ephrin-B2-sense-transfected cells had a significantly lower blood volume per gram of tissue than did tumours from pcDNA3-transfected cells (P=0.0288) (Figure 3B).
DISCUSSION
Ephrins consist of two classes: ephrin-As, which bind to the membrane via a glycosylphosphatidylinositol anchor, and ephrin-Bs, which interact with their receptors through the transmembrane cytoplasmic domain. Ephrins/Ephs are essential mediators in the development of the nervous (Flanagan and Vanderhaeghen, 1998) and cardiovascular systems (Wang et al, 1998).
Among the ephrin family members, ephrin-B2 is critically important for embryologic vascular development. Ephrin-B2 knockout mice resulted in severe vascular developmental defects during embryogenesis (Wang et al, 1998; Adams et al, 1999). In murine models, ephrin-B2 is expressed in the arterial ECs but not in the venous ECs (Wang et al, 1998; Adams et al, 1999). Ephrin-B2 is also expressed in the vascular smooth muscle cells of arteries (Shin et al, 2001). Wang et al (1998) reported that ephrin-B2s on arterial ECs interact with EphB4s on venous ECs, and Zhang et al (2001) showed that stromal cells expressing ephrin-B2 are involved in vascular network formation and proliferation of ECs. These data suggest that the interaction of ephrin-B2 and EphB4 leads to reciprocal signalling between arterial ECs, venous ECs, and surrounding vascular supportive cells (Wang et al, 1998; Zhang et al, 2001).
Further studies suggest that ephrin-B2 is involved in tumour angiogenesis. Shin et al (2001) observed ephrin-B2-expressing ECs within the vasculature of tumours, and ephrin-Bs and Ephs have been found to be expressed by a variety of human solid tumours and human tumour cell lines (Vogt et al, 1998; Tang et al, 1999a, 2000; Takai et al, 2001; Liu et al, 2002; Varelias et al, 2002). Overexpression of ephrin-Bs/Ephs is associated with increased tumour growth, increased tumorigenicity, and poorer prognosis in human osteosarcoma, endometrial cancer, and melanomas (Vogt et al, 1998; Takai et al, 2001; Varelias et al, 2002). Vogt et al (1998) reported that ephrin-B2 mRNA expression is higher in metastatic melanoma cell lines than in isogenic primary melanoma cell lines and higher in metastatic human tumours compared to primary melanomas. Vogt et al also showed a significant increase in ephrin-B2 mRNA expression in primary melanoma cell lines selected for increased tumorigenicity.
We transfected the full-length ephrin-B2 cDNA into the human colon cancer cell line KM12L4 and found that the in vivo growth of ephrin-B2-overexpressing tumours was significantly decreased compared to control tumours. Immunohistochemical staining showed that the tumours overexpressing ephrin-B2 had increased vessel counts but decreased tumour cell proliferation. Theoretically, decreases in tumour growth can be attributed to several broad categories of processes: (1) increased tumour cell death, (2) decreased tumour cell proliferation, and (3) alterations in angiogenesis that can affect both processes. Using Western blot analysis, we determined the protein expression profiles for regulators of the cell cycle (cyclins A, D1, D3, and E; cyclin-dependent kinases 2 and 4; and cyclin-dependent kinase inhibitors p21 and p27) and of apoptosis (the oncogene murine double minute 2; tumour suppressor genes pRB and p53; and apoptosis-related genes caspases 2, 3, and 7) in ephrin-B2-transfected and control cells. These studies were carried out to determine whether ephrin-B2 transfection altered any of these parameters that could directly affect tumour growth in vivo. None of these proteins was altered by ephrin-B2 transfection (data not shown). Furthermore, cell cycle analysis by fluorescence-activated cell sorting showed that the cell cycle distribution of ephrin-B2 transfectants was the same as that of control cells (data not shown). In addition, an in vitro analysis of cell proliferation/number (MTT assay) likewise did not show any differences among groups (data not shown). Therefore, based on these analyses, we concluded that the decrease in tumour growth observed in the current study was not due to changes in the cell cycle or tumour cell apoptosis. We therefore hypothesised that despite the increase in microvessel density, these vessels were dysfunctional. To evaluate the ‘functional’ tumour vascular volume, we injected 51Cr-labelled RBCs and harvested blood and tumours to obtain the relative radioactivity. Tumours from ephrin-B2-transfected cells had a significantly smaller tumour blood volume despite having an increase in microvessel density. These data demonstrated that overexpression of ephrin-B2 can increase tumour microvessel density but that these vessels may be ‘dysfunctional’. The fact that these tumour vessels were dysfunctional is similar to the findings of Jain and co-workers (Jain, 2001). Our observation suggests that vessel density, although typically associated with angiogenesis, does not always reflect the functional status of the vascular network in tumours. Functional studies such as noninvasive imaging techniques may more accurately reflect the true functional status of the tumour microvascular bed than do anatomic or morphologic studies.
In summary, our study demonstrated two novel findings with potentially important implications. The first was that overexpression of ephrin-B2 on tumour cells can markedly decrease the growth of SQ colon cancer xenografts. The second interesting finding was that this decrease in tumour growth occurred despite an increase in tumour vessel counts. Our experiment with 51Cr-labelled RBCs suggested that tumour vessel count does not always correlate with the ‘functional status’ of the vasculature. This further questions the value of vessel counts as a measure of angiogenic or antiantiangiogenic activity (Hlatky et al, 2002) and suggests that functional studies more accurately measuring blood flow may be a better indicator of the functional tumour vascular network than morphologic or anatomic studies.
Acknowledgments
We thank Melissa G. Burkett from the Department of Scientific Publications and Rita Hernandez from the Department of Surgical Oncology at The University of Texas M.D. Anderson Cancer Center for editorial assistance. We are grateful for the insight and technical expertise of Mollianne McGahren and Khandan Keyomarsi of the Department of Experimental Radiation Oncology at The University of Texas M.D. Anderson Cancer Center. This work was supported in part by grants from the Jon and Susie Hall Fund for Colon Cancer Research and the Gillson Longenbaugh Foundation (LME), NIH Grant T-32 CA09599 (SAA), and NIH Cancer Center Support Grant CA16672.
References
- Adams RH, Wilkinson GA, Weiss C, Diella F, Gale NW, Deutsch U, Risau W, Klein R (1999) Roles of ephrinB ligands and EphB receptors in cardiovascular development: demarcation of arterial/venous domains, vascular morphogenesis, and sprouting angiogenesis. Genes Dev 13: 295–306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmad SA, Liu W, Jung YD, Fan F, Wilson M, Reinmuth N, Shaheen RM, Bucana CD, Ellis LM (2001) The effects of angiopoietin-1 and -2 on tumor growth and angiogenesis in human colon cancer. Cancer Res 61: 1255–1259 [PubMed] [Google Scholar]
- Bruce V, Olivieri G, Eickelberg O, Miescher GC (1999) Functional activation of EphA5 receptor does not promote cell proliferation in the aberrant EphA5 expressing human glioblastoma U-118 MG cell line. Brain Res 821: 169–176 [DOI] [PubMed] [Google Scholar]
- Bruckner K, Pasquale EB, Klein R (1997) Tyrosine phosphorylation of transmembrane ligands for Eph receptors. Science 275: 1640–1643 [DOI] [PubMed] [Google Scholar]
- Ellis LM, Liu W, Wilson M (1996) Down-regulation of vascular endothelial growth factor in human colon carcinoma cell lines by antisense transfection decreases endothelial cell proliferation. Surgery 120: 871–878 [DOI] [PubMed] [Google Scholar]
- Eph_Nomenclature_Committee (1997) Unified nomenclature for Eph family receptors and their ligands, the ephrins. Eph Nomenclature Committee [letter]. Cell 90: 403–404 [DOI] [PubMed] [Google Scholar]
- Flanagan JG, Vanderhaeghen P (1998) The ephrins and Eph receptors in neural development. Annu Rev Neurosci 21: 309–345 [DOI] [PubMed] [Google Scholar]
- Gale NW, Yancopoulos GD (1999) Growth factors acting via endothelial cell-specific receptor tyrosine kinases: VEGFs, angiopoietins, and ephrins in vascular development. Genes Dev 13: 1055–1066 [DOI] [PubMed] [Google Scholar]
- Helbling PM, Saulnier DM, Brandli AW (2000) The receptor tyrosine kinase EphB4 and ephrin-B ligands restrict angiogenic growth of embryonic veins in Xenopus laevis. Development 127: 269–278 [DOI] [PubMed] [Google Scholar]
- Hlatky L, Hahnfeldt P, Folkman J (2002) Clinical application of antiangiogenic therapy: microvessel density, what it does and doesn't tell us. J Natl Cancer Inst 94: 883–893 [DOI] [PubMed] [Google Scholar]
- Holland SJ, Gale NW, Mbamalu G, Yancopoulos GD, Henkemeyer M, Pawson T (1996) Bidirectional signalling through the EPH-family receptor Nuk and its transmembrane ligands. Nature 383: 722–725 [DOI] [PubMed] [Google Scholar]
- Jain RK (2001) Normalizing tumor vasculature with anti-angiogenic therapy: a new paradigm for combination therapy. Nat Med 7: 987–989 [DOI] [PubMed] [Google Scholar]
- Kiyokawa E, Takai S, Tanaka M, Iwase T, Suzuki M, Xiang YY, Naito Y, Yamada K, Sugimura H, Kino I (1994) Overexpression of ERK, an EPH family receptor protein tyrosine kinase, in various human tumors. Cancer Res 54: 3645–3650 [PubMed] [Google Scholar]
- Liu W, Ahmad SA, Jung YD, Reinmuth N, Fan F, Bucana CD, Ellis LM (2002) Coexpression of ephrin-Bs and their receptors in colon carcinoma. Cancer 94: 934–939 [DOI] [PubMed] [Google Scholar]
- Morikawa K, Walker SM, Jessup JM, Fidler IJ (1988) In vivo selection of highly metastatic cells from surgical specimens of different primary human colon carcinomas implanted into nude mice. Cancer Res 48: 1943–1948 [PubMed] [Google Scholar]
- Nakamoto M, Bergemann AD (2002) Diverse roles for the Eph family of receptor tyrosine kinases in carcinogenesis. Microsc Res Tech 59: 58–67 [DOI] [PubMed] [Google Scholar]
- Ogawa K, Pasqualini R, Lindberg RA, Kain R, Freeman AL, Pasquale EB (2000) The ephrin-A1 ligand and its receptor, EphA2, are expressed during tumor neovascularization. Oncogene 19: 6043–6052 [DOI] [PubMed] [Google Scholar]
- Shin D, Garcia-Cardena G, Hayashi S, Gerety S, Asahara T, Stavrakis G, Isner J, Folkman J, Gimbrone Jr MA, Anderson DJ (2001) Expression of ephrinB2 identifies a stable genetic difference between arterial and venous vascular smooth muscle as well as endothelial cells, and marks subsets of microvessels at sites of adult neovascularization. Dev Biol 230: 139–150 [DOI] [PubMed] [Google Scholar]
- Takai N, Miyazaki T, Fujisawa K, Nasu K, Miyakawa I (2001) Expression of receptor tyrosine kinase EphB4 and its ligand ephrin-B2 is associated with malignant potential in endometrial cancer. Oncol Rep 8: 567–573 [DOI] [PubMed] [Google Scholar]
- Tang XX, Brodeur GM, Campling BG, Ikegaki N (1999a) Coexpression of transcripts encoding EPHB receptor protein tyrosine kinases and their ephrin-B ligands in human small cell lung carcinoma. Clin Cancer Res 5: 455–460 [PubMed] [Google Scholar]
- Tang XX, Evans AE, Zhao H, Cnaan A, London W, Cohn SL, Brodeur GM, Ikegaki N (1999b) High-level expression of EPHB6, EFNB2, and EFNB3 is associated with low tumor stage and high TrkA expression in human neuroblastomas. Clin Cancer Res 5: 1491–1496 [PubMed] [Google Scholar]
- Tang XX, Zhao H, Robinson ME, Cohen B, Cnaan A, London W, Cohn SL, Cheung NK, Brodeur GM, Evans AE, Ikegaki N (2000) Implications of EPHB6, EFNB2, and EFNB3 expressions in human neuroblastoma. Proc Natl Acad Sci USA 97: 10936–10941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varelias A, Koblar SA, Cowled PA, Carter CD, Clayer M (2002) Human osteosarcoma expresses specific ephrin profiles: implications for tumorigenicity and prognosis. Cancer 95: 862–869 [DOI] [PubMed] [Google Scholar]
- Vogt T, Stolz W, Welsh J, Jung B, Kerbel RS, Kobayashi H, Landthaler M, McClelland M (1998) Overexpression of Lerk-5/Eplg5 messenger RNA: a novel marker for increased tumorigenicity and metastatic potential in human malignant melanomas. Clin Cancer Res 4: 791–797 [PubMed] [Google Scholar]
- Walker-Daniels J, Coffman K, Azimi M, Rhim JS, Bostwick DG, Snyder P, Kerns BJ, Waters DJ, Kinch MS (1999) Overexpression of the EphA2 tyrosine kinase in prostate cancer. Prostate 41: 275–280 [DOI] [PubMed] [Google Scholar]
- Wang HU, Chen ZF, Anderson DJ (1998) Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4 [see comments]. Cell 93: 741–753 [DOI] [PubMed] [Google Scholar]
- Workman P, Twentyman P, Balkwill F, Balmain A, Chaplin D, Double J, Embleton J, Newell D, Raymond R, Stables J, Stephens T, Wallace J (1998) United Kingdom Co-ordinating Committee on Cancer Research (UKCCCR) guidelines for the welfare of animals in experimental neoplasia (second edition). Br J Cancer 77: 1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue Y, Widmer DA, Halladay AK, Cerretti DP, Wagner GC, Dreyer JL, Zhou R (1999) Specification of distinct dopaminergic neural pathways: roles of the Eph family receptor EphB1 and ligand ephrin-B2. J Neurosci 19: 2090–2101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XQ, Takakura N, Oike Y, Inada T, Gale NW, Yancopoulos GD, Suda T (2001) Stromal cells expressing ephrin-B2 promote the growth and sprouting of ephrin-B2(+) endothelial cells. Blood 98: 1028–1037 [DOI] [PubMed] [Google Scholar]