Abstract
Proteolysis-inducing factor (PIF) is a sulphated glycoprotein produced by cachexia-inducing tumours, which initiates muscle protein degradation through an increased expression of the ubiquitin–proteasome proteolytic pathway. The role of kinase C (PKC) in PIF-induced proteasome expression has been studied in murine myotubes as a surrogate model of skeletal muscle. Proteasome expression induced by PIF was attenuated by 4α-phorbol 12-myristate 13-acetate (100 nM) and by the PKC inhibitors Ro31-8220 (10 μM), staurosporine (300 nM), calphostin C (300 nM) and Gö 6976 (200 μM). Proteolysis-inducing factor-induced activation of PKCα, with translocation from the cytosol to the membrane at the same concentration as that inducing proteasome expression, and this effect was attenuated by calphostin C. Myotubes transfected with a constitutively active PKCα (pCO2) showed increased expression of proteasome activity, and a longer time course, compared with their wild-type counterparts. In contrast, myotubes transfected with a dominant-negative PKCα (pKS1), which showed no activation of PKCα in response to PIF, exhibited no increase in proteasome activity at any time point. Proteolysis-inducing factor-induced proteasome expression has been suggested to involve the transcription factor nuclear factor-κB (NF-κB), which may be activated through PKC. Proteolysis-inducing factor induced a decrease in cytosolic I-κBα and an increase in nuclear binding of NF-κB in pCO2, but not in pKS1, and the effect in wild-type cells was attenuated by calphostin C, confirming that it was mediated through PKC. This suggests that PKC may be involved in the phosphorylation and degradation of I-κBα, induced by PIF, necessary for the release of NF-κB from its inactive cytosolic complex.
Keywords: Proteolysis-inducing factor, protein kinase C, nuclear factor-κB, proteasome expression
Loss of skeletal muscle in cancer cachexia results in asthenia, immobility and eventually death through impairment of respiratory function. Nutritional supplementation alone is unable to reverse this wasting process (Evans et al, 1985), suggesting that the balance between protein synthesis and degradation is impaired, especially since visceral protein reserves are preserved and may even increase (Fearon, 1992). Thus, while protein synthesis in skeletal muscle of cachectic patients is impaired (Lundholm et al, 1976), there is also an increase in protein degradation (Lundholm et al, 1982). An increased activity of the ubiquitin–proteasome proteolytic pathway is considered to be the major factor for this increased protein degradation (Williams et al, 1999).
Tumour production of a sulphated glycoprotein called proteolysis-inducing factor (PIF) may be responsible for the progressive loss of skeletal muscle in cancer cachexia (Todorov et al, 1996). Proteolysis-inducing factor is only produced by cachexia-inducing tumours, and when purified and administered to mice it induces a specific loss of skeletal muscle, while visceral protein is maintained or even increased, as in cancer cachexia (Lorite et al, 1998). Using a surrogate model of skeletal muscle, PIF was shown to inhibit protein synthesis and increase protein degradation (Smith et al, 1999). The increased protein degradation was shown to arise from an increased expression of the ubiquitin–proteasome proteolytic pathway (Lorite et al, 2001).
Protein degradation induced by PIF was accompanied by an increased release of arachidonic acid from membrane phospholipids and its subsequent metabolism to 15-hydroxyeicosatetraenoic acid (15-HETE), which was shown to be related to protein catabolism. Both arachidonic acid and lipoxygenase metabolites have been shown to activate protein kinase C (PKC) (Fan et al, 1990), which may play a role in PIF-induced proteasome expression. Phosphorylation of proteasome subunits may be important in the regulation of proteasome activity, since some proteasome subunits have potential tyrosine (Tanaka et al, 1990) and serine/threonine (Heinemeyer et al, 1994) phosphorylation sites and dephosphorylation by acid phosphatase have been shown to lower proteasome activity significantly (Mason et al, 1996). At least one subunit (S4) has several potential PKC phosphorylation sites (Dubiel et al, 1992). Alternatively, PKC may play a role in activation of the transcription factor nuclear factor-κB (NF-κB), which has been shown to be involved in the production of interleukin-8 (IL-8), IL-6, C-reactive protein and ICAM-1 in liver cells (Watchorn et al, 2001), and may also be involved in PIF-induced proteasome expression (Whitehouse and Tisdale, 2003). Protein kinase C has been suggested as being an upstream activator of the I-κB kinase complex (IKK) (Lallena et al, 1999; Trushin et al, 1999; Vertegaal et al, 2000) leading to I-κBα phosphorylation and ubiquitination and the subsequent processing of the 26S proteasome followed by the translocation of NF-κB into the nucleus.
The present study investigates the role of PKC in PIF-induced proteasome expression and its relationship to activation of NF-κB in C2C12 murine myotubes.
MATERIALS AND METHODS
Materials
Foetal calf serum (FCS), horse serum (HS) and Dulbecco's modified Eagle's medium (DMEM) were purchased from Invitrogen (Paisley, Scotland). Mouse monoclonal antibodies to proteasome 20S α-subunits were from Affiniti Research Products (Exeter, UK). Rabbit polyclonal antisera to ubiquitin conjugating enzyme (E214k) were a gift from Dr Simon Wing, McGill University (Montreal, Canada). Rabbit polyclonal antisera to murine I-κBα, β-tubulin and PKCα were from Calbiochem (Herts, UK) as were PMA, Ro31-8220, calphostin C, staurosporine and Gö 6976. Rabbit polyclonal antisera to mouse actin were from Sigma-Aldridge (Dorset, UK). Peroxidase-conjugated goat antirabbit and rabbit antimouse secondary antibodies were from Dako Ltd (Cambridge, UK). Hybond™ nitrocellulose membranes and enhanced chemiluminescence (ECL) were from Amersham Life Science Products (Bucks, UK). Electrophoretic-mobility shift (EMSA) gel shift assay kits were from Panomics (California, USA). Escherichia coli DH5α cells were from Invitrogen (Paisley, Scotland). Constitutively active and mutant plasmids of PKCα were a gift from Prof. Peter Parker (Cancer Research, UK). The insert A25E PKCα is constitutively active due to a deletion of amino acids 22–28 in the N-terminal region and is expressed via the pCO2 vector (Pears et al, 1990). Protein kinase C α (T/A)3 is a dominant-negative mutant expressed in pKS1 (Bornancin and Parker, 1996). Plasmid DNA was purified using the WIZARD® PureFection purification system (Promega, Southampton, UK) according to the manufacturer's protocol. Primers for PCR analysis were from MWG Biotech (Ebersberg, Germany). GeneJuice™ for transfection studies was purchased from Calbiochem (Herts, UK).
Transformation of bacteria
E. coli DH5α were transformed with both constitutively active and mutant PKCα using heat shock, and selected with ampicillin (100 μg ml−1). Positive clones were identified using primers with homology to bovine PKC (forward 5′-CAC CTG TGA TAT GAA CGT GC-3′ reverse 5′-GAA GTT GAA GTC CGT GAG C-3′). The product was about 600 bp as determined on a 2% agarose gel. Plasmid DNA was extracted from positive colonies grown overnight in an LB medium containing ampicillin (100 μg ml−1).
Purification of PIF
PIF was purified from solid MAC16 tumours excised from mice with a weight loss between 20 and 25% as previously described (Todorov et al, 1996; Whitehouse and Tisdale, 2003). Tumours were homogenised in 10 mM Tris-HCl, pH 8.0, containing 0.5 mM phenylmethylsulphonyl fluoride, 0.5 mM EGTA and 1 mM dithiothreitol at a concentration of 5 ml g−1 tumour. The supernatant obtained after addition of ammonium sulphate (40% w v−1) was subjected to affinity chromatography using anti-PIF monoclonal antibody coupled to a solid matrix. The immunogenic fractions were concentrated and used for further studies.
Myogenic cell culture and transfection
The C2C12 myoblast cell line was grown in DMEM supplemented with 10% FCS plus 1% penicillin and streptomycin under an atmosphere of 10% CO2 in air. Transfection was carried out on cells at 50% confluency using GeneJuice™ transfection reagent, according to the manufacturer's protocol and selected by resistance to ampicillin (5 g l−1). Transfected myoblasts were stimulated to differentiate by replacing the growth medium with DMEM supplemented with 2% HS, when the cells reached confluence. Differentiation was allowed to continue for 5–9 days until myotubes were clearly visible, and used for the experiments described in results.
Measurement of proteasome ‘chymotrypsin-like activity
‘Chymotrypsin-like’ enzyme activity was determined fluorimetrically by the method of Orino et al (1991) as previously described (Lorite et al, 2001). Myotubes were washed with ice-cold phosphate-buffered saline (PBS) and sonicated in 20 mM Tris-HCl, pH 7.5, 2 mM ATP, 5 mM MgCl2 and 1 mM dithiothreitol at 4°C. The supernatant formed by centrifugation at 18 000 g for 10 min was used to measure the ‘chymotrypsin-like’ enzyme activity by the release of aminomethyl coumarin (AMC) from the fluorogenic peptide succinyl-LLVY-AMC (0.1 mM). Activity was measured in the presence and absence of the specific proteasome inhibitor lactacystin (10 μM). Only lactacystin-suppressible activity was considered to be proteasome specific.
Western blot analysis
Cytoplasmic proteins, obtained from the above assay, were also used for Western blotting, while the pellet was dissolved in sonicating buffer containing 0.1% Nonidet P40 and used as a source of cell membranes. Both extracts were loaded at 2–5 μg protein and resolved on 10% sodium dodecylsulphate: polyacrylamide gels and transferred to Hybond™ nitrocellulose membrane. Membranes were blocked with 5% Marvel in PBS. The primary antibodies for PKCα, E214k and β-tubulin were used at a dilution of 1 : 100, while antibodies for I-κBα were at 1 : 1000 and 20S proteasome α-subunits at 1 : 1500. The secondary antibodies were used at a dilution of 1 : 2000. Incubation was carried out for 2 h at room temperature, and development was by ECL.
Electrophoresis mobility shift assay
DNA-binding proteins were extracted from myotubes by the method of Andrews and Faller (1991), which utilises hypotonic lysis followed by high salt extraction of nuclei. The EMSA-binding assay was carried out using a Panomics EMSA ‘gel shift’ kit according to the manufacturer's instructions.
Statistical analysis
Differences as means between groups was determined by one-way ANOVA followed by Tukey–Kramer multiple comparison test.
RESULTS
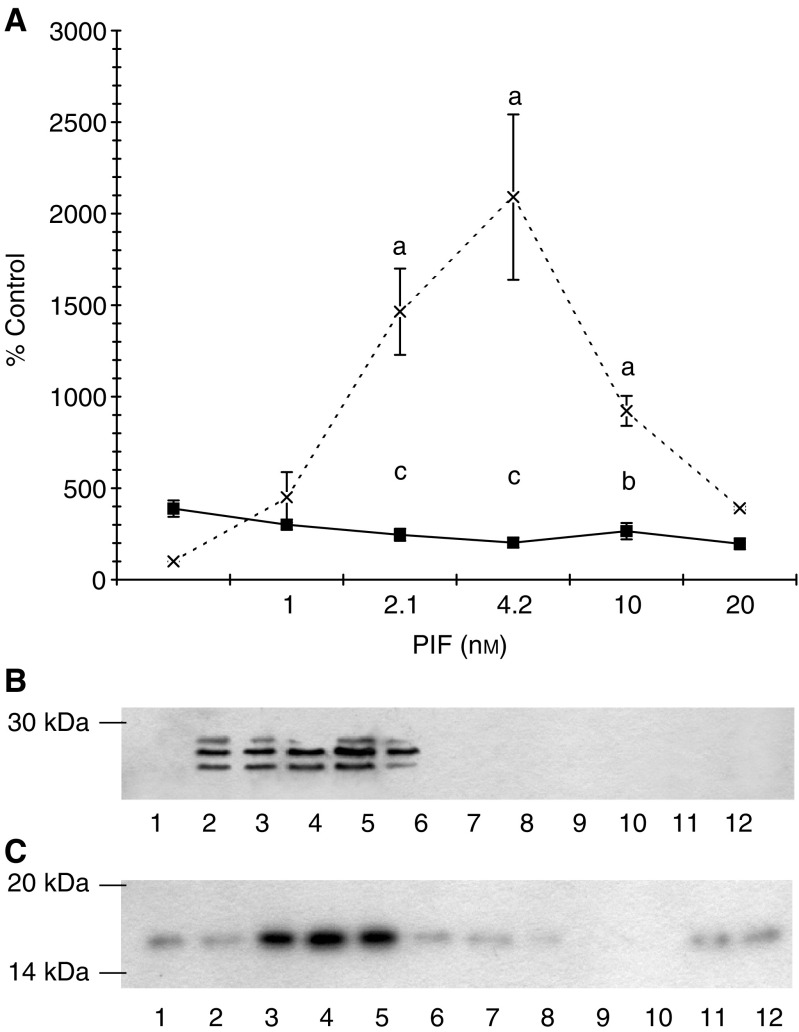
To evaluate the role of PKC in PIF-induced proteasome expression, the effect of excess 4α-phorbol 12-myristate 13-acetate (PMA) on ‘chymotrypsin-like’ enzyme activity, the predominant proteolytic activity of the proteasome (Figure 1A), and on expression of proteasome 20Sα subunits (Figure 1B) and the ubiquitin-conjugating enzyme (E214k) (Figure 1C) was determined in C2C12 myotubes 24 h after PIF addition. Proteolysis-inducing factor produced an increase in ‘chymotrypsin-like’ enzyme activity, proteasome 20Sα subunits and E214k with a maximal effect between 2.1 and 10 nM, and this effect was completely attenuated in myotubes pretreated with PMA. These results suggest that PKC may be important in PIF-induced proteasome expression.
Figure 1.
(A) Effect of PMA on PIF-induced chymotrypsin-like enzyme activity in murine myotubes. Cells were incubated either with PIF alone (×) or in the presence of PMA (100 nM), added 2 h prior to PIF (▪), and the chymotrypsin-like enzyme activity was determined after 24 h, as described in Materials and methods. The experiment was repeated three times (n=9). Differences from control are indicated as aP<0.001, while differences from cells incubated in the presence of PIF alone are shown as bP<0.05 and cP<0.001. (B) Western blot of the effect of PMA on proteasome 20S α-subunits and (C) E214k 24 h after addition of PIF. Cells were incubated with 0 (lanes 1 and 7), 1.0 (lanes 2 and 8), 2.1 (lanes 3 and 9), 4.2 (lanes 4 and 10), 10 (lanes 5 and 11) or 20 nM PIF (lanes 6 and 12) in the absence (lanes 1–6) or presence (lanes 7–12) of PMA (100 nM). A representative blot is shown and the experiment was repeated three times.
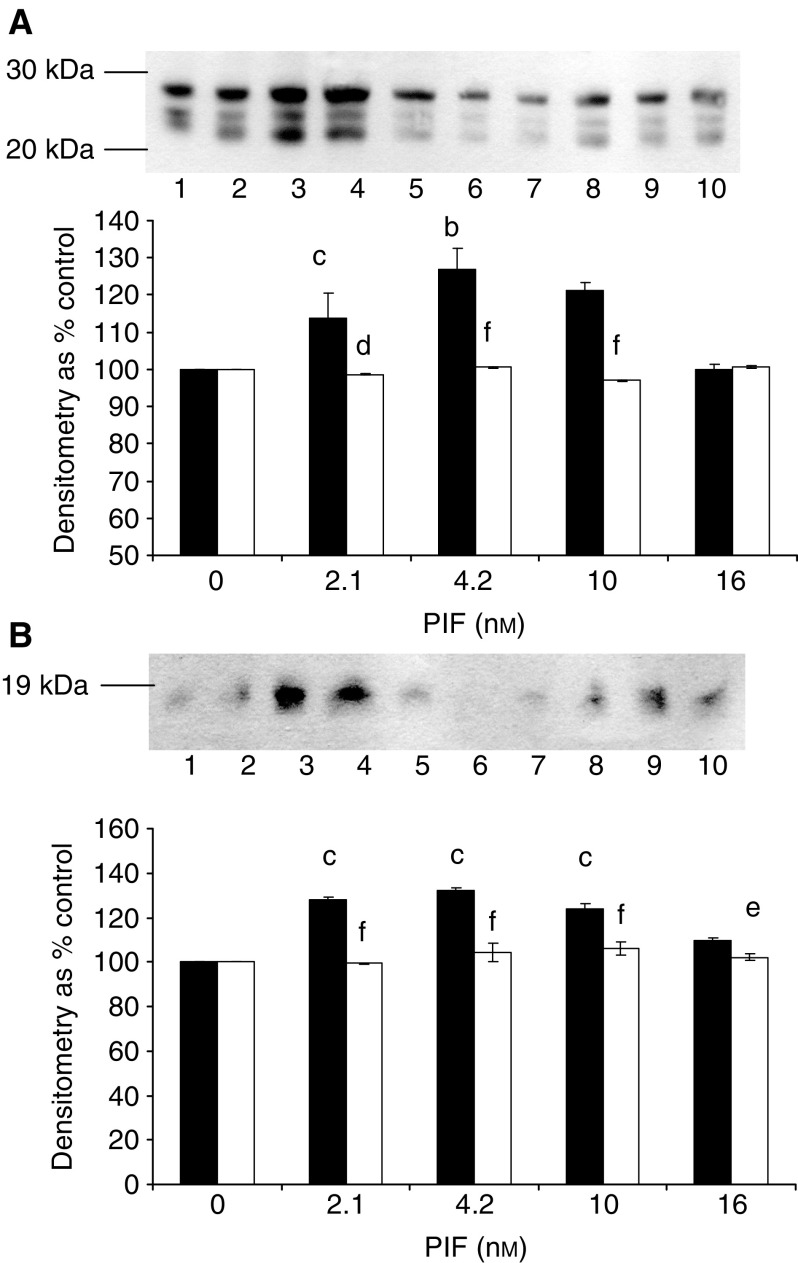
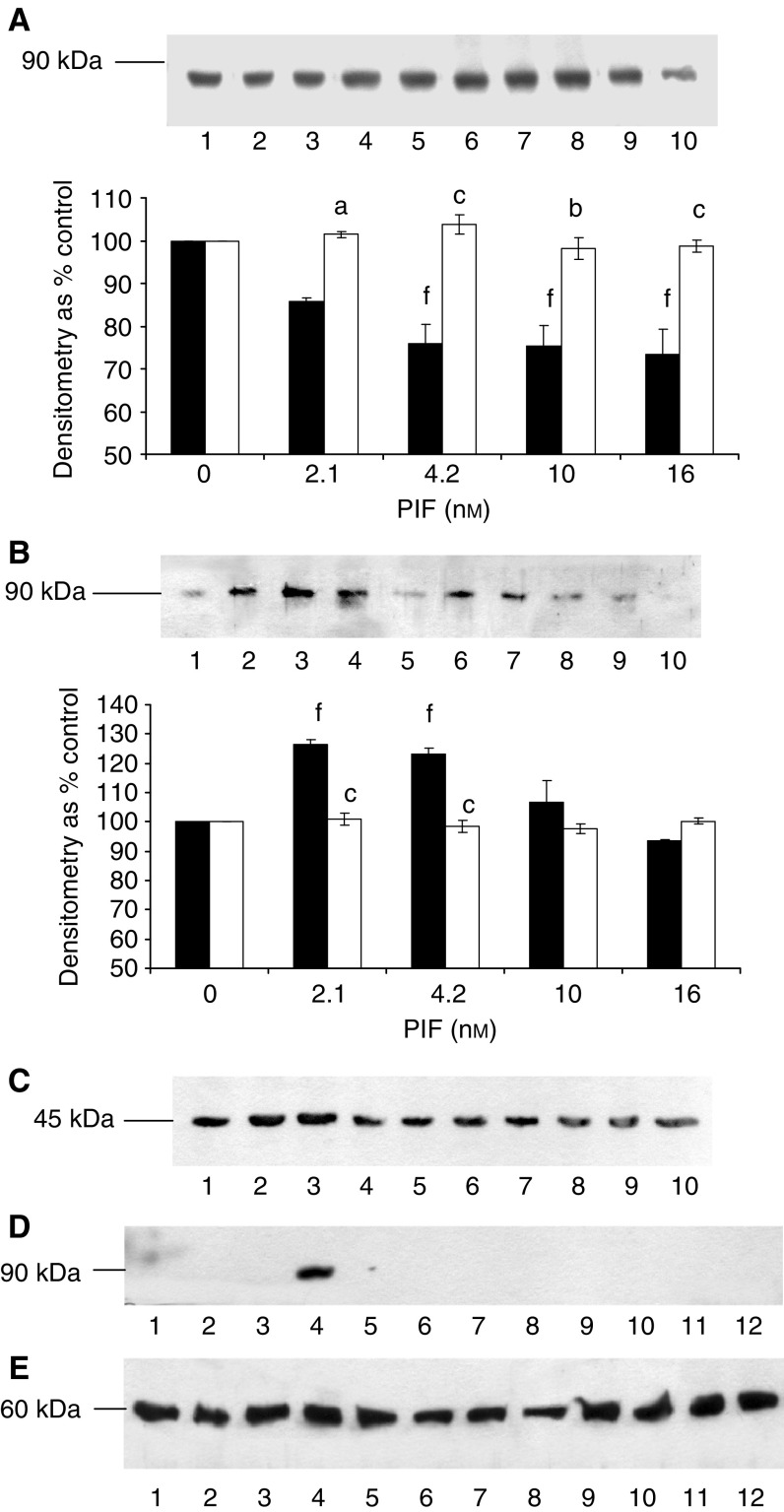
To confirm a role for PKC in this process, the effect of Ro31-8220, a competitive and selective PKC inhibitor (Beltman et al, 1996), staurosporine, a broad-spectrum inhibitor of protein kinases (Couldwell et al, 1994), calphostin C, a highly specific inhibitor of PKC (Jarvis et al, 1994), and Gö 6976, which selectively inhibits PKCα and β, isoenzymes (Wang et al, 1998), on the PIF-induced increase in ‘chymotrypsin-like’ enzyme activity was determined (Figure 2). The PIF-induced enzyme activity was completely attenuated by 10 μM Ro31-8220 (Figure 2A), 300 nM staurosporine (Figure 2B), 300 nM calphostin C (Figure 2C) and 200 μM Gö 6976 (Figure 2D). In addition, calphostin C completely attenuated the PIF-induced increase in proteasome 20S α-subunit expression (Figure 3A) and E214k (Figure 3B). Proteolysis-inducing factor induced a decrease in cytosolic PKC (Figure 4A) and an increase in membrane-bound PKCα (Figure 4B) at the same concentrations as those inducing proteasome expression (Figure 1) and this effect was attenuated by both calphostin C (Figure 4A and B) and eicosapentaenoic acid (EPA) (Figure 4D). These results confirm a role for PKC in PIF-induced proteasome expression, and suggest another mechanism by which EPA may attenuate PIF-induced protein degradation through inhibition of PKC.
Figure 2.
Effect of inhibitors of PKC on PIF-induced chymotrypsin-like enzyme activity. Myotubes were incubated with PIF alone (×) or with Ro 31-8220 (1 μM) (A); staurosporine (300 nM) (B); calphostin C (300 nM) (C); or with Go 6976 (200 μM) (D) added 2 h prior to PIF, and chymotrypsin-like enzyme activity was determined 24 h after addition of PIF. The experiment was repeated three times (n=9). Differences from control are indicated as aP<0.05 and bP<0.001, while differences from cells incubated in the presence of PIF alone are shown as cP<0.05, dP<0.01 and eP<0.001.
Figure 3.
Western blot of the effect of calphostin C on 20S proteasome α-subunit expression (A) and E214k (B) in the presence of PIF. Cells were incubated with 0 (lanes 1 and 6), 2.1 (lanes 2 and 7), 4.2 (lanes 3 and 8), 10 (lanes 4 and 9) or 16.8 nM PIF (lanes 5 and 10) either alone (lanes 1–5) or in the presence of calphostin C (300 nM) and expression was determined after 24 h. A representative blot is shown and the densitometric analysis is based on three replicate blots. Values in the presence of PIF are indicated as ▪ and in the presence of PIF and calphostin C as □. The densitometric analysis of the 20S subunits represents the average of the two major bands. Differences from control are indicated as aP<0.05, bP<0.01 and cP<0.001, while differences from the presence of PIF alone are shown as dP<0.05, eP<0.01 and fP<0.001.
Figure 4.
Effect of PIF on activation of PKCα in murine myotubes in the absence or presence of calphostin C (A, B) or EPA (C). (A) Cytoplasmic and (B) Membrane-bound PKCα after incubation with 0 (lanes 1 and 6), 2.1 (lanes 2 and 7), 4.2 (lanes 3 and 8), 10 (lanes 4 and 9) or 16.8 nM PIF (lanes 5 and 10) for 24 h in the absence (lanes 1–5) or presence (lanes 6–10) of calphostin C (300 nM). The densitometric analysis was based on three replicate blots, and values in the presence of PIF are shown as ▪ and in the presence of PIF and calphostin C as □. Differences from control are shown as cP<0.001, while differences from PIF alone are shown as dP<0.05, eP<0.01 and fP<0.001. (C) Actin loading control for the blots shown in (A, B). (C) Actin loading control for the blots shown in (A, B). (D) Effect of EPA (50 μM) on membrane-bound PKCα in the presence of PIF. Cells were loaded with 0 (lanes 1 and 7), 1.0 (lanes 2 and 8), 2.1 (lanes 3 and 9), 4.2 (lanes 4 and 10), 10 (lanes 5 and 11) or 20 nM PIF (lanes 6 and 12) either in the absence (lanes 1–6) or after 2 h pretreatment with EPA (50 μM), and membrane-bound PKCα was determined after 24 h. (E) β-tubulin loading control for the blot shown in (D).
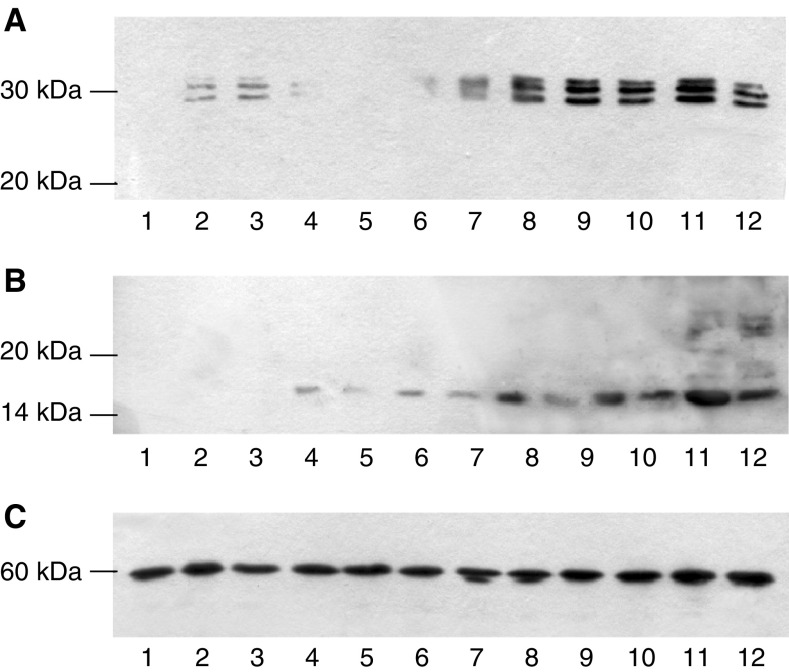
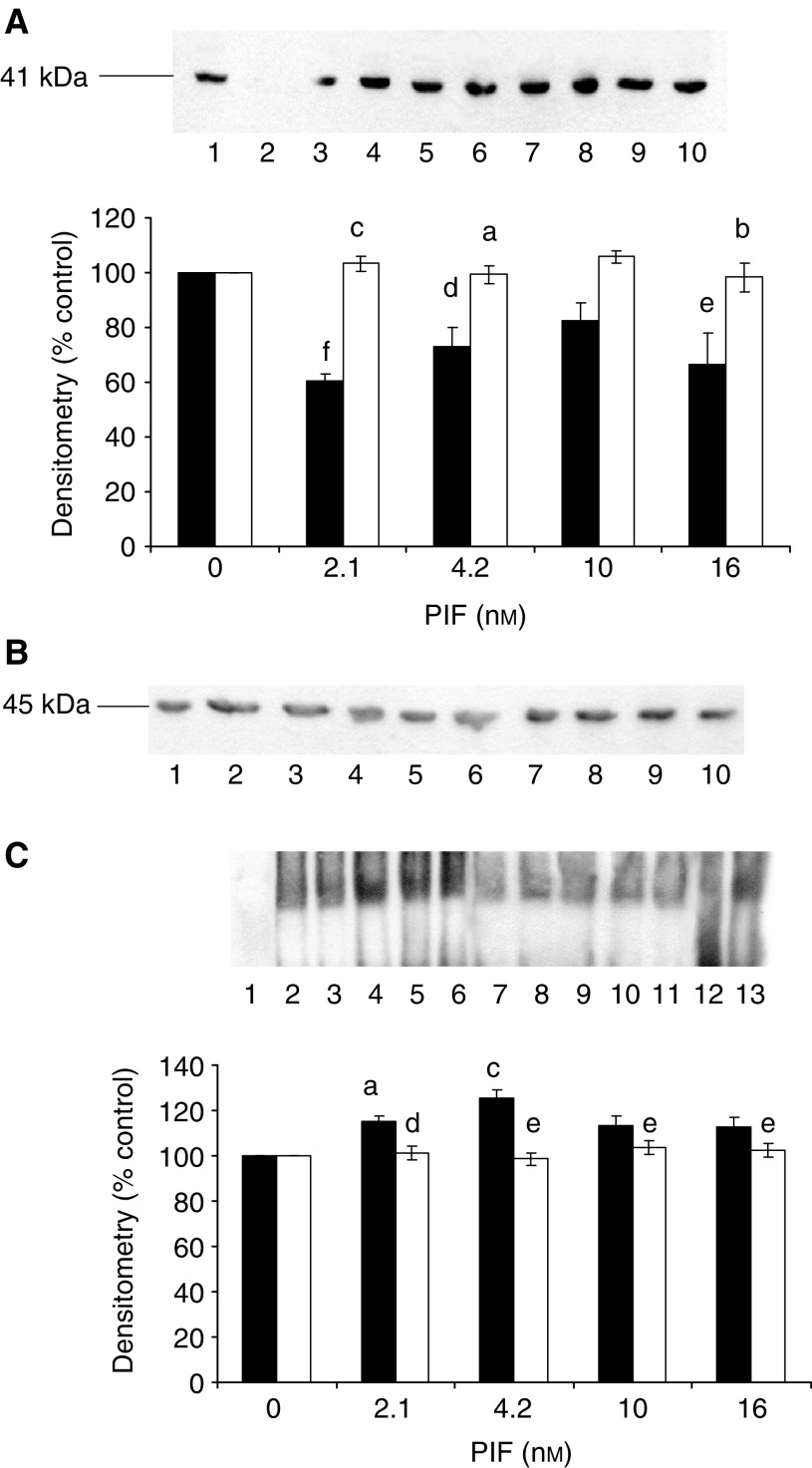
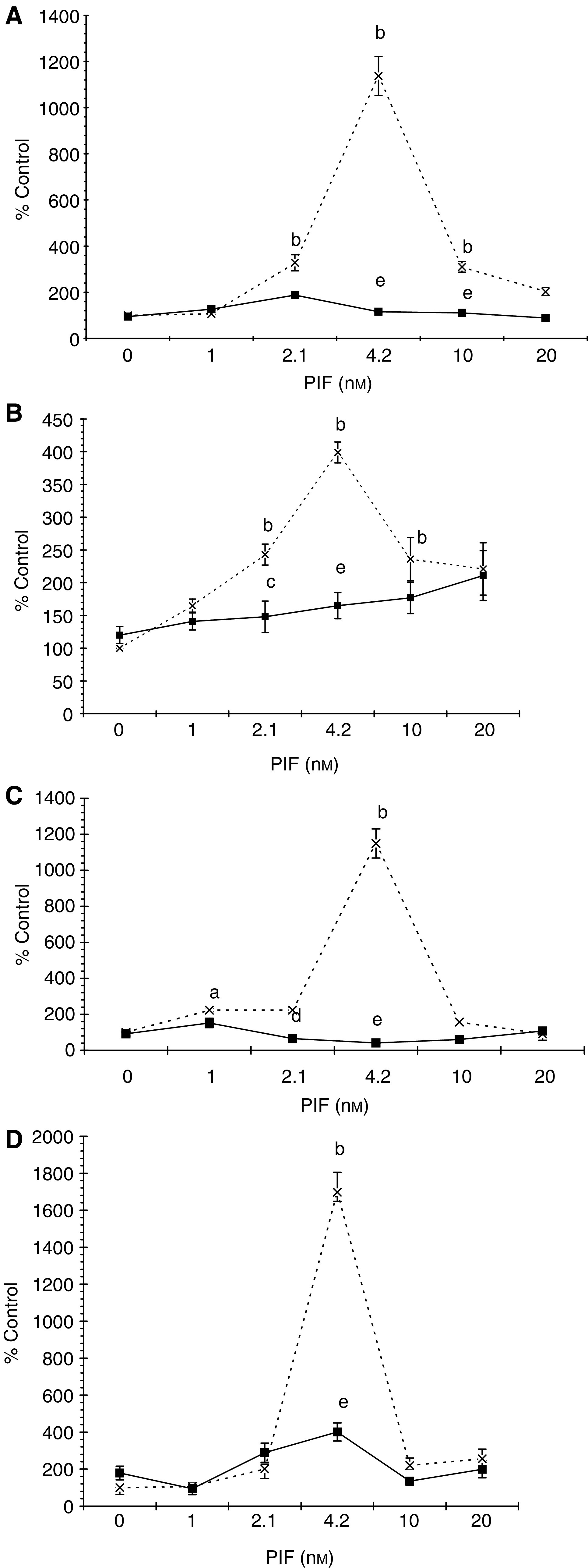
To further substantiate a role for PKC in the induction of proteasome expression by PIF C2C12, myoblasts were transfected with plasmids encoding constitutively active PKC-α (pCO2) and dominant-negative PKC-α (T/A)3 (pKS1) (Bornancin and Parker, 1996; Schonwasser et al, 1998), and induced to differentiate into myotubes. Myotubes transfected with pCO2 showed an increased sensitivity to PIF, as determined by the ‘chymotrypsin-like’ enzyme activity (Figure 5) in comparison with wild-type myotubes, with a significant increase within 3 h of PIF addition (Figure 5A) persisting up to 48 h (Figure 5D). In addition, the elevation of ‘chymotrypsin-like’ enzyme activity in myotubes transfected with pCO2 greatly exceeded that in wild type at all time points. In contrast, myotubes transfected with the dominant-negative PKCα, pKS1 showed no elevation in ‘chymotrypsin-like’ enzyme activity in response to PIF at any time point (Figure 5). These results were confirmed by Western blotting of cellular supernatants for 20S proteasome α-subunit expression (Figure 6A) and E214k (Figure 6B). Proteolysis-inducing factor induced an increase in both proteasome α-subunit expression and E214k in pCO2 but not pKS1, confirming a role for PKC in this process. The ability of PIF to activate PKCα in pCO2, but not in pKS1, was confirmed by Western blotting (Figure 7). The concentrations of PIF causing maximum activation of PKCα were the same as those inducing 20S proteasome α-subunit expression (Figure 6A).
Figure 5.
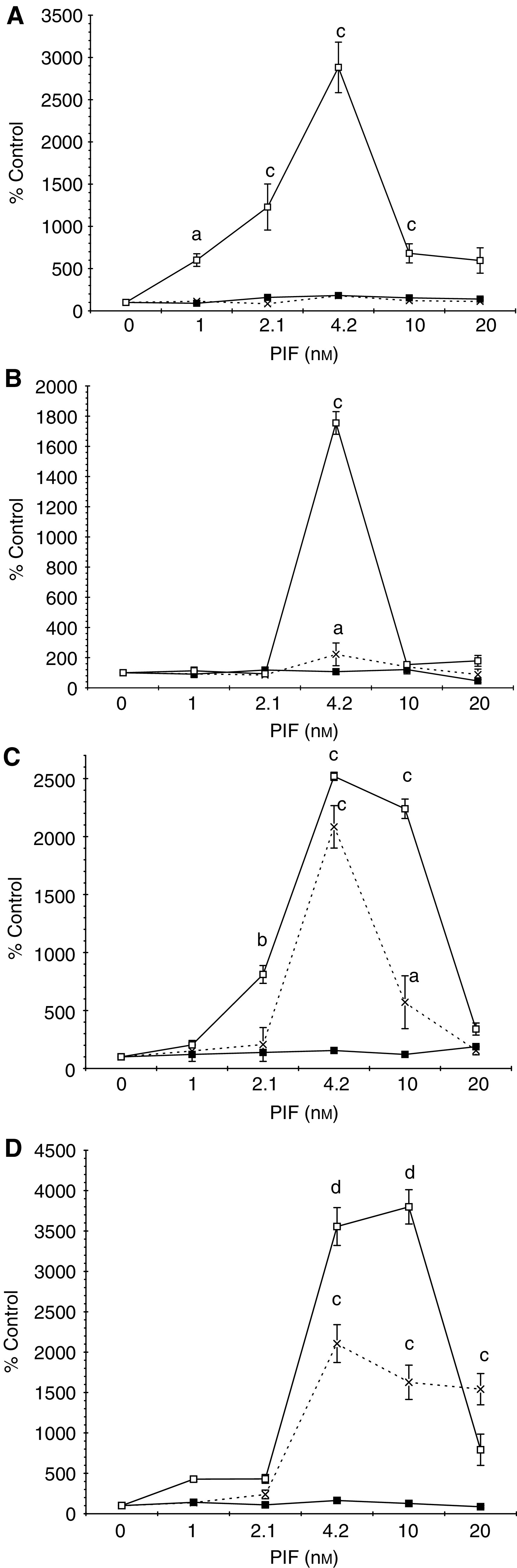
Time course for induction of chymotrypsin-like enzyme activity in wild-type myotubes (×) and in those transfected with constitutively active (pCO2 □) and mutant (pKS1 ▪) PKCα. Myotubes were treated with the indicated concentrations of PIF, and enzyme activity was determined at 3 h (A), 6 h (B), 24 h (C) and 48 h (D). The experiment was repeated three times (n=9). Differences from control or pKS1 are indicated as aP<0.05, bP<0.01 and cP<0.005, while differences from wild-type myotubes are indicated as dP<0.005.
Figure 6.
Western blot of the effect of PIF on 20S proteasome α-subunit expression (A) and E214k (B) in pKS1 (lanes 1–6) and pCO2 (lanes 7–12) after treatment with 0 (lanes 1 and 7), 1.0 (lanes 2 and 8), 2.1 (lanes 3 and 9), 4.2 (lanes 4 and 10), 10 (lanes 5 and 11) and 20 nM PIF (lanes 6 and 12) determined after 24 h. (C) β-tubulin loading control.
Figure 7.
Western blot of the effect of PIF on cytoplasmic (A) and membrane-bound (B) PKCα in pKS1 and pCO2 after 24 h. The lanes are the same as in Figure 6.
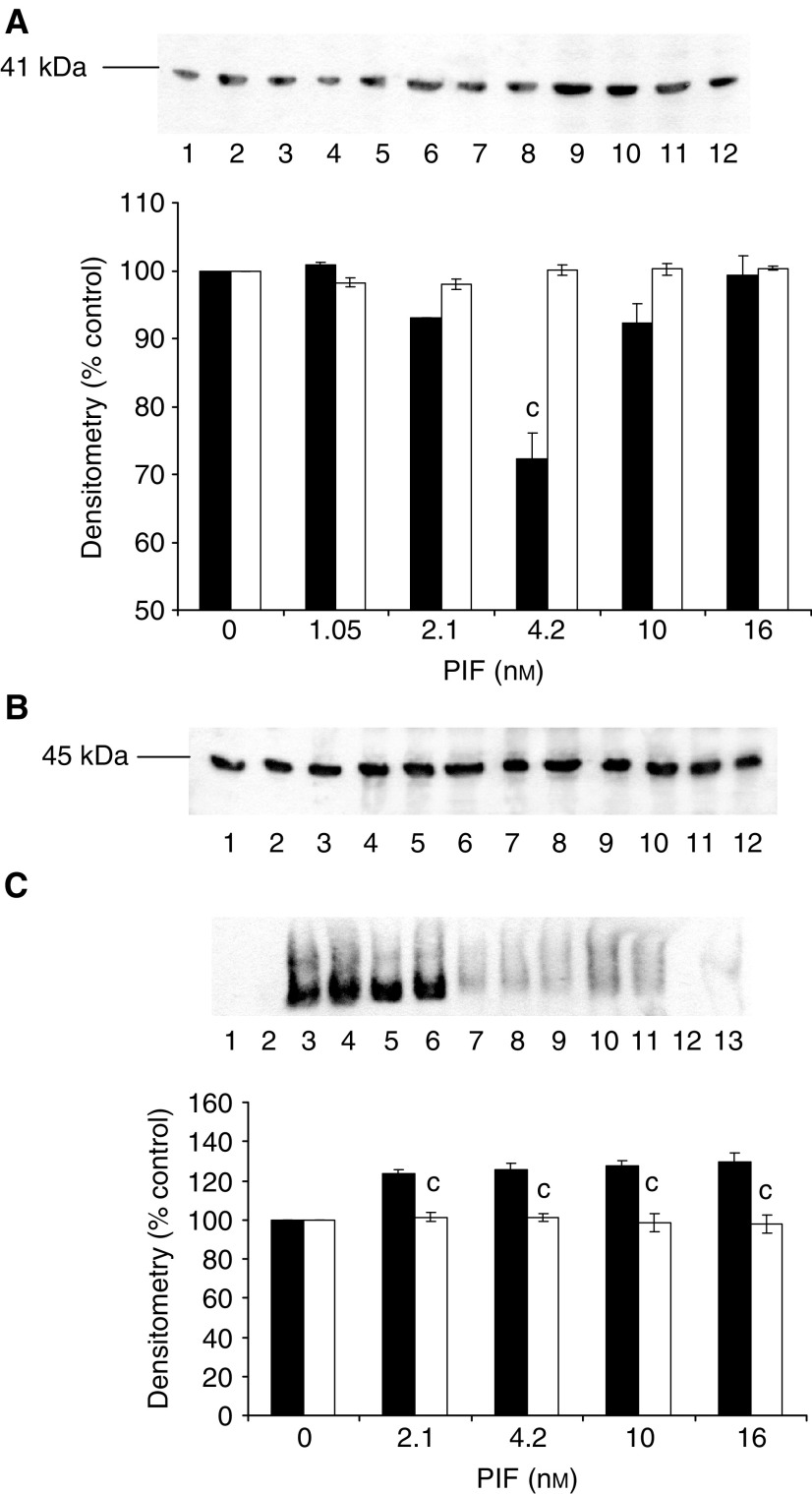
We have recently shown (Whitehouse and Tisdale, 2003) that PIF-induced proteasome expression appears to require activation of NF-κB. One mechanism by which PKC may function in the PIF signalling pathway is activation of IKK with subsequent phosphorylation and degradation of I-κB, and translocation of NF-κB from the cytosol to the nucleus (Vertegaal et al, 2000). Evidence for this hypothesis is provided by the following experiments. Proteolysis-inducing factor induced a decrease in cytoplasmic I-κBα within 30 min of addition to wild-type cells (Figure 8A), accompanied by nuclear accumulation of NF-κB (Figure 8C) and this effect was completely attenuated by calphostin C (Figure 8). In addition, PIF induced a decrease in I-κBα (Figure 9A) and an increase in DNA binding of NF-κB (Figure 9C) in myotubes transfected with constitutively active PKCα (pCO2), but not in those containing dominant-negative PKC-α (pKS1). These results suggest that activation of PKC by PIF in muscle cells leads to I-κBα degradation, nuclear accumulation of NF-κB and an increased proteasome expression leading to increased intracellular protein degradation (Whitehouse and Tisdale, 2003).
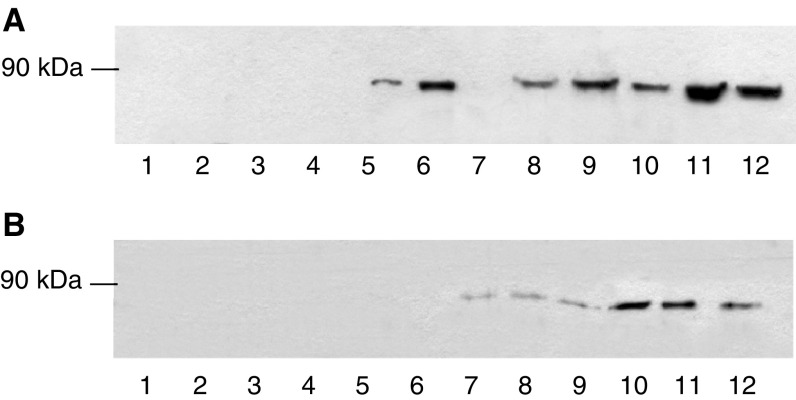
Figure 8.
Effect of PIF and calphostin C on cytoplasmic I-κBα (A) and nuclear-bound NF-κB (C) determined 30 min after PIF addition to murine myotubes. (A) Myotubes were treated with 0 (lanes 1 and 6), 2.1 (lanes 2 and 7), 4.2 (lanes 3 and 8), 10 (lanes 4 and 9) or 16.8 nM PIF (lanes 5 and 10) in the absence (lanes 1–5) or presence (lanes 6–10) of calphostin C (300 nM) added 2 h prior to PIF. (B) Actin loading control for the blot shown in (A). (C) Nuclear levels of NF-κB in myotubes in the absence (lanes 2–6) or presence (lanes 7–11) of calphostin C. Lane 1 is a negative control and lane 13 a positive control and lane 12 contains excess unlabelled NF-κB. The other lanes were the same as in (A). The densitometric analysis is an average of three replicate EMSAs. Differences from control are indicated as aP<0.05 and cP<0.001, while differences in the presence of calphostin C are indicated as dP<0.01 and eP<0.001.
Figure 9.
Effect of PIF on cytoplasmic I-κBα (A) and nuclear-bound NF-κB (C) determined 30 min after PIF addition to murine myotubes transfected with constitutively active (pCO2 □) and mutant (pKS1 ▪) PKCα. (A) Myotubes transfected with either pCO2 (lanes 1–6) or pKS1 (lanes 7–12) were treated with 0 (lanes 1 and 7), 1.05 (lanes 2 and 8), 2.1 (lanes 3 and 9), 4.2 (lanes 4 and 10), 10 (lanes 5 and 11) or 16.8 nM PIF (lanes 6 and 12). The densitometric analysis is an average of three replicate blots. Differences from 0 nM PIF are shown as cP<0.001. (B) Actin loading control for the blot shown in (A). (C) EMSA of NF-κB nuclear binding in murine myotubes transfected with pCO2 (lanes 2 and 6) and pK1 (lanes 7–11). Myotubes were treated with 0 (lanes 2 and 7), 2.1 (lanes 3 and 8), 4.2 (lanes 4 and 9), 10 (lanes 5 and 10) and 16.8 nM PIF (lanes 6 and 11). Lane 1 is a negative control containing the labelled probe without a nuclear extract; lane 12 contains a 100-fold excess of unlabelled NF-κB probe and lane 13 is a positive control for NF-κB (supplied by the manufacturers of the kit). The densitometric analysis is an average of three replicate EMSAs. Differences from control are indicated as bP<0.01 and cP<0.001.
DISCUSSION
Although increased intracellular protein catabolism is a common feature of many disease states, there is little knowledge of the cellular signalling pathways involved, which may be useful in therapeutic intervention. Initial studies suggested that prostaglandin E2 (PGE2) was involved in total protein degradation in skeletal muscle, based on the demonstration in a variety of muscle types that tyrosine release was stimulated by arachidonic acid and PGE2 (Rodemann and Goldberg, 1982) and blocked by prostaglandin synthesis inhibitors (Strelkov et al, 1989). However, other studies (Hasselgren et al, 1990) found no evidence that total or myofibrillar protein breakdown in normal or septic muscle is regulated by PGE2. Studies with PIF showed that total protein breakdown was related to the release of arachidonic acid and formation of PGE2, but that PGE2 was not the eicosanoid responsible for the effect (Smith et al, 1999). Although the arachidonic acid was converted into a range of PGs and HETEs, only one metabolite 15-HETE alone was capable of inducing protein degradation. Further studies showed that 15-HETE induced an increase in expression of the ubiquitin–proteasome pathway, which was responsible for the initiation of protein catabolism and that this process involved the transcription factor NF-κB (Whitehouse et al, 2003).
The present study has investigated the possibility that PKC may act as an intermediate in the PIF signalling pathway transmitting the rise in 15-HETE into activation of NF-κB. The results support the suggestion that PKC plays a central role in the induction of proteasome expression by PIF and thus protein degradation. Previous studies (Smith and Tisdale, 2003) have shown that PIF induces activation of phospholipase C (PLC) as an important signalling event in inducing proteasome expression. Activation of PLC would result in the generation of diacylglycerol (DAG), which would then induce translocation of PKC from the cytosol to the membrane, resulting in the complete activation of the kinase. Indeed, PIF has been shown to induce translocation of PKCα from the cytosol to the membrane at the same concentrations as those inducing proteasome expression. The importance of this step to the induction of proteasome expression by PIF is shown by the attenuation of this process by a range of inhibitors of PKC. In addition, myotubes transfected with a dominant-negative mutant of PKCα also showed no induction of proteasome expression in the presence of PIF. Interestingly, myotubes transfected with constitutively active PKCα showed an increased induction of proteasome expression compared with their wild-type counterparts, confirming the importance of this pathway in the signalling cascade. At present, it is not known which particular isoenzymes of PKC are involved in this process, or indeed whether activation of PKC occurs through production of DAG via PLC or directly through production of 15-HETE.
Attenuation of PIF-induced activation of PKC provides another control point where EPA may interfere with the signalling cascade leading to increased proteasome expression. Eicosapentaenoic acid is an effective anticachectic agent both in murine models of cachexia (Beck et al, 1991) and in weight-losing patients with pancreatic cancer (Barber et al, 1999), and effectively attenuates PIF-induced proteasome expression in murine myotubes (Whitehouse and Tisdale, 2003). Eicosapentaenoic acid inhibits both the release of arachidonic acid from membrane phospholipids and formation of 15-HETE in response to PIF (Smith et al, 1999), and stabilised the NF-κB/I-κB complex in the cytosol, preventing nuclear accumulation of NF-κB (Whitehouse and Tisdale, 2003). This study shows that EPA also attenuates PIF-induced activation of PKC, which may be due to reduced generation of DAG (Sperling et al, 1993). In addition, DAG with an n-3 polyunsaturated fatty acid (PUFA) occupying the sn-2 position were found to be less effective in activating PKC than DAG with an n-6 PUFA, and n-3 PUFA decreased the effectiveness of activation of PKC and binding of phosphatidyl serine in the cell membrane (Terano et al, 1996).
Protein kinase C α is an upstream activator of the I-κB kinase complex (IKK) (Vertegaal et al, 2000), which phosphorylates I-κBα at serines-32 and -36 leading to ubiquitination and subsequent proteasome proteolysis. This suggests a mechanism by which PIF may induce degradation of I-κBα and stimulate nuclear binding of NF-κB (Whitehouse and Tisdale, 2003). Nuclear factor-κB regulates the transcription of a number of genes and has been shown (Li and Reed, 2000) to be an essential mediator of TNF-α-induced protein catabolism in differentiated muscle cells. This study shows that degradation of I-κBα and translocation of NF-κB to the nucleus in response to PIF is attenuated by calphostin C and is not seen in myotubes expressing mutant PKCα. This suggests that PKC acts as an important mediator in activation of NF-κB in response to PIF. It is not known whether NF-κB acts alone or in concert with other transcriptional activators in PIF-induced proteasome expression and future studies will be aimed at identifying the role of NF-κB in this process.
Acknowledgments
This work has been supported by the Lustgarten Foundation for Pancreatic cancer research.
References
- Andrews NC, Faller DV (1991) A rapid micropreparation technique for extraction of DNA-binding proteins from limiting numbers of mammalian cells. Nucleic Acid Res 19: 2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber MD, Ross JA, Voss AC, Tisdale MJ, Fearon KCH (1999) The effect of an oral nutritional supplement enriched with fish oil on weight-loss in patients with pancreatic cancer. Br J Cancer 81: 80–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck SA, Smith KL, Tisdale MJ (1991) Anticachectic and antitumor effect of eicosapentaenoic acid and its effect on protein turnover. Cancer Res 51: 6089–6093 [PubMed] [Google Scholar]
- Beltman J, McCormick F, Cook SJ (1996) The selective protein kinase C inhibitor, Ro 31-8220 inhibits mitogen-activated protein kinase phosphate-1 (MKP-1) expression, induces c-Jun expression and activates Jun N-terminal kinase. J Biol Chem 43: 27018–27024 [DOI] [PubMed] [Google Scholar]
- Bornancin F, Parker P (1996) Phosphorylation of threonine 638 critically controls dephosphorylation and inactivation of protein kinase C α. Curr Biol 6: 1114–1123 [DOI] [PubMed] [Google Scholar]
- Couldwell WT, Hinton DR, He S, Chen TC, Sebat I, Weiss MH, Law RE (1994) Protein kinase C inhibitors induce apoptosis in human malignant glioma cell lines. FEBS Lett 345: 43–46 [DOI] [PubMed] [Google Scholar]
- Dubiel W, Ferrell K, Pratt G, Rechsteiner M (1992) Subunit 4 of the 26S proteasome is a member of a novel eukaryotic ATPase family. J Biol Chem 267: 22699–22702 [PubMed] [Google Scholar]
- Evans WK, Makuch R, Clamon GH, Feld K, Weiner RS, Moran E, Blum R, Shepherd FA, Jeejeebhoy KW, DeWys WD (1985) Limited impact of total parenteral nutrition on nutritional status during treatment for small cell lung cancer. Cancer Res 45: 3347–3353 [PubMed] [Google Scholar]
- Fan X, Hunag X, DaSilva C, Castagna M (1990) Arachidonic acid and related methyl ester mediate protein kinase C activation in intact platelets through the arachidonate metabolism pathways. Biochem Biophys Res Commun 109: 933–940 [DOI] [PubMed] [Google Scholar]
- Fearon KCH (1992) The mechanisms and treatment of weight loss in cancer. Proc Nutr Soc 51: 251–265 [DOI] [PubMed] [Google Scholar]
- Hasselgren P-O, Zamir O, James JH, Fischer JE (1990) Prostaglandin E2 does not regulate total or myofibrillar protein breakdown in incubated skeletal muscle from normal or septic rats. Biochem J 270: 45–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinemeyer W, Trondle N, Albrecht G, Wolf DH (1994) PRE5 and PRE6, the last missing genes encoding 20S proteasome subunits from yeast? Indication for a set of 14 different subunits in the eukaryotic proteasome core. Biochem 33: 12229–12237 [DOI] [PubMed] [Google Scholar]
- Jarvis WD, Turner AJ, Povrik LF, Taylor RS, Grant S (1994) Induction of apoptotic DNA fragmentation and cell death in HL-60 human promyelocitic leukaemia cells by pharmacological inhibitors of protein kinase C. Cancer Res 54: 1707–1714 [PubMed] [Google Scholar]
- Lallena M-J, Diaz-Meco MT, Bren G, Paya CV, Moscat J (1999) Activation of IκB kinase β by protein kinase C isoforms. Mol Cell Biol 19: 2180–2188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y-P, Reed MB (2000) NF-κB mediates the protein loss induced by TNF-α in differentiated skeletal muscle myotubes. Am J Physiol 279: R1165–R1170 [DOI] [PubMed] [Google Scholar]
- Lorite MJ, Smith HJ, Arnold JA, Morris A, Thompson MG, Tisdale MJ (2001) Activation of ATP-ubiquitin-dependent proteolysis in skeletal muscle in vivo and murine myoblasts in vitro by a proteolysis-inducing factor (PIF). Br J Cancer 85: 297–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorite MJ, Thompson MG, Drake JL, Carling G, Tisdale MJ (1998) Mechanism of muscle protein degradation induced by a cancer cachectic factor. Br J Cancer 78: 850–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundholm K, Bennegard K, Eden E, Rennie MJ (1982) Efflux of 3-methylistidine from the leg in cancer patients who experience weight loss. Cancer Res 42: 4809–4818 [PubMed] [Google Scholar]
- Lundholm K, Bylund AC, Holm J, Schersten T (1976) Skeletal muscle metabolism in patients with malignant tumour. Eur J Cancer 12: 465–473 [DOI] [PubMed] [Google Scholar]
- Mason GG, Hendil KB, Rivett AJ (1996) Phosphorylation of proteasomes in mammalian cells. Identification of two phosphorylated subunits and the effect of phosphorylation on activity. Biochemistry 238: 453–462 [DOI] [PubMed] [Google Scholar]
- Orino E, Tanaka K, Tamura T, Sone S, Ogura T, Ichihara A (1991) ATP-dependent reversible association of proteasomes with multiple protein components to form 26S complexes that degrade ubiquitinated proteins in human HL-60 cells. FEBS Lett 284: 206–210 [DOI] [PubMed] [Google Scholar]
- Pears CJ, Kour G, House C, Kemp BE, Parker PJ (1990) Mutagenesis of pseudosubstrate site of protein kinase C leads to activation. Eur J Cancer 194: 89–94 [DOI] [PubMed] [Google Scholar]
- Rodemann HP, Goldberg AL (1982) Arachidonic acid, prostaglandin E2 and F2α influence rates of protein turnover in skeletal and cardiac muscle. J Biol Chem 257: 1632–1638 [PubMed] [Google Scholar]
- Schonwasser DC, Marais RM, Marshall CJ, Parker PJ (1998) Activation of the mitogen-activated protein kinase/extracellular signal-regulated kinase pathway by conventional, novel and atypical protein kinase C isotypes. Mol Cell Biol 18: 790–798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith HJ, Lorite MJ, Tisdale MJ (1999) Effect of a cancer cachectic factor on protein synthesis/degradation in murine C2C12 myoblasts: modulation by eicosapentaenoic acid. Cancer Res 59: 5507–5513 [PubMed] [Google Scholar]
- Smith HJ, Tisdale MJ (2003) Signal transduction pathways involved in proteolysis-inducing factor induced proteasome expression in murine myotubes. Br J Cancer 89: 1783–1788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling RI, Benincaso AI, Knoell CT, Larkin JK, Austen KF, Robinson DR (1993) Dietary omega-3 polyunsaturated fatty acids inhibit phosphoinositide formation and chemotaxis in neutrophils. J Clin Invest 91: 651–660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strelkov AB, Fields ALA, Baracos VE (1989) Effects of systemic inhibition of prostaglandin production on protein metabolism in tumor-bearing rats. Am J Physiol 257: C261–C269 [DOI] [PubMed] [Google Scholar]
- Tanaka K, Fujiwara T, Kumatori A, Shin S, Yoshimura T, Ichihara A, Tokunaga F, Aruga R, Iwanaga S, Kakizuka A, Nakanishi S (1990) Molecular cloning of cDNA for proteasome from rat liver: primary structure of component C3 with a possible tyrosine phosphorylation site. Biochemistry 29: 3777–3785 [DOI] [PubMed] [Google Scholar]
- Terano T, Shiina T, Tamura Y (1996) Eicosapentaenoic acid suppressed the proliferation of vascular smooth muscle cells through modulation of various steps of growth signals. Lipids 31: S301–S304 [DOI] [PubMed] [Google Scholar]
- Todorov P, Cariuk P, McDevitt T, Coles B, Fearon K, Tisdale M (1996) Characterization of a cancer cachectic factor. Nature 379: 739–742 [DOI] [PubMed] [Google Scholar]
- Trushin SA, Pennington KN, Algecirao-Schimnich A, Paya CV (1999) Protein kinase C and calcineurin synergize to activate IκB kinase and NF-κB in T lymphocytes. J Biol Chem 274: 22923–22931 [DOI] [PubMed] [Google Scholar]
- Vertegaal ACO, Kuiperij HB, Yamaoka S, Courtois G, van der Eb AJ, Zantema A (2000) Protein kinase C-α is an upstream activator of the IκB kinase complex in the TPA signal transduction pathway to NF-κB in U20S cells. Cell Signal 12: 759–768 [DOI] [PubMed] [Google Scholar]
- Watchorn TM, Waddell ID, Dowidar N, Ross JA (2001) Proteolysis-inducing factor regulates hepatic gene expression via the transcription factors NF-κB and STAT3. FASEB J 15: 562–564 [DOI] [PubMed] [Google Scholar]
- Wang D, Yu X, Brecher P (1998) Nitric oxide and N-acetylcysteine inhibit the activation of mitogen-activated protein kinases by angiotensin II in rat cardiac fibroblasts. J Biol Chem 273: 33027–33034 [DOI] [PubMed] [Google Scholar]
- Whitehouse AS, Khal J, Tisdale MJ (2003) Induction of protein catabolism in myotubes by 15(S)-hydroxyeicosatetraenoic acid through increased expression of the ubiquitin-proteasome pathway. Br J Cancer 89: 737–745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehouse AS, Tisdale MJ (2003) Increased expression of the ubiquitin–proteasome pathway in murine myotubes by proteolysis-inducing factor (PIF) is associated with activation of the transcription factor NF-κB. Br J Cancer 89: 1116–1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams A, Sun X, Fischer JE, Hasselgren P-O (1999) The expression of genes in the ubiquitin–proteasome proteolytic pathway is increased in skeletal muscle from patients with cancer. Surgery 126: 744–750 [PubMed] [Google Scholar]