Abstract
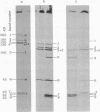
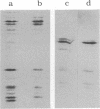
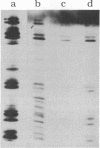
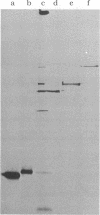
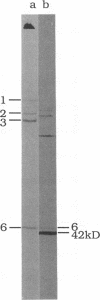
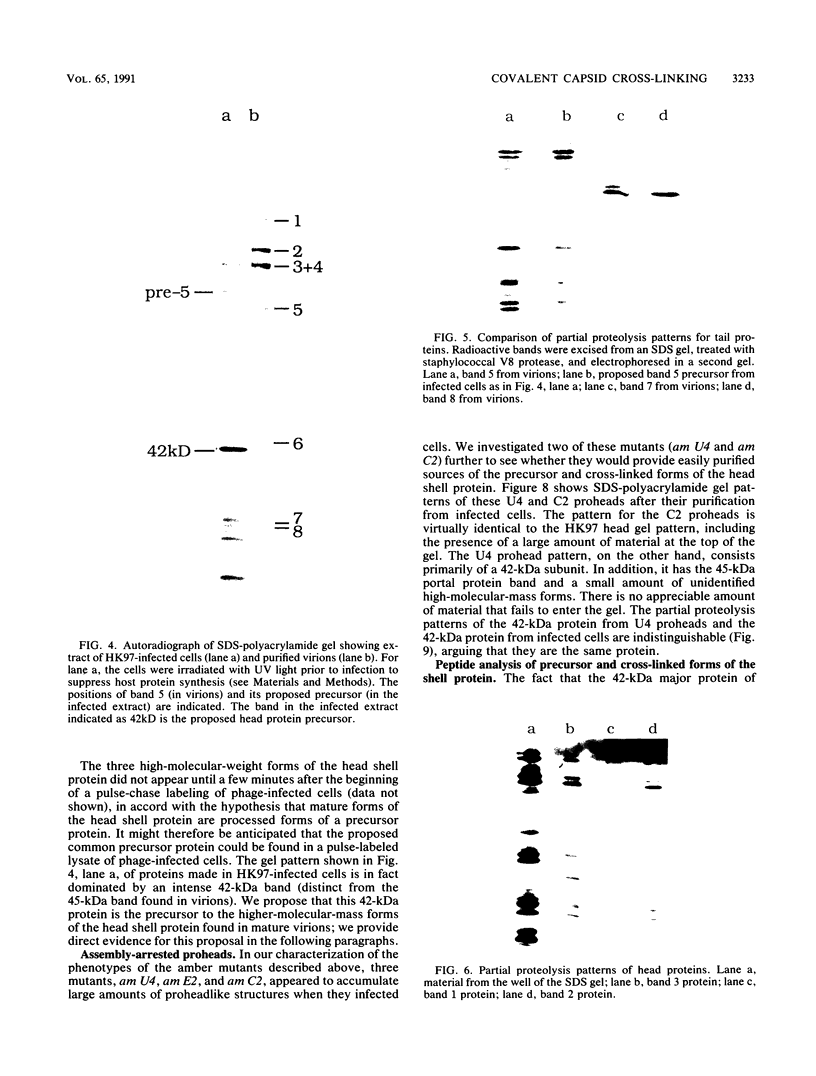
We describe initial genetic and structural characterizations of HK97, a temperate bacteriophage of Escherichia coli. We isolated 28 amber mutants, characterized them with respect to what phage-related structures they make, and mapped many of them to restriction fragments of genomic DNA. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis of HK97 virions revealed nine different protein species plus a substantial amount of material that failed to enter the gel, apparently because it is too large. Five proteins are tail components and are assigned functions as tail fiber subunit, tail length template, and major shaft subunit (two and possibly three species). The four remaining proteins and the material that did not enter the gel are head components. One of these proteins is assigned as the portal subunit, and the remaining three head proteins in the gel and the material that did not enter the gel are components of the head shell. All of the head shell protein species have apparent molecular masses well in excess of 100 kDa; they share amino acid sequence with each other and also with a 42-kDa protein that is found in infected lysates and as the major component of prohead structures that accumulate in infections by one of the amber mutants. We propose that all of the head shell species found in mature heads are covalently cross-linked oligomers derived from the 42-kDa precursor during head shell maturation.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allet B., Katagiri K. J., Gesteland R. F. Characterization of polypeptides made in vitro from bacteriophage lambda DNA. J Mol Biol. 1973 Aug 25;78(4):589–600. doi: 10.1016/0022-2836(73)90281-7. [DOI] [PubMed] [Google Scholar]
- Bazinet C., King J. The DNA translocating vertex of dsDNA bacteriophage. Annu Rev Microbiol. 1985;39:109–129. doi: 10.1146/annurev.mi.39.100185.000545. [DOI] [PubMed] [Google Scholar]
- Blattner F. R., Williams B. G., Blechl A. E., Denniston-Thompson K., Faber H. E., Furlong L., Grunwald D. J., Kiefer D. O., Moore D. D., Schumm J. W. Charon phages: safer derivatives of bacteriophage lambda for DNA cloning. Science. 1977 Apr 8;196(4286):161–169. doi: 10.1126/science.847462. [DOI] [PubMed] [Google Scholar]
- CASPAR D. L., KLUG A. Physical principles in the construction of regular viruses. Cold Spring Harb Symp Quant Biol. 1962;27:1–24. doi: 10.1101/sqb.1962.027.001.005. [DOI] [PubMed] [Google Scholar]
- Carrington D. M., Auffret A., Hanke D. E. Polypeptide ligation occurs during post-translational modification of concanavalin A. Nature. 1985 Jan 3;313(5997):64–67. doi: 10.1038/313064a0. [DOI] [PubMed] [Google Scholar]
- Casjens S. Molecular organization of the bacteriophage P22 coat protein shell. J Mol Biol. 1979 Jun 15;131(1):1–14. doi: 10.1016/0022-2836(79)90298-5. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Collmer C. W., Vogt V. M., Zaitlin M. H protein, a minor protein of TMV virions, contains sequences of the viral coat protein. Virology. 1983 Apr 30;126(2):429–448. doi: 10.1016/s0042-6822(83)80002-6. [DOI] [PubMed] [Google Scholar]
- Dhillon E. K., Dhillon T. S., Lai A. N., Linn S. Host range, immunity and antigenic properties of lambdoid coliphage HK97. J Gen Virol. 1980 Sep;50(1):217–220. doi: 10.1099/0022-1317-50-1-217. [DOI] [PubMed] [Google Scholar]
- Dhillon T. S., Dhillon E. K. Temperate coliphage HK022. Clear plaque mutants and preliminary vegetative map. Jpn J Microbiol. 1976 Oct;20(5):385–396. doi: 10.1111/j.1348-0421.1976.tb01004.x. [DOI] [PubMed] [Google Scholar]
- Earnshaw W. C., Hendrix R. W., King J. Structural studies of bacteriophage lambda heads and proheads by small angle X-ray diffraction. J Mol Biol. 1979 Nov 5;134(3):575–594. doi: 10.1016/0022-2836(79)90368-1. [DOI] [PubMed] [Google Scholar]
- Enquist L. W., Weisberg R. A. The red plaque test: a rapid method for identification of excision defective variants of bacteriophage lambda. Virology. 1976 Jul 1;72(1):147–153. doi: 10.1016/0042-6822(76)90319-6. [DOI] [PubMed] [Google Scholar]
- Fullmer C. S., Wasserman R. H. Analytical peptide mapping by high performance liquid chromatography. Application to intestinal calcium-binding proteins. J Biol Chem. 1979 Aug 10;254(15):7208–7212. [PubMed] [Google Scholar]
- Georgopoulos C. P., Hendrix R. W., Casjens S. R., Kaiser A. D. Host participation in bacteriophage lambda head assembly. J Mol Biol. 1973 May 5;76(1):45–60. doi: 10.1016/0022-2836(73)90080-6. [DOI] [PubMed] [Google Scholar]
- Hawke D., Yuan P. M., Shively J. E. Microsequence analysis of peptides and proteins. II. Separation of amino acid phenylthiohydantoin derivatives by high-performance liquid chromatography on octadecylsilane supports. Anal Biochem. 1982 Mar 1;120(2):302–311. doi: 10.1016/0003-2697(82)90351-7. [DOI] [PubMed] [Google Scholar]
- Henderson L. E., Oroszlan S., Konigsberg W. A micromethod for complete removal of dodecyl sulfate from proteins by ion-pair extraction. Anal Biochem. 1979 Feb;93(1):153–157. [PubMed] [Google Scholar]
- Hendrix R. W., Casjens S. R. Assembly of bacteriophage lambda heads: protein processing and its genetic control in petit lambda assembly. J Mol Biol. 1975 Jan 15;91(2):187–199. doi: 10.1016/0022-2836(75)90159-x. [DOI] [PubMed] [Google Scholar]
- Hendrix R. W., Casjens S. R. Protein cleavage in bacteriophage lambda tail assembly. Virology. 1974 Sep;61(1):156–159. doi: 10.1016/0042-6822(74)90250-5. [DOI] [PubMed] [Google Scholar]
- Hendrix R. W., Casjens S. R. Protein fusion: a novel reaction in bacteriophage lambda head assembly. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1451–1455. doi: 10.1073/pnas.71.4.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KAISER A. D., JACOB F. Recombination between related temperate bacteriophages and the genetic control of immunity and prophage localization. Virology. 1957 Dec;4(3):509–521. doi: 10.1016/0042-6822(57)90083-1. [DOI] [PubMed] [Google Scholar]
- Kane P. M., Yamashiro C. T., Wolczyk D. F., Neff N., Goebl M., Stevens T. H. Protein splicing converts the yeast TFP1 gene product to the 69-kD subunit of the vacuolar H(+)-adenosine triphosphatase. Science. 1990 Nov 2;250(4981):651–657. doi: 10.1126/science.2146742. [DOI] [PubMed] [Google Scholar]
- Katsura I. Determination of bacteriophage lambda tail length by a protein ruler. Nature. 1987 May 7;327(6117):73–75. doi: 10.1038/327073a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Piez K. A. Cross-linking of collagen and elastin. Annu Rev Biochem. 1968;37:547–570. doi: 10.1146/annurev.bi.37.070168.002555. [DOI] [PubMed] [Google Scholar]
- Ptashne M. ISOLATION OF THE lambda PHAGE REPRESSOR. Proc Natl Acad Sci U S A. 1967 Feb;57(2):306–313. doi: 10.1073/pnas.57.2.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfer R., Huber U., Franklin R. M. Chemical and physical properties of mycobacteriophage D29. Eur J Biochem. 1977 Feb 15;73(1):239–246. doi: 10.1111/j.1432-1033.1977.tb11312.x. [DOI] [PubMed] [Google Scholar]
- Steven A. C., Couture E., Aebi U., Showe M. K. Structure of T4 polyheads. II. A pathway of polyhead transformation as a model for T4 capsid maturation. J Mol Biol. 1976 Sep 5;106(1):187–221. doi: 10.1016/0022-2836(76)90307-7. [DOI] [PubMed] [Google Scholar]
- Steven A. C., Serwer P., Bisher M. E., Trus B. L. Molecular architecture of bacteriophage T7 capsid. Virology. 1983 Jan 15;124(1):109–120. doi: 10.1016/0042-6822(83)90294-5. [DOI] [PubMed] [Google Scholar]
- Stockley P. G., Kirsh A. L., Chow E. P., Smart J. E., Harrison S. C. Structure of turnip crinkle virus. III. Identification of a unique coat protein dimer. J Mol Biol. 1986 Oct 20;191(4):721–725. doi: 10.1016/0022-2836(86)90456-0. [DOI] [PubMed] [Google Scholar]
- Susskind M. M., Botstein D. Superinfection exclusion by lambda prophage in lysogens of Salmonella typhimurium. Virology. 1980 Jan 15;100(1):212–216. doi: 10.1016/0042-6822(80)90571-1. [DOI] [PubMed] [Google Scholar]
- Walker J. E., Auffret A. D., Carne A., Gurnett A., Hanisch P., Hill D., Saraste M. Solid-phase sequence analysis of polypeptides eluted from polyacrylamide gels. An aid to interpretation of DNA sequences exemplified by the Escherichia coli unc operon and bacteriophage lambda. Eur J Biochem. 1982 Apr 1;123(2):253–260. doi: 10.1111/j.1432-1033.1982.tb19761.x. [DOI] [PubMed] [Google Scholar]
- Weigle J. Assembly of phage lambda in vitro. Proc Natl Acad Sci U S A. 1966 Jun;55(6):1462–1466. doi: 10.1073/pnas.55.6.1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams R. C., Richards K. E. Letter: Capsid structure of bacteriophage lambda. J Mol Biol. 1974 Sep 15;88(2):547–550. doi: 10.1016/0022-2836(74)90501-4. [DOI] [PubMed] [Google Scholar]
- Yanofsky C., Ito J. Nonsense codons and polarity in the tryptophan operon. J Mol Biol. 1966 Nov 14;21(2):313–334. doi: 10.1016/0022-2836(66)90102-1. [DOI] [PubMed] [Google Scholar]