Abstract
The convergence point of growth-signalling pathways that control cell proliferation is the initiation of genome replication, the core of which is the assembly of pre-replicative complexes (pre-RCs), resulting in chromatin being ‘licensed’ for DNA replication in the subsequent S phase. The Mcm2–7 complex is a core constituent of the pre-RC, whose recruitment to replication origins is dependent on the Cdt1 loading factor. Geminin is a potent inhibitor of the initiation of DNA replication by preventing Mcm2–7 assembly at origins via its interaction with Cdt1, ensuring genomic integrity through suppression of re-initiation events in S phase. Here we investigate the regulation of Ki67, Mcm2, p21, caspase 3 and Geminin in a series of 55 oligodendrogliomas to provide an integrated picture of how cellular proliferation and programmed cell death are dysregulated in these tumours. Geminin does not behave as an inhibitor of cell proliferation, its labelling index rising with increasing growth fraction as defined by Ki67 or Mcm2 expression. Geminin is expressed in a higher proportion of cells in higher grade tumours (P<0.001) and shows a strong correlation to proliferation and replication licensing (P<0.01), but not apoptosis. Increasing tumour anaplasia is not associated with loss of Geminin. Importantly, the G1 phase of the proliferative cell cycle, as assessed by the Geminin/Ki67 ratio, shortens with increasing anaplasia, providing new potential algorithms for prognostic assessment. Origin licensing proteins thus provide powerful novel tools for assessment of tumour cell cycle kinetics in routinely processed surgical biopsy material.
Keywords: Ki67, MCM, Geminin, p21, DNA replication licensing, oligodendroglioma
The initiation of chromosomal replication is a critical decision point in cell proliferation downstream of cell signalling and DNA transcription. This final and critical step in growth control lies at the convergence point of all oncogenic signalling and transduction pathways that trigger cell proliferation (Williams and Stoeber, 1999; Stoeber et al, 2001). Initiation of chromosomal replication is dependent on sequential assembly of multi-protein complexes, referred to as pre-replicative complexes (pre-RCs), at replication origins along the chromosomes during late mitosis and early G1 phase of the cell cycle. Assembly of pre-RCs onto chromatin results in origins being ‘licensed’ for replication during the subsequent S phase (Blow and Hodgson, 2002). The initial step in origin licensing is the binding of the origin recognition complex (ORC) to chromatin. The origin recognition complex functions as a landing platform for two loading factors, cell division cycle (Cdc)6 and Cdt1, which in turn recruit the minichromosome maintenance (MCM) complex comprised of subunits Mcm2–7 to the origin (Lei and Tye, 2001). At the G1 to S phase transition, bi-directional replication forks are established at licensed origins by the concerted action of cyclin-dependent kinases (CDKs) (Nishitani and Lygerou, 2002) and the ASK-dependent Cdc7 kinase (Masai and Arai, 2002). Firing of origins triggers a conformational change in the macromolecular origin-licensing complex, and results in recruitment of the single-strand binding protein RPA and additional initiation/elongation factors to origins. During this process, the DNA helix is unwound by the helicase activity of the MCM complex (Labib and Diffley, 2001), and replication is initiated by the primase activity of DNA polymerase-α (Bell and Dutta, 2002).
The initiation of chromosomal replication is tightly coupled to removal of the license and thus prevention of re-licensing following origin firing. This step is critical as replication must occur once, and only once, per cell cycle to ensure genomic stability. As such, human cells have adopted several strategies for preventing origin re-licensing. These include elevated CDK activity during the latter half of the proliferative cycle, resulting in activation and/or removal of replication-licensing factors, changes in gene expression and/or cell-cycle-regulated ubiquitin-mediated proteolysis of replication-licensing factors, and expression of a negative regulator of origin licensing known as Geminin during the S, G2 and M phases (Blow and Hodgson, 2002; Nishitani and Lygerou, 2002). Geminin acts as an inhibitor of DNA replication initiation via its interaction with the loading factor Cdt1 and subsequent inhibition of MCM loading onto chromatin (Wohlschlegel et al, 2000; Tada et al, 2001).
We have previously shown that repression of origin licensing is a ubiquitous route by which the proliferative capacity of cells is lowered as cells exit from cycle. Withdrawal from cycle into quiescent, differentiated or senescent states is coupled to downregulation of the MCM helicase proteins and the Cdc6 loading factor (Stoeber et al, 2001; Blow and Hodgson, 2002). Importantly, we have demonstrated that dysregulation of these replicative factors occurs in dysplastic states, indicating that it is an early event in tumorigenesis (Williams et al, 1998; Freeman et al, 1999; Stoeber et al, 1999, 2002; Going et al, 2002). Although Geminin is a potential inhibitor of cell proliferation (McGarry and Kirschner, 1998; Wohlschlegel et al, 2000), a regulator of differentiation (Kroll et al, 1998), and may be required for maintenance of genomic integrity (Saxena and Dutta, 2003), its role in tumour progression remains to be determined.
Here we investigate the regulation of DNA replication-licensing factors Mcm2 and Geminin in a series of oligodendrogliomas to examine the potential linkages between aberrant Geminin expression and tumour progression. These tumours are relatively homogeneous, facilitating accurate assessment of proliferation indices. Moreover, we have previously demonstrated that expression of the Mcm2 origin-licensing factor rises with increasing tumour grade and other markers of proliferation in this tumour type (Wharton et al, 2001). Our study includes a comparison of the regulation of Geminin with the bona fide cell cycle inhibitor p21/WAF and an analysis of potential linkages between these cell cycle regulators and apoptosis.
MATERIALS AND METHODS
Clinical material
A total of 55 surgical specimens of oligodendroglioma were retrieved from the files of the Histopathology Department of the Royal Hallamshire Hospital for the period 1985–1997. Ethical approval for the study was obtained from the local research ethics committee. Eight of the cases showed focal areas of astrocytic differentiation. In all, 47 of the cases were first diagnoses and eight were recurrent tumours. All of the cases were reviewed by a neuropathologist for confirmation of diagnosis and histological grade according to the World Health Organisation (WHO) criteria (Reifenberger et al, 2000a, 2000b). In total, 25 cases were graded as WHO grade II and 30 as anaplastic tumours, WHO grade III. The biopsies were derived from 22 female and 33 male patients. World Health Organisation grade II tumours were from patients with a mean age of 37.4 years (s.d. 15.6), while WHO grade III tumours tended to be from older patients, of mean age 44.6 years (s.d. 14.5).
Cell culture conditions
MOLT-4 human leukaemic lymphocytes (ATCC CRL-1582; Rockville, MD, USA) were cultured in CO2-independent media (Leibovitz (L-15)) supplemented with 50 U ml−1 penicillin G, 50 μg ml−1 streptomycin sulphate and 10% foetal bovine serum (all from Invitrogen, Paisley, UK) and maintained at 37°C. Using a novel method for cell synchronisation (membrane elution), minimally disturbed early G1-phase cells were collected and followed during synchronous growth as previously described (Thornton et al, 2002; Helmstetter et al, 2003). Samples from these synchronous batch cultures were removed at specific cell cycle time points for determination of cell concentrations, cell sizes, and analyses of DNA content and protein. Cell density and size were determined using a Coulter Z2 Particle Count and Size Analyzer (Beckman-Coulter, Hialeah, FL, USA). HL-60 cells were obtained from the European Collection of Cell Cultures (ECACC). Asynchronous HL-60 cells were maintained between 1 and 5 × 105 cells ml−1 at 37°C in a humidified 5% CO2 atmosphere in RPMI 1640 medium supplemented with 2 mM glutamine and 10% FCS.
Generation of rabbit polyclonal antisera against full-length human Geminin
pET15b-human (hs)Geminin (a generous gift from Anindya Dutta; Wohlschlegel et al, 2000) was expressed in Escherichia coli strain BL21(De3) and purified by Ni-NTA metal affinity chromatography following the manufacturers’ instructions (Qiagen, Crawley, UK). Recombinant hsGeminin was further purified using an (HPLC) Hi-load Q sepharose 16/10 column in NaPi buffer and eluted with varying concentrations of 1 M NaCl. Four rabbits were injected with 125 μg of purified hsGeminin protein, and received three boost injections over a period of 80 days following a standard protocol (Eurogentech, Seraing, Belgium). The sera were collected and affinity-purified on a CNBr column against 10 mg of recombinant hsGeminin protein, eluted with 0.1 M glycine (pH 2.5) and dialysed into PBS, 1% BSA and 0.1% sodium azide. An equal volume of sterile glycerol was added and affinity-purified polyclonal antibodies G92 and G95 were stored at −20°C. Antibody purification was quality controlled by SDS–PAGE for purity and by ELISA. Specificity of the affinity-purified antibodies was demonstrated by immunoblotting of cell lysates and by quenching all immunohistochemical staining after incubating antibodies diluted at working concentrations with equal or less than molar amount of recombinant hsGeminin protein for 1 h prior to a standard immunohistochemical staining protocol.
Flow cytometric analysis of DNA content, Ki67 and Geminin
For cell cycle analysis of DNA content, cell samples were fixed in 80% ethanol and stained with propidium iodide as previously described (Helmstetter et al, 2003) before flow cytometric analysis. For determination of Ki67 and Geminin content, cells were fixed as described above, and permeabilised with nonionic detergent using a method modified from Gong et al (1995). After centrifugation, cell pellets were resuspended in D-PBS, and either 10 μl of FITC-conjugated Ki67 monoclonal antibody (clone B56) or the matched nonspecific staining control (FITC-conjugated IgG; each from BD PharMingen™, Oxford, UK), or 0.1 μg of affinity-purified rabbit polyclonal anti-Geminin antibody G95, or G95 and recombinant hsGeminin protein (1 : 10 ratio). After 2 h incubation at 4°C, cells were washed with D-PBS containing 1% BSA, concentrated via centrifugation and stained with propidium iodide. Analyses of light-scatter properties and DNA/protein content were performed using a FACSCalibur flow cytometer (BD Biosciences, San Jose, CA, USA). Cell doublets were excluded by gating on a dot plot of the width vs the area of DNA fluorescence intensity (Erlanson and Landberg, 1998). In most samples, 104 cells were examined and data were stored and analysed using CellQuest™ software (BD Biosciences), Cylchred (V.1.0.0.1) and WinMDI (V.2.7).
Preparation of protein extracts and immunoblotting
Whole-cell lysates (2 × 106 cells) were prepared for immunoblotting from asynchronous or synchronous batch cultures using a method modified from Harlow and Lane (1999). Cell pellets were resuspended by vigorous vortexing in sample buffer containing 3% SDS, 100 mM DTT, 60 mM Tris (pH 6.8), 0.01% bromophenol blue and 10% glycerol. Equal loading of MOLT-4 (or HL-60) cell lysates was achieved by using the extract from equivalent cell numbers in each lane. Immunoblots were performed as previously described (Stoeber et al, 2001) using affinity-purified rabbit polyclonal anti-Geminin antibodies, and antibodies obtained from commercial sources against the following antigens: Mcm2 (clone 46) and PCNA (Clone 24; BD Transduction Laboratories™, Lexington, KY, USA), Cyclin A (C-19; Santa Cruz; CA, USA). Total cell extracts from equivalent cell numbers were separated on 10% SDS–PAGE gels (Invitrogen), before transfer to Hybond-P membranes (Amersham, Little Chalfont, UK), incubation with primary and secondary antibodies (HRP-conjugated anti-mouse or anti-rabbit antibodies (DAKO, Glostrup, Denmark)), and visualisation of immune complexes using enhanced chemiluminescence (Amersham).
Immunohistochemistry and expression profile analysis
All available slides from each case were reviewed and a block was selected containing a representative area of oligodendroglioma. Immunohistochemistry was performed using antibodies against Mcm2, Ki67, activated caspase 3 and p21, as shown in Table 1 . Apoptotic bodies were assessed on H&E-stained sections. Immunohistochemistry was performed using an ABC method and the signal visualised using diaminobenzidine. Serial sections were cut at 4 μm onto APES-coated slides, or at 3 μm onto ChemMate slides (DAKO) for Ki67, Mcm2 and Geminin preparations. Areas of highest cellularity and expression were used for quantitation, as previously described (Wharton et al, 1998). For each marker, a percentage labelling index was obtained from a count of at least 1000 cells using an eyepiece graticule.
Table 1. Conditions for immunohistochemistry.
| Antibody | Source | Dilution | Incubation | Ag retrieval |
|---|---|---|---|---|
| Ki67 | Novocastra | 1 : 25 | 1 h RT | MW 20 min Na citrate pH 6 |
| p21 | DAKO | 1 : 25 | 1 h RT | MW 20 min Na citrate pH 6 |
| Caspase 3 | R&D systems | 1 : 600 | O/N 4°C | MW 20 min Na citrate pH 6 |
| Mcm2 | Transduction laboratories | 1:1000 | 1 h RT | PC 2 min Na citrate pH 6 |
| G92 | See text | |||
| G95 | See text |
RT=room temperature, O/N=overnight, MW=microwave, PC=pressure cooker.
Statistical analysis
Statistical analyses were performed using the Statistical Package for the Social Science (SPSS, V.10.1). Labelling indices between grades were compared using the Mann–Whitney U-test and correlation determined using Pearson's coefficient of correlation.
RESULTS
Generation and characterisation of rabbit polyclonal antibodies against human Geminin
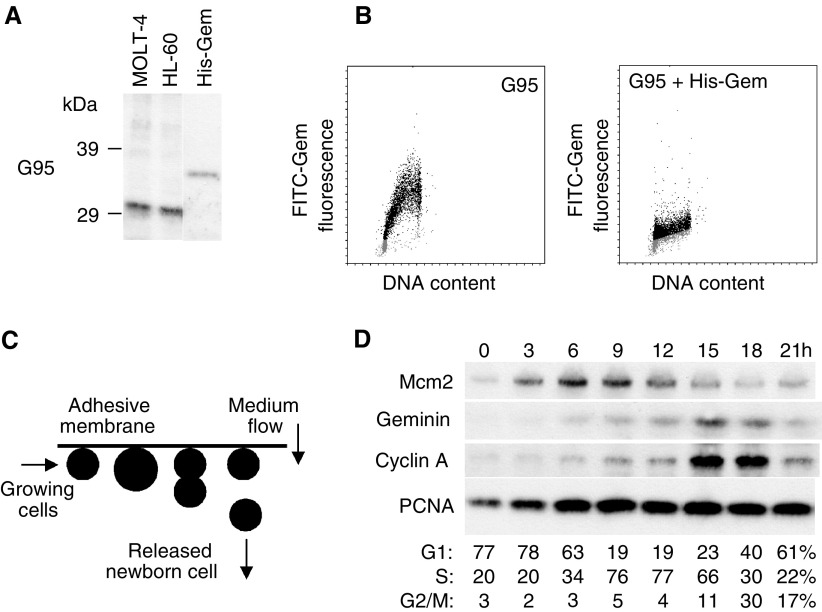
We raised rabbit polyclonal antisera G92 and G95 against bacterially expressed full-length human Geminin. In immunoblots of total cell lysates from asynchronously proliferating human MOLT-4 leukaemic lymphocytes (Figure 1A, lane 1) and human promyelocytic HL-60 cells (Figure 1A, lane 2), affinity-purified antibody G95 detected a single protein with an estimated molecular mass of ∼33 kDa, consistent with the reported electrophoretic mobility of human Geminin (McGarry and Kirschner, 1998; Wohlschlegel et al, 2000), while the pre-immune sera did not (data not shown). Antibody G95 also recognised recombinant His6-tagged Geminin with a molecular mass of ∼34 kDa (Figure 1A, lane 3). In addition, bivariate flow cytometric analyses of Geminin expression and DNA content in asynchronously proliferating MOLT-4 cells showed that Geminin was absent in cells (represented as dots) with a 2C (or G1 phase) DNA content (grey dots) and increased during S and G2 phases of the cell cycle (black dots; Figure 1B), consistent with previous studies in HeLa cells (McGarry and Kirschner, 1998; Wohlschlegel et al, 2000). Pre-incubation of antibody G95 with a 1 : 10 ratio of recombinant Geminin resulted in loss of detection of this cell cycle-specific expression (Figure 1B). Affinity-purified antibody G92 also recognised the ∼33 kDa band in total cell lysates, but showed weak crossreactivity with nonspecific bands of higher molecular mass (data not shown). Antibody G95 was therefore chosen for subsequent detailed immunoblot analysis of Geminin expression during the proliferative cell cycle.
Figure 1.
(A) Immunoblots of total cell lysates prepared from asynchronously proliferating MOLT-4 and HL-60 cells and rec. human Geminin with anti-Geminin antibody G95. Rabbit polyclonal affinity-purified antibody G95 detected a single protein with a molecular mass of ∼33 kDa in total lysates from MOLT-4 (lane 1) and HL-60 (lane 2) cells, and recognised nanogram quantities of rec. Geminin (lane 3; ∼34 kDa). (B) Bivariate flow cytometry of asynchronous MOLT-4 cells with G95 alone, or G95 plus rec. Geminin, further supports the notion that the antibody is specific for human Geminin. Note that cells in G1 (2C; grey dots) are Geminin-negative, whereas cells in S–G2–M (black dots) are positive for Geminin. Pre-incubation of G95 with rec. Geminin results in loss of cell cycle periodicity and little Geminin expression is detected at any stage of the cell cycle. (C) Schematic of membrane elution. Asynchronously growing MOLT-4 cell cultures are immobilised on surfaces such that gravity coupled with cell division results in release of one daughter cell, while the other remains surface-bound. Newborn early G1-phase cells are continuously released in the effluent and grow synchronously without evidence of disturbance. (D) Immunoblots of origin-licensing factors and control proteins in synchronously proliferating MOLT-4 cells. Protein levels of Mcm2, Geminin, Cyclin A and PCNA were determined in lysates from equivalent numbers of cells isolated from synchronous batch cultures at the indicated times.
Cell cycle expression of origin-licensing factors and tumour cell kinetics
Determination of the molecular and temporal events comprising the process of mammalian DNA replication often requires the use of synchronous cell populations. We have examined expression of the standard proliferation marker Ki67, the DNA replication-licensing factor Mcm2 and the repressor of origin-licensing Geminin during the proliferative cell cycle, using a novel, nonchemical method for cell synchronisation (membrane elution; Thornton et al, 2002; Helmstetter et al, 2003; Figure 1C). We have employed membrane elution because use of chemical methods for cell synchronisation may account in part for reported discrepancies in temporal periodicities and subcellular localisation of replication proteins such as Orc1 (Pak et al, 1997; Kreitz et al, 2001; Okuno et al, 2001; Li and DePamphilis, 2002) and Cdc6 (Fujita et al, 1999; Jiang et al, 1999; Mendez and Stillman, 2000; Petersen et al, 2000).
Newborn (early G1-phase) MOLT-4 cells isolated by membrane elution progressed through a full cycle of synchronous growth, as demonstrated by FACS analysis of the DNA content (Figure 1D). In total cell extracts prepared from these synchronous batch cultures, protein levels of the DNA replication-licensing factor Mcm2 increase linearly in early G1 phase up to a maximum in S phase before decreasing during the latter part of the cell cycle (Figure 1D). Moreover, cell synchronisation by membrane elution has revealed a similar cell cycle-dependent periodicity in Mcm5 and Mcm7 expression (Eward et al, manuscript submitted), findings that may have been previously obscured by metabolic perturbation due to the use of chemical synchronisation agents. Previous work has suggested that Geminin is present during S, G2 and early mitosis of the proliferative cell cycle before ubiquitination by the anaphase-promoting complex (McGarry and Kirschner, 1998; Wohlschlegel et al, 2000; Nishitani et al, 2001; Tada et al, 2001). Using our newly generated affinity-purified polyclonal anti-Geminin antibody G95 (Figure 1A), we found little to no expression of this repressor of origin licensing during G1 phase, with detectable levels at the G1/S transition and increasing linearly during the remainder of the cycle before mitotic degradation (Figure 1D). Notably, the Geminin expression profile is similar to Cyclin A, another oscillating cell cycle regulator which, like Geminin, is degraded at the metaphase to anaphase transition via the ubiquitin–proteasome pathway (Figure 1D) (Yam et al, 2002). In contrast to the proteins described above, protein levels of proliferating cell nuclear antigen (PCNA) remained constant during synchronous progression through the cell cycle (Figure 1D). Moreover, the fraction of MOLT-4 cells expressing the standard proliferation marker Ki67 during synchronous growth (85–95%; determined by FACS analysis) remained constant and was similar to the proportion that was Ki67-positive in asynchronous culture (data not shown). Geminin therefore provides a precise estimate of the S-G2-M growth fraction in dynamic cell populations and the ratio of Geminin/Ki67 provides information about the relative length of the G1 phase of the proliferative cell cycle (Figure 1D).
Correlation of Geminin expression to replication licensing and cell proliferation
Ki67 and Mcm2 both demonstrated an increased labelling index in WHO grade III compared to grade II tumours (P<0.01), reflecting greater proliferation with increasing anaplasia (Table 2 ). Values for Mcm2 obtained in this study showed a pattern of increasing expression with grade similar to that observed previously by us in a separate series of oligodendroglial tumours (Wharton et al, 2001).
Table 2. Labelling indices – descriptive statistics.
| Grade | Statistic | Ki67 | Mcm2 | AI | Csp3 | p21 | G92 | G95 |
|---|---|---|---|---|---|---|---|---|
| II | Mean (s.d.) | 11.4 (8.5) | 16.0 (15.3) | 0.8 (0.5) | 1.8 (2.5) | 2.3 (2.7) | 2.5 (1.8) | 2.4 (1.9) |
| Median (IQR) | 8.8 (11.7) | 10.2 (16.8) | 0.6 (0.8) | 0.9 (1.6) | 1.5 (3.4) | 2.5 (2.5) | 2.3 (2.8) | |
| III | Mean (s.d.) | 22.1 (16.3) | 40.0 (25.1) | 1.1 (0.7) | 2.4 (2.5) | 4.4 (5.4) | 8.9 (9.8) | 8.5 (6.9) |
| Median (IQR) | 21.3 (18.2) | 37.8 (44.6) | 0.9 (0.8) | 1.6 (3.2) | 3.2 (4.5) | 6.4 (5.1) | 6.4 (7.2) | |
| P-value* | 0.002 | <0.001 | 0.058 | 0.335 | 0.061 | <0.001 | <0.001 |
AI=apoptosis index based on H&E counts, IQR=interquartile range, P-value*=for grade III vs grade II tumours, Mann–Whitney U-test.
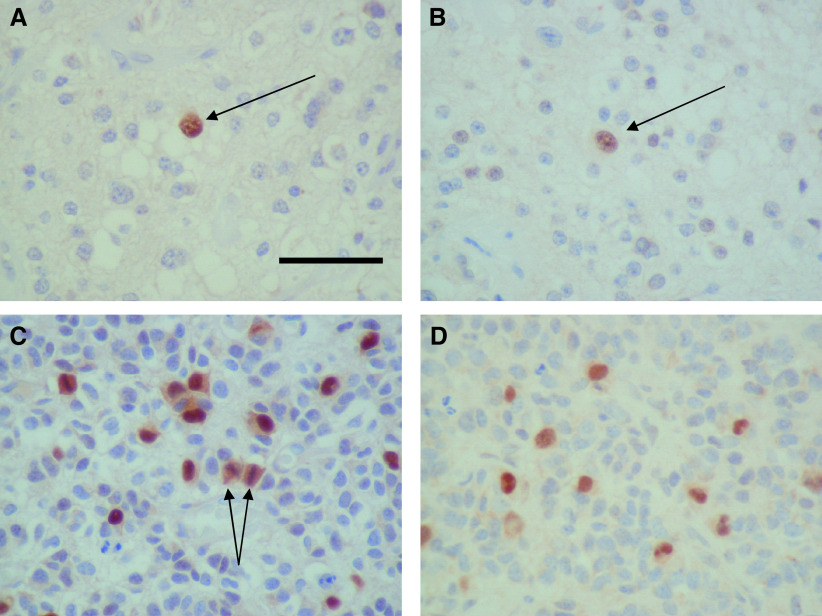
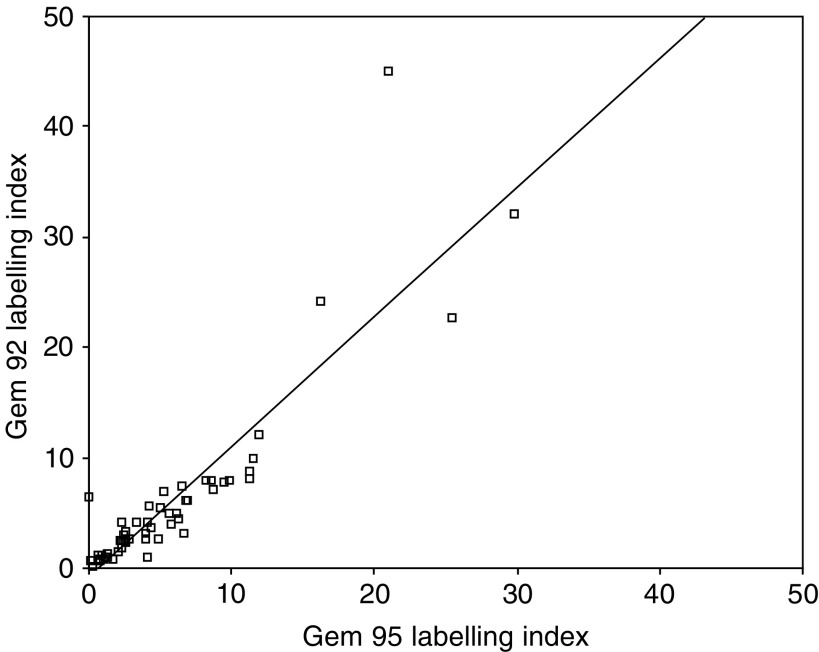
Both Geminin antibodies, G92 and G95, gave strong, predominantly nuclear staining (Figure 2). Cytoplasmic staining was noted in some cases, especially with antibody G92. Quantitation of staining with the two antibodies produced comparable results with very good correlation (r=0.89, P<0.01) (Figure 3). Although comparable, staining with G95 was stronger and the values for this antibody have been used for subsequent analyses.
Figure 2.
Geminin immunohistochemistry. (A) Low-grade oligodendroglioma showing a single Geminin-positive nucleus (arrow) stained with G95. (B) G92 showing a similar nuclear pattern of staining. (C) Anaplastic oligodendroglioma showing multiple nuclei stained with G95. Diffuse cell staining is observed in mitotic figures (arrows). (D) Anaplastic oligodendroglioma stained with G92.
Figure 3.
Scatter plot showing a close correlation between labelling indices obtained with G92 and G95.
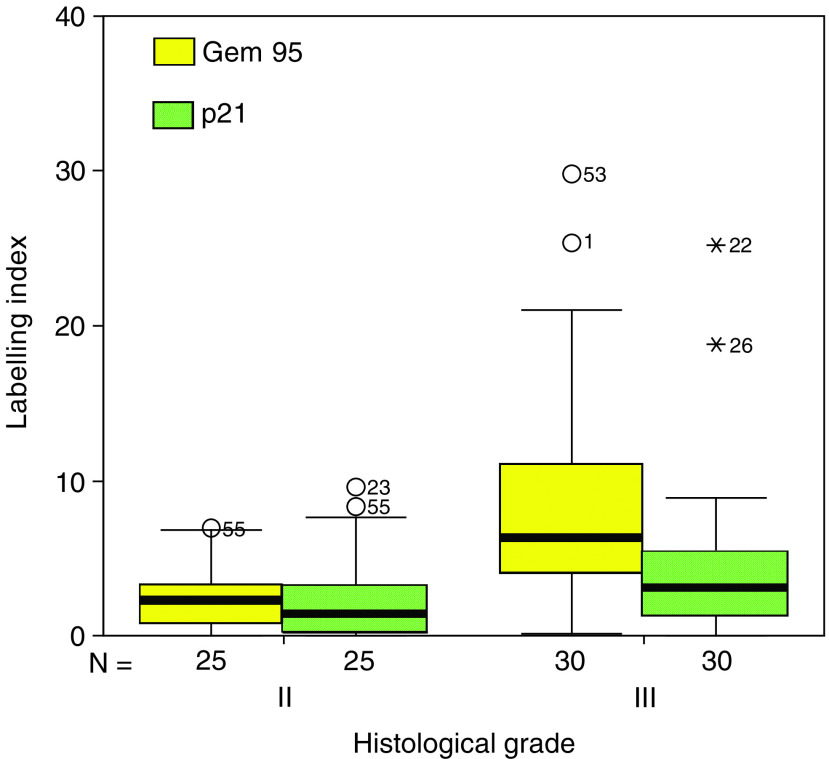
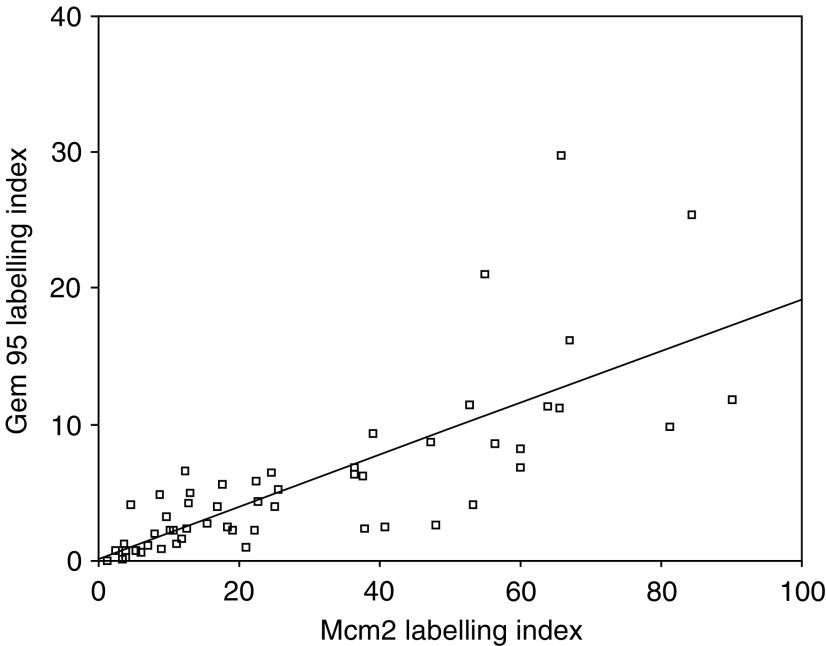
Geminin expression showed a clear relationship to grade, with greater labelling indices for both antibodies for grade III vs grade II tumours (P<0.001), and with a greater variation for the higher grade tumours (Figure 4, Table 2). Geminin expression showed a close relationship to licensing for proliferation, with a strong quantitative relationship to Mcm2 labelling index (r=0.76, P<0.01) (Figure 5). Examination of the scatter plot did not suggest the presence of any distinct clustered subgroup in which the relationship of Geminin expression to chromosome licensing was differently defined.
Figure 4.
Box-whisker plot showing labelling indices for G95 and p21 in relation to histological grade. Median values are shown as heavy lines within the boxes. Outlying values (>1.5 box lengths from the upper end of the box) are represented as stars or circles.
Figure 5.
Scatter plot showing a strong linear correlation between Geminin and Mcm2 expression.
Relationship of Geminin to p21/WAF1 expression
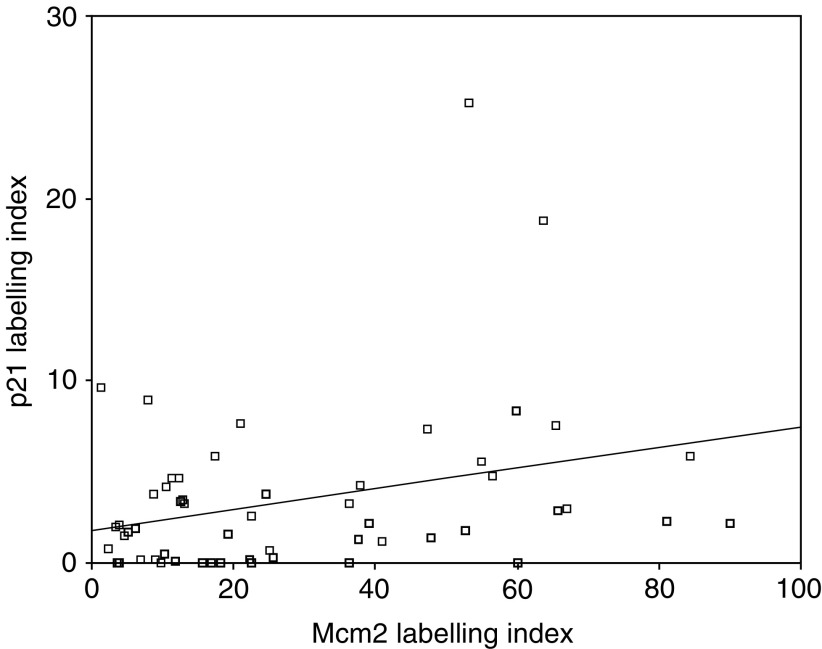
We sought to determine whether Geminin behaved similarly to the cell cycle inhibitor p21. Although the p21 labelling index showed a trend towards increased labelling with higher grade, this was not significant. Similarly, although p21 showed a correlation with the Mcm2 proliferation marker, this was very weak (r=0.3, P<0.05) (Figure 6). There was no correlation between p21 and Geminin (r=0.17, P=0.224).
Figure 6.
Scatter plot showing a poor relationship between p21 and Mcm2 labelling indices.
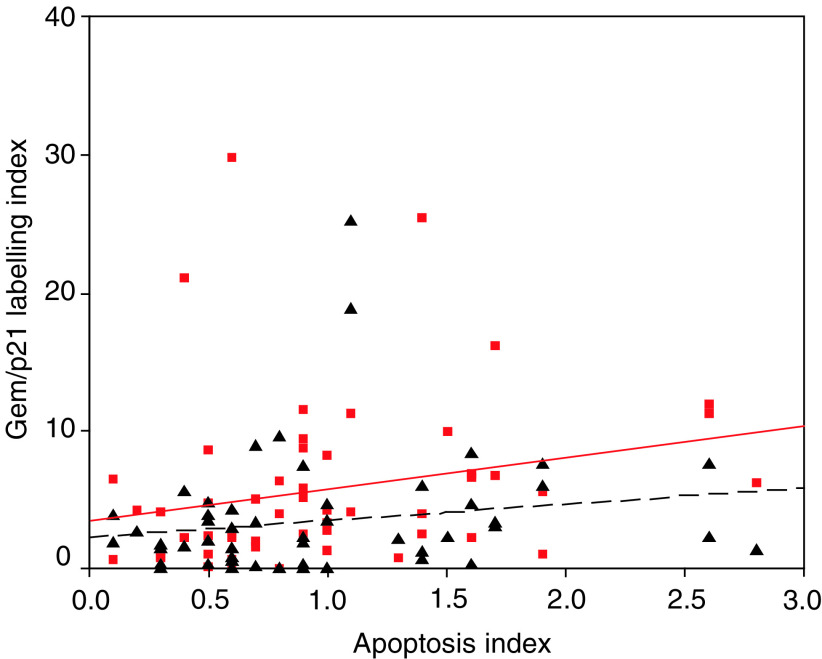
Induction of p21 may occur due to activation of p53 and may therefore relate to increasing cellular stress. We therefore sought to determine whether p21 or Geminin expression might correlate with degree of apoptosis. Apoptosis was assayed by means of counts of apoptotic bodies and labelling index for activated caspase 3. Both of these markers demonstrated a nonsignificant trend towards higher labelling with grade. Neither p21 nor Geminin expression demonstrated a significant correlation to levels of apoptosis (Figure 7).
Figure 7.
Scatter plot showing the relationship of apoptosis index with G95 (squares and solid line) and with p21 (triangle and dashed line).
The Geminin/Ki67 ratio is a new parameter for determining the relative length of G1 phase in dynamic cell populations
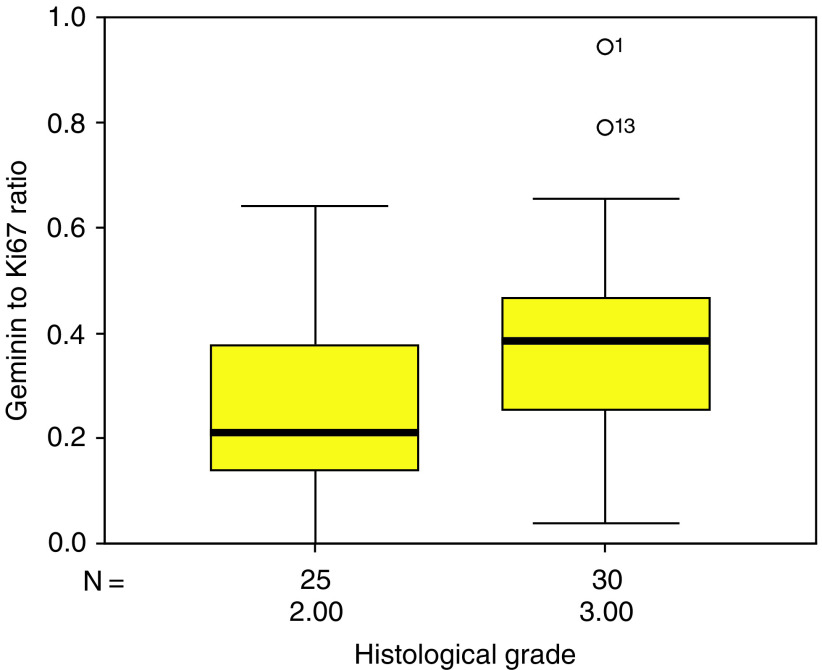
A comparison of Geminin/Ki67 ratios between low- and high-grade tumours reveals significant differences. The ratio is significantly higher for the grade III tumours (mean 0.39, s.d. 0.2) than grade II tumours (mean 0.25, s.d. 0.17; P=0.005) (Figure 8), indicating a relatively shorter G1 phase in these tumours. There was no significant correlation between Geminin/Ki67 ratio and apoptosis.
Figure 8.
Box plot showing Geminin to Ki67 ratio for grade II and grade III oligodendrogliomas.
DISCUSSION
We have used two polyclonal affinity-purified antibodies to detect the expression of Geminin in a series of human oligodendrogliomas. Both antibodies produced very similar quantitative results, and demonstrated a predominantly nuclear pattern of expression, as previously reported in proliferating cell populations (Wohlschlegel et al, 2002). Cytoplasmic expression was noted in some cases, particularly using the G92 antibody. The labelling index for Geminin was higher for grade III than for grade II tumours, and showed a strong positive correlation with markers of proliferation (Ki67) and DNA replication licensing (Mcm2). Our data show that expression of this origin licensing repressor is tightly coupled to proliferation in these tumours and do not support the alternative hypothesis that Geminin is an inhibitor of cell proliferation. The loss of Geminin therefore does not appear to be rate-limiting in the establishment of high-grade anaplastic tumours and may indicate that re-licensing of DNA replication does not contribute to genetic instability in these tumours. A similar pattern of expression, whereby Geminin appears to be expressed in proliferating cells, in a manner analogous to cell-cycle progression factors, has been noted in proliferating normal tissues (Wohlschlegel et al, 2002).
Oligodendrogliomas may be subdivided into two molecular types, according to whether there is deletion from chromosome 1p and 19q. The presence of 1p/19q loss predicts a longer progression-free survival and a better response to combination chemotherapy (Cairncross et al, 1998; Smith et al, 2000; Ino et al, 2001). Similar cytogenetic abnormalities are observed in tumours which also have areas of astrocytic differentiation (Kraus et al, 1995; Maintz et al, 1997). These molecular subgroups appear histologically similar (Sasaki et al, 2002). It is conceivable, however, that such subgroups differ in their patterns of cell cycle dysregulation. Inspection of scatter plots for our data does not reveal any distinct tumour subgroups in which the relationship of Geminin to that of proliferation licensing might be differently defined, but this is a hypothesis that we are exploring in further work.
We also sought to determine in this tumour type whether Geminin behaved in a similar way to a known cell-cycle inhibitor, p21. p21 (WAF1/CIP1) and p27 bind to, and inhibit, a range of Cyclin/CDK complexes, resulting in inhibition of cell cycle progression (Coqueret, 2003). Previous studies have demonstrated p21 expression in oligodendrogliomas. Some studies have suggested that p21 expression increases with increased tumour grade, with lack of p21 expression associated with a better prognosis (Miettinen et al, 2001), although other studies have found little difference in expression between low- and high-grade tumours (Korshunov and Golanov, 2001). In our series, we have shown a nonsignificant trend towards higher p21 labelling indices with higher tumour grade. In contrast, Geminin expression is strongly related to higher tumour grade and growth fraction. This suggests that Geminin behaves somewhat differently from classical cell cycle inhibitors. Data from normal tissue suggest that, whereas Geminin is particularly associated with proliferating cells, p21 is more typically seen in quiescence (Wohlschlegel et al, 2002). Similarly, in our study, the lower expression of Geminin in low-grade tumours suggests that this origin-licensing repressor is not required for maintaining tumour cells in quiescence.
Induction of apoptosis in tumours may also reflect cell stress and in oligodendrogliomas, as in many other tumour types, there is a trend towards greater levels of apoptosis in higher grade tumours (Schiffer et al, 1997; Wharton et al, 1998). Activation of cell cycle inhibitors in higher grade tumours may be a response to greater cell stress. Expression of p21 is induced by p53 (Prives and Hall, 1999), and it is possible that such a pathway might operate in high-grade oligodendrogliomas (Miettinen et al, 2001). We therefore hypothesised that there may be a potential linkage between expression of cell cycle inhibitors and apoptosis. We estimated apoptosis using counts of apoptotic bodies in H&E preparations, which correlates well with the TUNEL method (Wharton et al, 1998), and by immunohistochemistry to activated caspase 3. Activated caspase 3 has been used as a marker for apoptosis, with a good correlation with other apoptotic indices (Duan et al, 2003). In this series, neither p21 nor Geminin showed a statistical relationship to these markers of apoptosis.
The differential expression patterns of Ki67, Mcm2–7 and Geminin during the mitotic cell cycle and their tight downregulation in out-of-cycle states (Stoeber et al, 2001; Wohlschlegel et al, 2002) provide a novel and powerful multi-parameter analysis for assessment of growth fraction and cell cycle kinetics in human tissues and tumours. Using membrane elution, we have shown that Geminin expression is restricted to the S–G2–M phase of the proliferative cell cycle in human cells (Figure 1D). Previous assessment of the S-phase fraction has relied on methodologies such as flow cytometry, in vitro incorporation of 3H-thymidine (Quinn and Wright, 1990) or in vitro DNA synthesis (Mills et al, 2000), which are relatively complex, requiring fresh tissue. Here we have demonstrated that the S–G2–M fraction can now be easily assessed in glial tumour specimens simply through application of anti-Geminin antibodies to archival specimens. The Ki67 labelling index identifies all phases of the proliferative cell cycle (G1, S, G2, M), and the Geminin labelling index identifies the fraction of cells in S–G2–M. The Geminin/Ki67 ratio is therefore an indicator of the relative length of the G1 phase. Proliferating cells with a short G1 phase will approximate to a Geminin/Ki67 ratio of ∼1, whereas cells with a prolonged G1 phase will approximate to a Geminin/Ki67 ratio of ∼0. Interestingly, significant differences are observed in the Geminin/Ki67 ratio between grade II and grade III oligodendrogliomas, indicative of an accelerated G1 phase in anaplastic tumours. This novel approach thus allows a detailed assessment of tumour cell cycle kinetics that can be routinely applied to archival specimens. We and others have already demonstrated that the MCM replication-licensing factors can be exploited as prognostic markers in cancer diagnosis (Meng et al, 2001; Ramnath et al, 2001; Wharton et al, 2001; Gonzalez et al, 2003; Kruger et al, 2003). Analysis of a range of different tumour types including glial tumours is now in progress to determine whether the Geminin origin-licensing repressor and/or the Geminin/Ki67 ratio can be exploited as independent predictors of disease-free survival.
In summary, we have shown that Geminin expression correlates most strongly with proliferation in oligodendrogliomas and not with expression of the p21/WAF1 cell cycle inhibitor. Thus, despite being a potent inhibitor of DNA synthesis, Geminin does not appear to exert an antiproliferative effect in this tumour type. Our studies of the DNA replication-licensing pathway have revealed that high-grade tumours are associated with an accelerated G1 phase. Analysis of constituents of the DNA-licensing pathway in archival material thus provides novel tools for assessment of proliferative capacity and potential new algorithms for improved treatment decisions and prognostication in the management of oligodendroglial tumours.
Acknowledgments
We are grateful to Charles Helmstetter for advice about membrane elution. We wish to thank Craig Williams and Hyo-Il Jung for technical assistance in the expression and purification of recombinant Geminin and the generation of Geminin-specific antibodies. We thank Ellen Obermann for technical assistance in the membrane elution experiments. This work has been jointly funded by a Cancer Research UK programme grant and the John Hall fund.
References
- Bell SP, Dutta A (2002) DNA replication in eukaryotic cells. Annu Rev Biochem 71: 333–374 [DOI] [PubMed] [Google Scholar]
- Blow JJ, Hodgson B (2002) Replication licensing – defining the proliferative state? Trends Cell Biol 12: 72–78 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairncross JG, Ueki K, Zlatescu MC, Lisle DK, Finkelstein DM, Hammond RR, Silver JS, Stark PC, Macdonald DR, Ino Y, Ramsay DA, Louis DN (1998) Specific genetic predictors of chemotherapeutic response and survival in patients with anaplastic oligodendrogliomas. J Natl Cancer Inst 90: 1473–1479 [DOI] [PubMed] [Google Scholar]
- Coqueret O (2003) New roles for p21 and p27 cell-cycle inhibitors: a function for each cell compartment? Trends Cell Biol 13: 65–70 [DOI] [PubMed] [Google Scholar]
- Duan WR, Garner DS, Williams SD, Funckes-Shippy CL, Spath IS, Blomme EA (2003) Comparison of immunohistochemistry for activated caspase-3 and cleaved cytokeratin 18 with the TUNEL method for quantification of apoptosis in histological sections of PC-3 subcutaneous xenografts. J Pathol 199: 221–228 [DOI] [PubMed] [Google Scholar]
- Erlanson M, Landberg G (1998) Flow cytometric quantification of cyclin E in human cell lines and hematopoietic malignancies. Cytometry 32: 214–222 [PubMed] [Google Scholar]
- Freeman A, Morris LS, Mills AD, Stoeber K, Laskey RA, Williams GH, Coleman N (1999) Minichromosome maintenance proteins as biological markers of dysplasia and malignancy. Clin Cancer Res 5: 2121–2132 [PubMed] [Google Scholar]
- Fujita M, Yamada C, Goto H, Yokoyama N, Kuzushima K, Inagaki M, Tsurumi T (1999) Cell cycle regulation of human CDC6 protein. Intracellular localization, interaction with the human mcm complex, and CDC2 kinase-mediated hyperphosphorylation. J Biol Chem 274: 25927–25932 [DOI] [PubMed] [Google Scholar]
- Going JJ, Keith WN, Neilson L, Stoeber K, Stuart RC, Williams GH (2002) Aberrant expression of minichromosome maintenance proteins 2, 5 and Ki-67 in dysplastic squamous oesophageal epithelium and Barrett's mucosa. Gut 50: 373–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong J, Traganos F, Darzynkiewicz Z (1995) Growth imbalance and altered expression of cyclins B1, A, E, and D3 in MOLT-4 cells synchronized in the cell cycle by inhibitors of DNA replication. Cell Growth Differ 6: 1485–1493 [PubMed] [Google Scholar]
- Gonzalez MA, Pinder SE, Callagy G, Vowler SL, Morris LS, Bird K, Bell JA, Laskey RA, Coleman N (2003) Minichromosome maintenance protein 2 is a strong independent prognostic marker in breast cancer. J Clin Oncol 21: 4306–4313 [DOI] [PubMed] [Google Scholar]
- Harlow E, Lane DP (1999) Using Antibodies; A Laboratory Manual. Cold Spring Harbor: Cold Spring Harbor Laboratory Press [Google Scholar]
- Helmstetter CE, Thornton M, Romero A, Eward KL (2003) Synchrony in human, mouse and bacterial cell cultures – a comparison. Cell Cycle 2: 42–45 [DOI] [PubMed] [Google Scholar]
- Ino Y, Betensky RA, Zlatescu MC, Sasaki H, Macdonald DR, Stemmer-Rachamimov AO, Ramsay DA, Cairncross JG, Louis DN (2001) Molecular subtypes of anaplastic oligodendroglioma: implications for patient management at diagnosis. Clin Cancer Res 7: 839–845 [PubMed] [Google Scholar]
- Jiang W, Wells NJ, Hunter T (1999) Multistep regulation of DNA replication by Cdk phosphorylation of HsCdc6. Proc Natl Acad Sci USA 96: 6193–6198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korshunov A, Golanov A (2001) The prognostic significance of DNA topoisomerase II-alpha (Ki-S1), p21/Cip-1, and p27/Kip-1 protein immunoexpression in oligodendrogliomas. Arch Pathol Lab Med 125: 892–898 [DOI] [PubMed] [Google Scholar]
- Kraus JA, Koopmann J, Kaskel P, Maintz D, Brandner S, Schramm J, Louis DN, Wiestler OD, von Deimling A (1995) Shared allelic losses on chromosomes 1p and 19q suggest a common origin of oligodendroglioma and oligoastrocytoma. J Neuropathol Exp Neurol 54: 91–95 [DOI] [PubMed] [Google Scholar]
- Kreitz S, Ritzi M, Baack M, Knippers R (2001) The human origin recognition complex protein 1 dissociates from chromatin during S phase in HeLa cells. J Biol Chem 276: 6337–6342 [DOI] [PubMed] [Google Scholar]
- Kroll KL, Salic AN, Evans LM, Kirschner MW (1998) Geminin, a neuralizing molecule that demarcates the future neural plate at the onset of gastrulation. Development 125: 3247–3258 [DOI] [PubMed] [Google Scholar]
- Kruger S, Thorns C, Stocker W, Muller-Kunert E, Bohle A, Feller AC (2003) Prognostic value of MCM2 immunoreactivity in stage T1 transitional cell carcinoma of the bladder. Eur Urol 43: 138–145 [DOI] [PubMed] [Google Scholar]
- Labib K, Diffley JF (2001) Is the Mcm2–7 complex the eukaryotic DNA replication fork helicase? Curr Opin Genet Dev 11: 64–70 [DOI] [PubMed] [Google Scholar]
- Lei M, Tye BK (2001) Initiating DNA synthesis: from recruiting to activating the MCM complex. J Cell Sci 114: 1447–1454 [DOI] [PubMed] [Google Scholar]
- Li CJ, DePamphilis ML (2002) Mammalian Orc1 protein is selectively released from chromatin and ubiquitinated during the S-to-M transition in the cell division cycle. Mol Cell Biol 22: 105–116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maintz D, Fiedler K, Koopmann J, Rollbrocker B, Nechev S, Lenartz D, Stangl AP, Louis DN, Schramm J, Wiestler OD, von Deimling A (1997) Molecular genetic evidence for subtypes of oligoastrocytomas. J Neuropathol Exp Neurol 56: 1098–1104 [DOI] [PubMed] [Google Scholar]
- Masai H, Arai K (2002) Cdc7 kinase complex: a key regulator in the initiation of DNA replication. J Cell Physiol 190: 287–296 [DOI] [PubMed] [Google Scholar]
- McGarry TJ, Kirschner MW (1998) Geminin, an inhibitor of DNA replication, is degraded during mitosis. Cell 93: 1043–1053 [DOI] [PubMed] [Google Scholar]
- Mendez J, Stillman B (2000) Chromatin association of human origin recognition complex, cdc6, and minichromosome maintenance proteins during the cell cycle: assembly of prereplication complexes in late mitosis. Mol Cell Biol 20: 8602–8612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng MV, Grossfeld GD, Williams GH, Dilworth S, Stoeber K, Mulley TW, Weinberg V, Carroll PR, Tlsty TD (2001) Minichromosome maintenance protein 2 expression in prostate: characterization and association with outcome after therapy for cancer. Clin Cancer Res 7: 2712–2718 [PubMed] [Google Scholar]
- Miettinen HE, Paunu N, Rantala I, Kalimo H, Paljarvi L, Helin H, Haapasalo H (2001) Cell cycle regulators (p21, p53, pRb) in oligodendrocytic tumors: a study by novel tumor microarray technique. J Neurooncol 55: 29–37 [DOI] [PubMed] [Google Scholar]
- Mills AD, Coleman N, Morris LS, Laskey RA (2000) Detection of S-phase cells in tissue sections by in situ DNA replication. Nat Cell Biol 2: 244–245 [DOI] [PubMed] [Google Scholar]
- Nishitani H, Lygerou Z (2002) Control of DNA replication licensing in a cell cycle. Genes Cells 7: 523–534 [DOI] [PubMed] [Google Scholar]
- Nishitani H, Taraviras S, Lygerou Z, Nishimoto T (2001) The human licensing factor for DNA replication Cdt1 accumulates in G1 and is destabilized after initiation of S-phase. J Biol Chem 276: 44905–44911 [DOI] [PubMed] [Google Scholar]
- Okuno Y, McNairn AJ, den Elzen N, Pines J, Gilbert DM (2001) Stability, chromatin association and functional activity of mammalian pre-replication complex proteins during the cell cycle. EMBO J 20: 4263–4277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pak DT, Pflumm M, Chesnokov I, Huang DW, Kellum R, Marr J, Romanowski P, Botchan MR (1997) Association of the origin recognition complex with heterochromatin and HP1 in higher eukaryotes. Cell 91: 311–323 [DOI] [PubMed] [Google Scholar]
- Petersen BO, Wagener C, Marinoni F, Kramer ER, Melixetian M, Denchi EL, Gieffers C, Matteucci C, Peters JM, Helin K (2000) Cell cycle- and cell growth-regulated proteolysis of mammalian CDC6 is dependent on APC-CDH1. Genes Dev 14: 2330–2343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prives C, Hall PA (1999) The p53 pathway. J Pathol 187: 112–126 [DOI] [PubMed] [Google Scholar]
- Quinn CM, Wright NA (1990) The clinical assessment of proliferation and growth in human tumours: evaluation of methods and applications as prognostic variables (see comments). J Pathol 160: 93–102 [DOI] [PubMed] [Google Scholar]
- Ramnath N, Hernandez FJ, Tan DF, Huberman JA, Natarajan N, Beck AF, Hyland A, Todorov IT, Brooks JS, Bepler G (2001) MCM2 is an independent predictor of survival in patients with non-small-cell lung cancer. J Clin Oncol 19: 4259–4266 [DOI] [PubMed] [Google Scholar]
- Reifenberger G, Kros JM, Burger PC, Louis DN, Collins VP (2000a) Anaplastic oligodendroglioma. In Tumours of the Nervous System, Kleihues P, Cavanee WK (eds) pp 62–64, Lyon: IARC Press [Google Scholar]
- Reifenberger G, Kros JM, Burger PC, Louis DN, Collins VP (2000b) Oligodendroglioma. In Tumours of the Nervous System, Kleihues P, Cavanee WK (eds) pp 56–61, Lyon: IARC Press [Google Scholar]
- Sasaki H, Zlatescu MC, Betensky RA, Johnk LB, Cutone AN, Cairncross JG, Louis DN (2002) Histopathological–molecular genetic correlations in referral pathologist-diagnosed low-grade ‘oligodendroglioma’. J Neuropathol Exp Neurol 61: 58–63 [DOI] [PubMed] [Google Scholar]
- Saxena S, Dutta A (2003) Geminin and p53: deterrents to rereplication in human cancer cells. Cell Cycle 2: 283–286 [PubMed] [Google Scholar]
- Schiffer D, Dutto A, Cavalla P, Chio A, Migheli A, Piva R (1997) Role of apoptosis in the prognosis of oligodendrogliomas. Neurochem Int 31: 245–250 [DOI] [PubMed] [Google Scholar]
- Smith JS, Perry A, Borell TJ, Lee HK, O’Fallon J, Hosek SM, Kimmel D, Yates A, Burger PC, Scheithauer BW, Jenkins RB (2000) Alterations of chromosome arms 1p and 19q as predictors of survival in oligodendrogliomas, astrocytomas, and mixed oligoastrocytomas. J Clin Oncol 18: 636–645 [DOI] [PubMed] [Google Scholar]
- Stoeber K, Halsall I, Freeman A, Swinn R, Doble A, Morris L, Coleman N, Bullock N, Laskey RA, Hales CN, Williams GH (1999) Immunoassay for urothelial cancers that detects DNA replication protein Mcm5 in urine (letter). Lancet 354: 1524–1525 [DOI] [PubMed] [Google Scholar]
- Stoeber K, Swinn R, Prevost AT, de Clive-Lowe P, Halsall I, Dilworth SM, Marr J, Turner WH, Bullock N, Doble A, Hales CN, Williams GH (2002) Diagnosis of genito-urinary tract cancer by detection of minichromosome maintenance 5 protein in urine sediments. J Natl Cancer Inst 94: 1071–1079 [DOI] [PubMed] [Google Scholar]
- Stoeber K, Tlsty TD, Happerfield L, Thomas GA, Romanov S, Bobrow L, Williams ED, Williams GH (2001) DNA replication licensing and human cell proliferation. J Cell Sci 114: 2027–2041 [DOI] [PubMed] [Google Scholar]
- Tada S, Li A, Maiorano D, Mechali M, Blow JJ (2001) Repression of origin assembly in metaphase depends on inhibition of RLF-B/Cdt1 by geminin. Nat Cell Biol 3: 107–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton M, Eward KL, Helmstetter CE (2002) Production of minimally disturbed synchronous cultures of hematopoietic cells. Biotechniques 32: 1098–1100 1102, 1105 [DOI] [PubMed] [Google Scholar]
- Wharton SB, Chan KK, Anderson JR, Stoeber K, Williams GH (2001) Replicative Mcm2 protein as a novel proliferation marker in oligodendrogliomas and its relationship to Ki67 labelling index, histological grade and prognosis. Neuropathol Appl Neurobiol 27: 305–313 [DOI] [PubMed] [Google Scholar]
- Wharton SB, Hamilton FA, Chan WK, Chan KK, Anderson JR (1998) Proliferation and cell death in oligodendrogliomas. Neuropathol Appl Neurobiol 24: 21–28 [PubMed] [Google Scholar]
- Williams G, Stoeber K (1999) Clinical applications of a novel mammalian cell-free DNA replication system. Br J Cancer 80(Suppl 1): 20–24 [PubMed] [Google Scholar]
- Williams GH, Romanowski P, Morris L, Madine M, Mills AD, Stoeber K, Marr J, Laskey RA, Coleman N (1998) Improved cervical smear assessment using antibodies against proteins that regulate DNA replication. Proc Natl Acad Sci USA 95: 14932–14937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohlschlegel JA, Dwyer BT, Dhar SK, Cvetic C, Walter JC, Dutta A (2000) Inhibition of eukaryotic DNA replication by geminin binding to cdt1. Science 290: 2309–2312 [DOI] [PubMed] [Google Scholar]
- Wohlschlegel JA, Kutok JL, Weng AP, Dutta A (2002) Expression of geminin as a marker of cell proliferation in normal tissues and malignancies. Am J Pathol 161: 267–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yam CH, Fung TK, Poon RY (2002) Cyclin A in cell cycle control and cancer. Cell Mol Life Sci 59: 1317–1326 [DOI] [PMC free article] [PubMed] [Google Scholar]