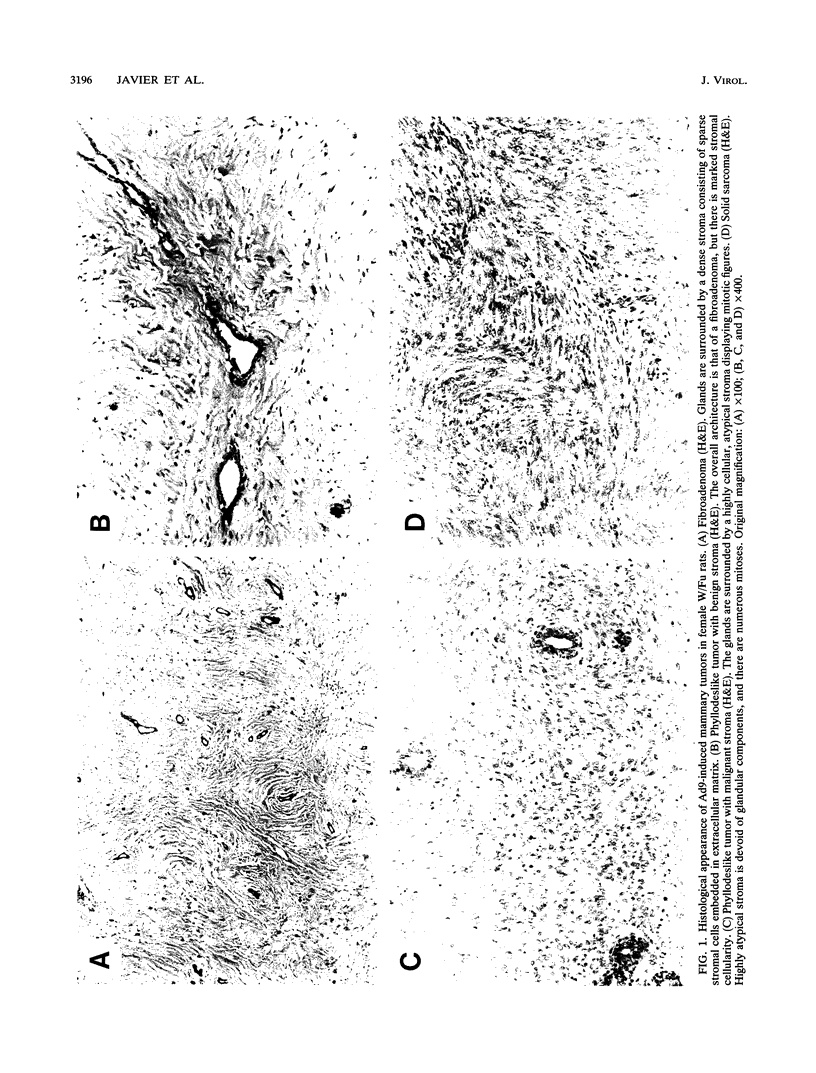
Abstract
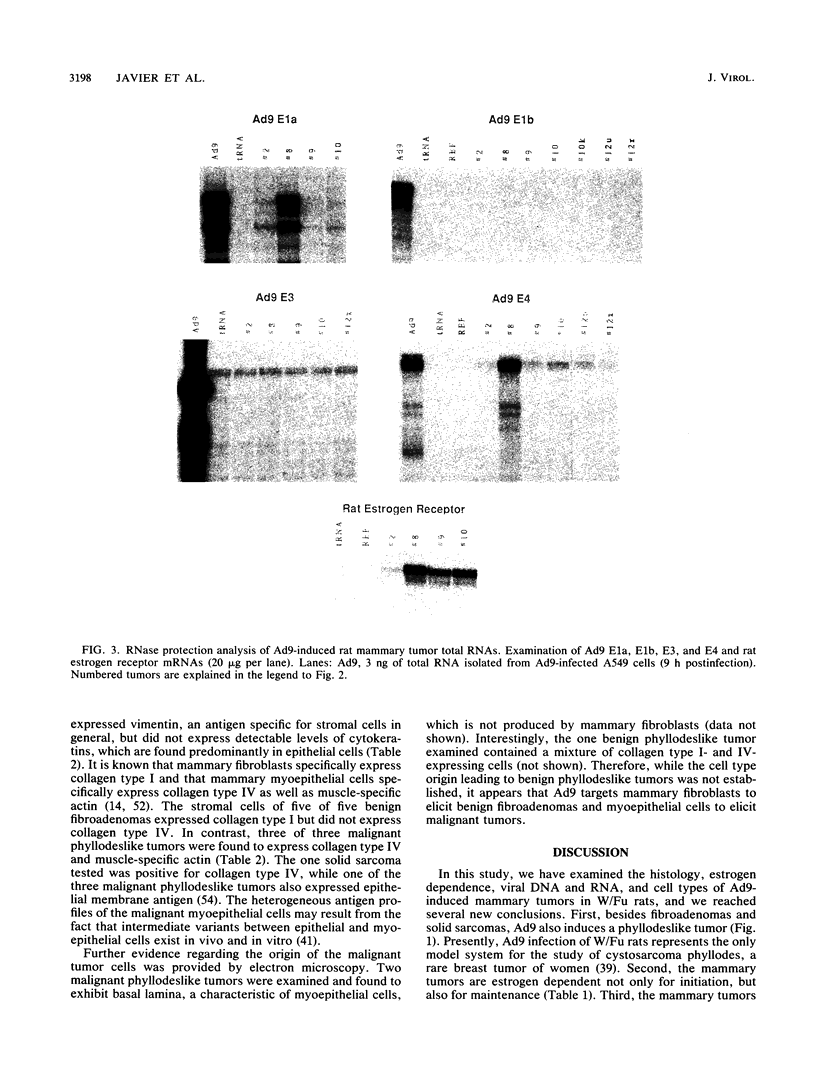
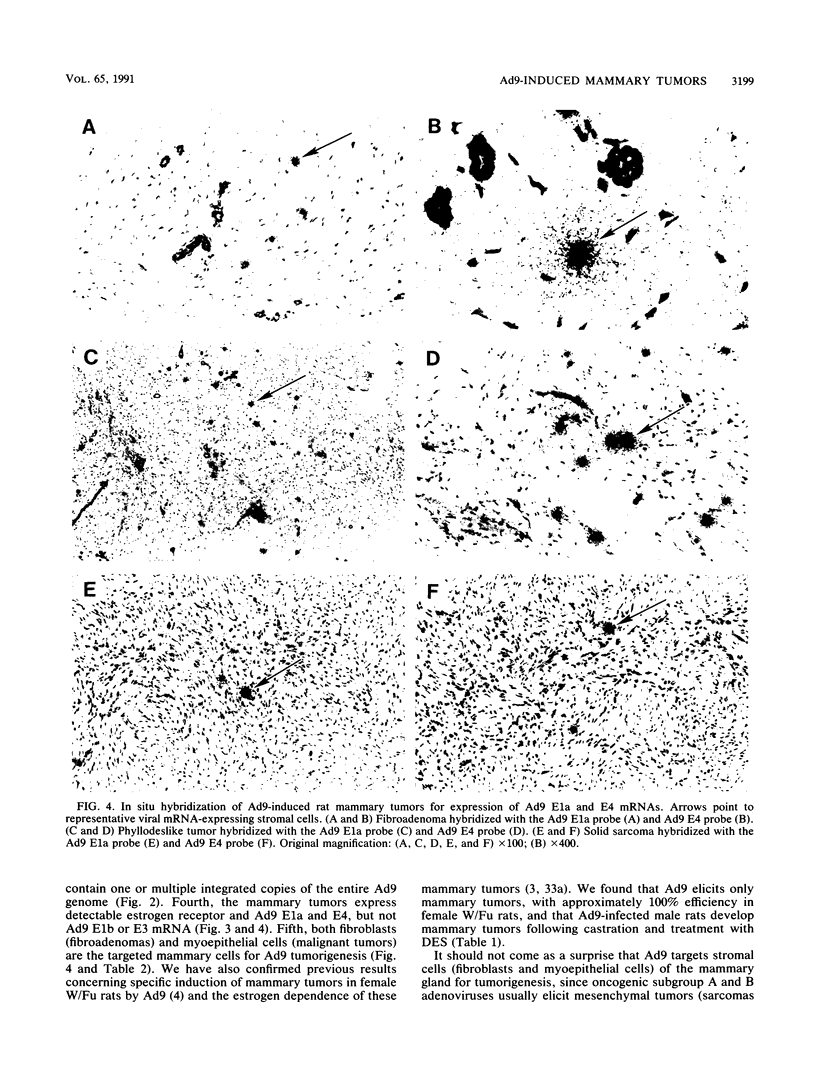
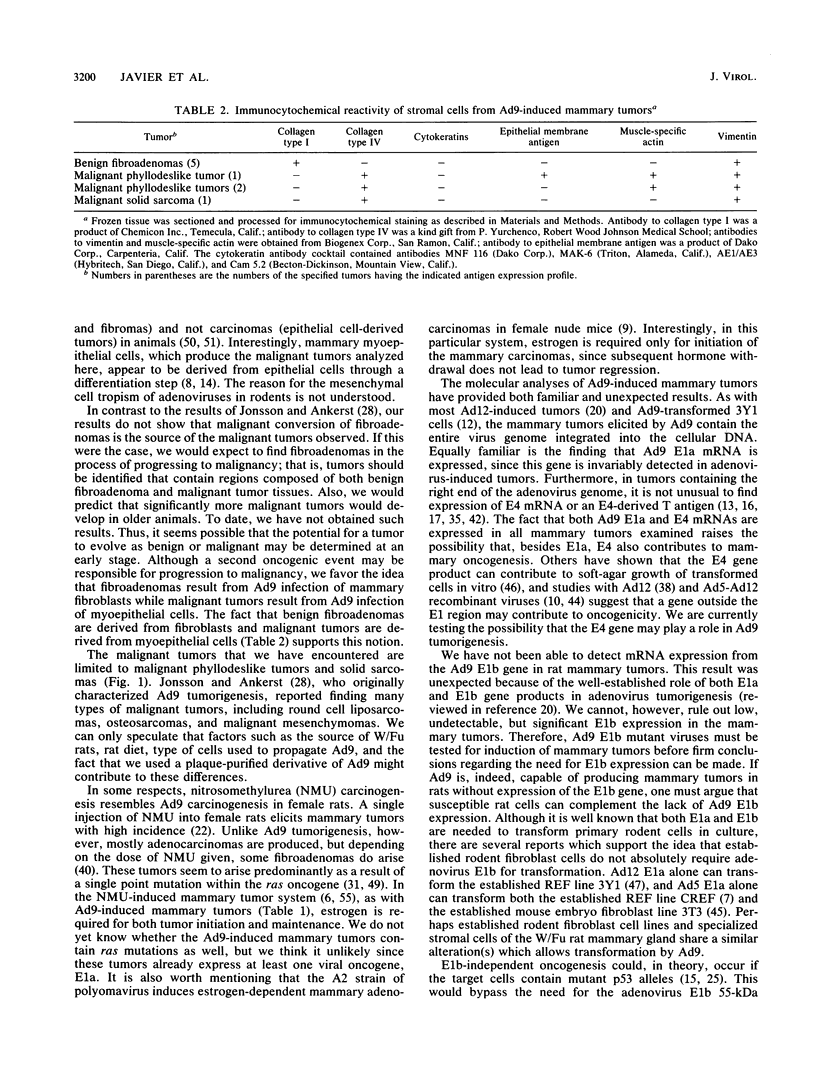
Following subcutaneous inoculation of newborn Wistar-Furth rats with human adenovirus type 9 (Ad9), 16 of 16 female and 0 of 11 male rats developed mammary tumors. Tumor-positive animals usually developed tumors in multiple glands. Histopathological analyses indicated that three general categories of tumor could be identified. Mammary fibroadenomas were the most common tumor type encountered, but phyllodeslike tumors and solid sarcomas were also frequently found. In situ hybridization and immunohistochemical techniques established that benign fibroadenomas were derived from mammary fibroblasts (collagen type I- and vimentin-positive cells) and that malignant tumors were derived from myoepithelial cells (collagen type IV-, vimentin-, and muscle-specific actin-positive cells). The fact that mammary tumors were limited to female rats suggested that female hormones are essential for tumor growth and development. In this regard, ovariectomy of Ad9-infected female rats prevented tumor development, while subsequent diethylstilbestrol (DES) treatment elicited tumor formation. In addition, Ad9-infected and castrated male rats which received DES also developed mammary tumors. Established male mammary tumors regressed when DES treatment was stopped and reappeared after DES treatment was resumed. Together, these results indicate that estrogen is required for both initiation and maintenance of Ad9-induced mammary tumors. Southern blot analysis of high-molecular-weight tumor DNA showed that mammary tumor cells contained single or multiple integrated copies of the entire Ad9 genome. RNase protection experiments established that estrogen receptor as well as Ad9 E1a and E4 mRNAs were expressed in mammary tumors, but Ad9 E3 and, surprisingly, E1b mRNAs were not expressed at detectable levels.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adrian T., Bastian B., Benoist W., Hierholzer J. C., Wigand R. Characterization of adenovirus 15/H9 intermediate strains. Intervirology. 1985;23(1):15–22. doi: 10.1159/000149562. [DOI] [PubMed] [Google Scholar]
- Angerer L. M., Angerer R. C. Detection of poly A+ RNA in sea urchin eggs and embryos by quantitative in situ hybridization. Nucleic Acids Res. 1981 Jun 25;9(12):2819–2840. doi: 10.1093/nar/9.12.2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ankerst J., Jonsson N. Adenovirus type 9-induced tumorigenesis in the rat mammary gland related to sex hormonal state. J Natl Cancer Inst. 1989 Feb 15;81(4):294–298. doi: 10.1093/jnci/81.4.294. [DOI] [PubMed] [Google Scholar]
- Ankerst J., Jonsson N., Kjellén L., Norrby E., Sjögren H. O. Induction of mammary fibroadenomas in rats by adenovirus type 9. Int J Cancer. 1974 Mar 15;13(3):286–290. doi: 10.1002/ijc.2910130303. [DOI] [PubMed] [Google Scholar]
- Ankerst J., Steele G., Jr, Sjögren H. O. Cross-reacting tumor-associated antigen(s) of adenovirus type 9-induced fibroadenomas and a chemically induced mammary carcinoma in rats. Cancer Res. 1974 Aug;34(8):1794–1800. [PubMed] [Google Scholar]
- Arafah B. M., Gullino P. M., Manni A., Pearson O. H. Effect of ovariectomy on hormone receptors and growth of N-nitrosomethylurea-induced mammary tumors in the rat. Cancer Res. 1980 Dec;40(12):4628–4630. [PubMed] [Google Scholar]
- Babiss L. E., Liaw W. S., Zimmer S. G., Godman G. C., Ginsberg H. S., Fisher P. B. Mutations in the E1a gene of adenovirus type 5 alter the tumorigenic properties of transformed cloned rat embryo fibroblast cells. Proc Natl Acad Sci U S A. 1986 Apr;83(7):2167–2171. doi: 10.1073/pnas.83.7.2167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett D. C., Peachey L. A., Durbin H., Rudland P. S. A possible mammary stem cell line. Cell. 1978 Sep;15(1):283–298. doi: 10.1016/0092-8674(78)90104-6. [DOI] [PubMed] [Google Scholar]
- Berebbi M., Martin P. M., Berthois Y., Bernard A. M., Blangy D. Estradiol dependence of the specific mammary tissue targeting of polyoma virus oncogenicity in nude mice. Oncogene. 1990 Apr;5(4):505–509. [PubMed] [Google Scholar]
- Bernards R., de Leeuw M. G., Vaessen M. J., Houweling A., van der Eb A. J. Oncogenicity by adenovirus is not determined by the transforming region only. J Virol. 1984 Jun;50(3):847–853. doi: 10.1128/jvi.50.3.847-853.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brahic M., Haase A. T. Detection of viral sequences of low reiteration frequency by in situ hybridization. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6125–6129. doi: 10.1073/pnas.75.12.6125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brusca J. S., Jannun R., Chinnadurai G. Efficient transformation of rat 3Y1 cells by human adenovirus type 9. Virology. 1984 Jul 30;136(2):328–337. doi: 10.1016/0042-6822(84)90169-7. [DOI] [PubMed] [Google Scholar]
- Downey J. F., Rowe D. T., Bacchetti S., Graham F. L., Bayley S. T. Mapping of a 14,000-dalton antigen to early region 4 of the human adenovirus 5 genome. J Virol. 1983 Feb;45(2):514–523. doi: 10.1128/jvi.45.2.514-523.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulbecco R., Henahan M., Bowman M., Okada S., Battifora H., Unger M. Generation of fibroblast-like cells from cloned epithelial mammary cells in vitro: a possible new cell type. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2345–2349. doi: 10.1073/pnas.78.4.2345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliyahu D., Goldfinger N., Pinhasi-Kimhi O., Shaulsky G., Skurnik Y., Arai N., Rotter V., Oren M. Meth A fibrosarcoma cells express two transforming mutant p53 species. Oncogene. 1988 Sep;3(3):313–321. [PubMed] [Google Scholar]
- Esche H., Siegmann B. Expression of early viral gene products in adenovirus type 12-infected and -transformed cells. J Gen Virol. 1982 May;60(Pt 1):99–113. doi: 10.1099/0022-1317-60-1-99. [DOI] [PubMed] [Google Scholar]
- Esche H. Viral gene products in adenovirus type-2 transformed hamster cells. J Virol. 1982 Mar;41(3):1076–1082. doi: 10.1128/jvi.41.3.1076-1082.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Green M. R., Chinnadurai G., Mackey J. K., Green M. A unique pattern of integrated viral genes in hamster cells transformed by highly oncogenic human adenovirus 12. Cell. 1976 Mar;7(3):419–428. doi: 10.1016/0092-8674(76)90172-0. [DOI] [PubMed] [Google Scholar]
- Gullino P. M., Pettigrew H. M., Grantham F. H. N-nitrosomethylurea as mammary gland carcinogen in rats. J Natl Cancer Inst. 1975 Feb;54(2):401–414. [PubMed] [Google Scholar]
- Hanahan D., Gluzman Y. Rescue of functional replication origins from embedded configurations in a plasmid carrying the adenovirus genome. Mol Cell Biol. 1984 Feb;4(2):302–309. doi: 10.1128/mcb.4.2.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi M., Ninomiya Y., Parsons J., Hayashi K., Olsen B. R., Trelstad R. L. Differential localization of mRNAs of collagen types I and II in chick fibroblasts, chondrocytes, and corneal cells by in situ hybridization using cDNA probes. J Cell Biol. 1986 Jun;102(6):2302–2309. doi: 10.1083/jcb.102.6.2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinds P., Finlay C., Levine A. J. Mutation is required to activate the p53 gene for cooperation with the ras oncogene and transformation. J Virol. 1989 Feb;63(2):739–746. doi: 10.1128/jvi.63.2.739-746.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibelgaufts H., Doerfler W., Scheidtmann K. H., Wechsler W. Adenovirus type 12-induced rat tumor cells of neuroepithelial origin: persistence and expression of the viral genome. J Virol. 1980 Jan;33(1):423–437. doi: 10.1128/jvi.33.1.423-437.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jannun R., Chinnadurai G. Functional relatedness between the E1a and E1b regions of group C and group D human adenoviruses. Virus Res. 1987 Feb;7(1):33–48. doi: 10.1016/0168-1702(87)90056-6. [DOI] [PubMed] [Google Scholar]
- Jonsson N., Ankerst J. Studies on adenovirus type 9-induced mammary fibroadenomas in rats and their malignant transformation. Cancer. 1977 Jun;39(6):2513–2519. doi: 10.1002/1097-0142(197706)39:6<2513::aid-cncr2820390631>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Kemp M. C., Hierholzer J. C., Cabradilla C. P., Obijeski J. F. The changing etiology of epidemic keratoconjunctivitis: antigenic and restriction enzyme analyses of adenovirus types 19 and 37 isolated over a 10-year period. J Infect Dis. 1983 Jul;148(1):24–33. doi: 10.1093/infdis/148.1.24. [DOI] [PubMed] [Google Scholar]
- Koike S., Sakai M., Muramatsu M. Molecular cloning and characterization of rat estrogen receptor cDNA. Nucleic Acids Res. 1987 Mar 25;15(6):2499–2513. doi: 10.1093/nar/15.6.2499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar R., Sukumar S., Barbacid M. Activation of ras oncogenes preceding the onset of neoplasia. Science. 1990 Jun 1;248(4959):1101–1104. doi: 10.1126/science.2188364. [DOI] [PubMed] [Google Scholar]
- Lawrence J. B., Singer R. H. Quantitative analysis of in situ hybridization methods for the detection of actin gene expression. Nucleic Acids Res. 1985 Mar 11;13(5):1777–1799. doi: 10.1093/nar/13.5.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. C., Mak S. Adenovirus type 12 DNA sequences in primary hamster tumors. J Virol. 1977 Oct;24(1):408–411. doi: 10.1128/jvi.24.1.408-411.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak I., Mak S., Benchimol S. Expression of the cellular p53 protein in cells transformed by adenovirus 12 and viral DNA fragments. Virology. 1988 Mar;163(1):201–204. doi: 10.1016/0042-6822(88)90248-6. [DOI] [PubMed] [Google Scholar]
- Matsuo T., Hashimoto S., Wold W. S., Symington J., Rankin A., Green M. Identification of adenovirus 2 early region 4 polypeptides by in vitro translation and tryptic peptide map analysis. J Virol. 1982 Jan;41(1):334–339. doi: 10.1128/jvi.41.1.334-339.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister R. M., Nicolson M. O., Reed G., Kern J., Gilden R. V., Huebner R. J. Transformation of rodent cells by adenovirus 19 and other group D adenoviruses. J Natl Cancer Inst. 1969 Oct;43(4):917–923. [PubMed] [Google Scholar]
- Raska K., Jr, Morrongiello M. P., Föhring B. Adenovirus type-12 tumor antigen. III. Tumorigenicity and immune response to syngeneic rat cells transformed with virions and isolated transforming fragment of adenovirus 12 DNA. Int J Cancer. 1980 Jul 15;26(1):79–86. doi: 10.1002/ijc.2910260113. [DOI] [PubMed] [Google Scholar]
- Rose D. P., Pruitt B., Stauber P., Ertürk E., Bryan G. T. Influence of dosage schedule on the biological characteristics of N-nitrosomethylurea-induced rat mammary tumors. Cancer Res. 1980 Feb;40(2):235–239. [PubMed] [Google Scholar]
- Rudland P. S., Barraclough R. Stem cells in mammary gland differentiation and cancer. J Cell Sci Suppl. 1988;10:95–114. doi: 10.1242/jcs.1988.supplement_10.8. [DOI] [PubMed] [Google Scholar]
- Sarnow P., Hearing P., Anderson C. W., Reich N., Levine A. J. Identification and characterization of an immunologically conserved adenovirus early region 11,000 Mr protein and its association with the nuclear matrix. J Mol Biol. 1982 Dec 15;162(3):565–583. doi: 10.1016/0022-2836(82)90389-8. [DOI] [PubMed] [Google Scholar]
- Sarnow P., Ho Y. S., Williams J., Levine A. J. Adenovirus E1b-58kd tumor antigen and SV40 large tumor antigen are physically associated with the same 54 kd cellular protein in transformed cells. Cell. 1982 Feb;28(2):387–394. doi: 10.1016/0092-8674(82)90356-7. [DOI] [PubMed] [Google Scholar]
- Sawada Y., Raska K., Jr, Shenk T. Adenovirus type 5 and adenovirus type 12 recombinant viruses containing heterologous E1 genes are viable, transform rat cells, but are not tumorigenic in rats. Virology. 1988 Sep;166(1):281–284. doi: 10.1016/0042-6822(88)90175-4. [DOI] [PubMed] [Google Scholar]
- Senear A. W., Lewis J. B. Morphological transformation of established rodent cell lines by high-level expression of the adenovirus type 2 E1a gene. Mol Cell Biol. 1986 Apr;6(4):1253–1260. doi: 10.1128/mcb.6.4.1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiroki K., Hashimoto S., Saito I., Fukui Y., Fukui Y., Kato H., Shimojo H. Expression of the E4 gene is required for establishment of soft-agar colony-forming rat cell lines transformed by the adenovirus 12 E1 gene. J Virol. 1984 Jun;50(3):854–863. doi: 10.1128/jvi.50.3.854-863.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiroki K., Shimojo H., Sawada Y., Uemizu Y., Fujinaga K. Incomplete transformation of rat cells by a small fragment of adenovirus 12 DNA. Virology. 1979 May;95(1):127–136. doi: 10.1016/0042-6822(79)90407-0. [DOI] [PubMed] [Google Scholar]
- Sukumar S., Notario V., Martin-Zanca D., Barbacid M. Induction of mammary carcinomas in rats by nitroso-methylurea involves malignant activation of H-ras-1 locus by single point mutations. Nature. 1983 Dec 15;306(5944):658–661. doi: 10.1038/306658a0. [DOI] [PubMed] [Google Scholar]
- TRENTIN J. J., YABE Y., TAYLOR G. The quest for human cancer viruses. Science. 1962 Sep 14;137(3533):835–841. doi: 10.1126/science.137.3533.835. [DOI] [PubMed] [Google Scholar]
- Trentin J. J., Van Hoosier G. L., Jr, Samper L. The oncogenicity of human adenoviruses in hamsters. Proc Soc Exp Biol Med. 1968 Mar;127(3):683–689. doi: 10.3181/00379727-127-32773. [DOI] [PubMed] [Google Scholar]
- Warburton M. J., Mitchell D., Ormerod E. J., Rudland P. Distribution of myoepithelial cells and basement membrane proteins in the resting, pregnant, lactating, and involuting rat mammary gland. J Histochem Cytochem. 1982 Jul;30(7):667–676. doi: 10.1177/30.7.6179984. [DOI] [PubMed] [Google Scholar]
- Wigand R., Gelderblom H., Ozel M., Distler H., Adrian T. Characteristics of mastadenovirus h 8, the causative agent of epidemic keratoconjunctivitis. Arch Virol. 1983;76(4):307–319. doi: 10.1007/BF01311198. [DOI] [PubMed] [Google Scholar]
- Wilkinson M. J., Howell A., Harris M., Taylor-Papadimitriou J., Swindell R., Sellwood R. A. The prognostic significance of two epithelial membrane antigens expressed by human mammary carcinomas. Int J Cancer. 1984 Mar 15;33(3):299–304. doi: 10.1002/ijc.2910330304. [DOI] [PubMed] [Google Scholar]
- Williams J. C., Gusterson B., Humphreys J., Monaghan P., Coombes R. C., Rudland P., Neville A. M. N-methyl-N-nitrosourea-induced rat mammary tumors. Hormone responsiveness but lack of spontaneous metastasis. J Natl Cancer Inst. 1981 Jan;66(1):147–155. [PubMed] [Google Scholar]
- Zantema A., Schrier P. I., Davis-Olivier A., van Laar T., Vaessen R. T., van der EB A. J. Adenovirus serotype determines association and localization of the large E1B tumor antigen with cellular tumor antigen p53 in transformed cells. Mol Cell Biol. 1985 Nov;5(11):3084–3091. doi: 10.1128/mcb.5.11.3084. [DOI] [PMC free article] [PubMed] [Google Scholar]