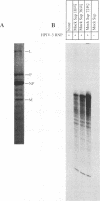
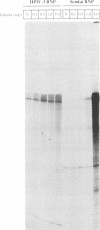
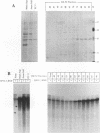
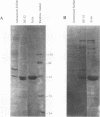
Abstract
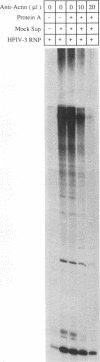
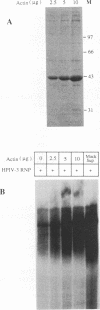
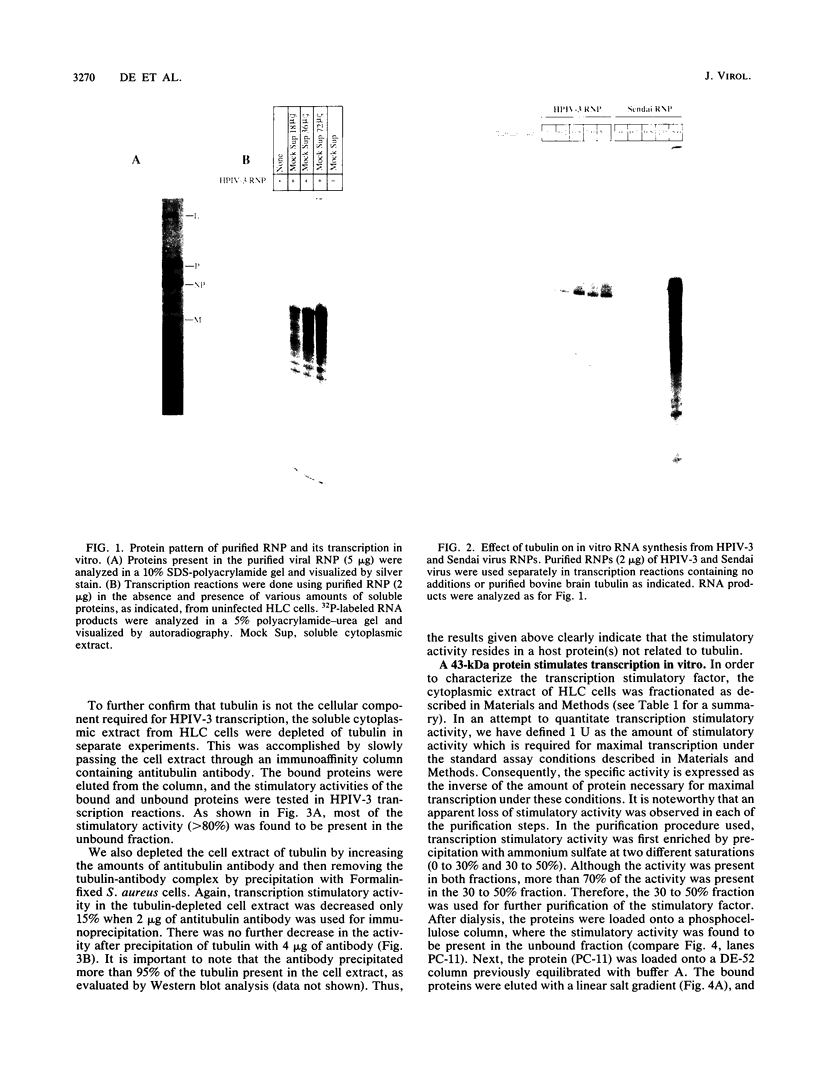
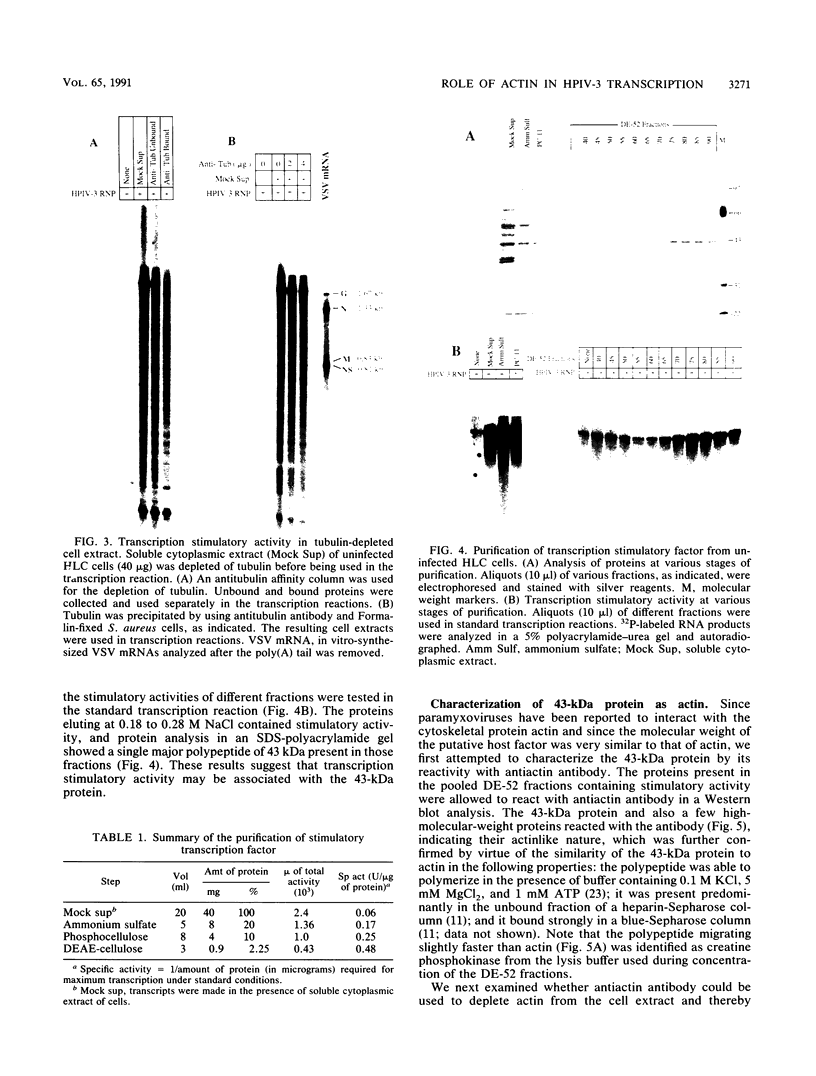
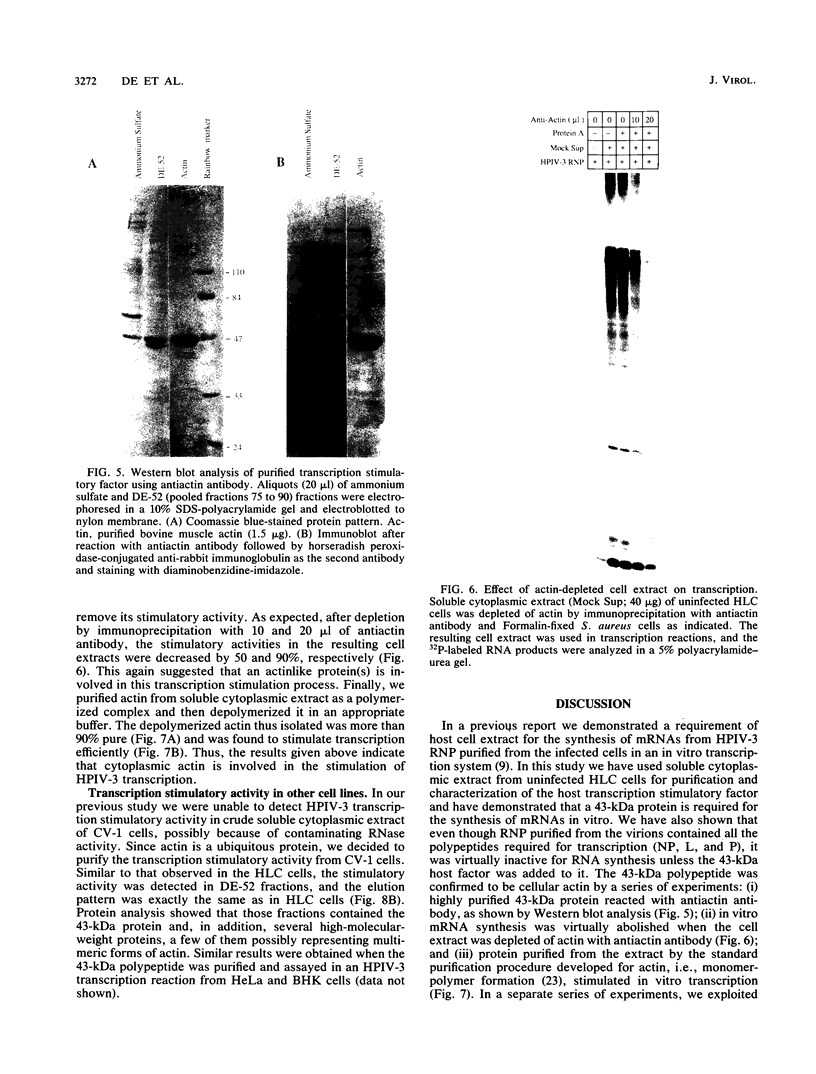
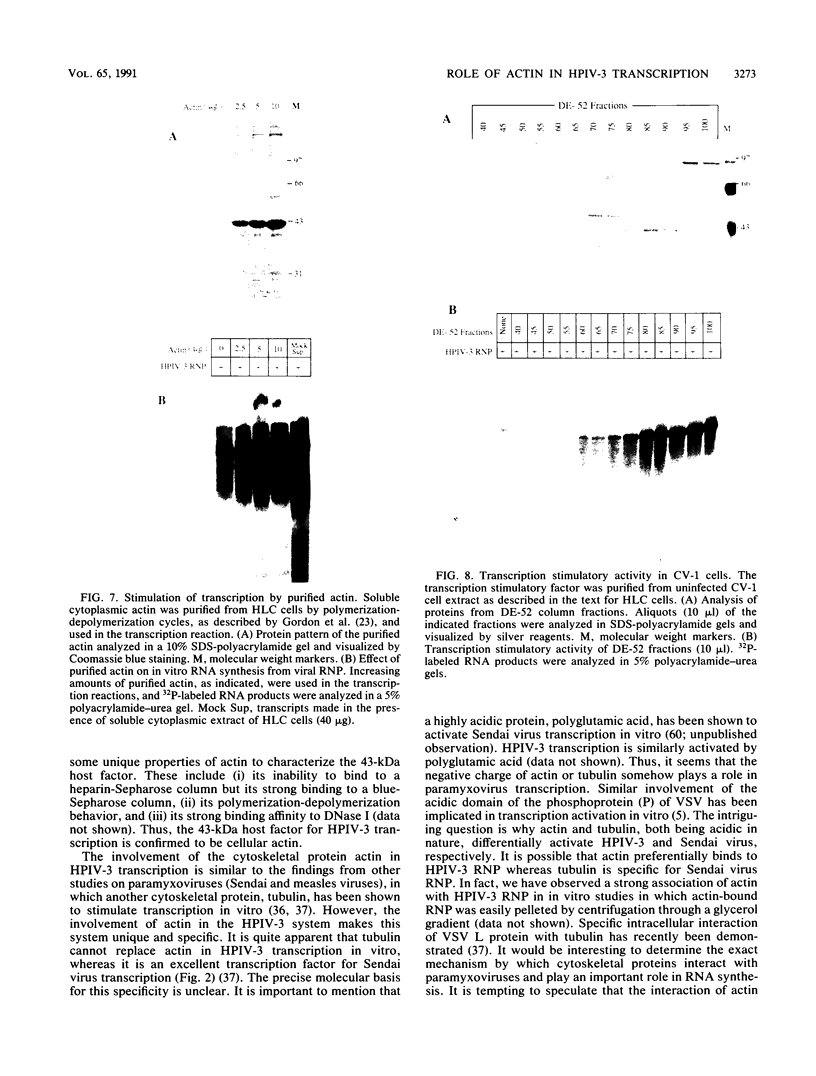
Purified ribonucleoprotein complexes of human parainfluenza virus type 3 (HPIV-3) virions required, in addition to the viral proteins, soluble cytoplasmic proteins from uninfected cells for the synthesis of mRNAs in vitro. In contrast to Sendai virus transcription, in vitro RNA synthesis from HPIV-3 ribonucleoprotein complexes was not stimulated significantly by purified tubulin. Moreover, cytoplasmic extract depleted of tubulin by immunoprecipitation stimulated HPIV-3 transcription effectively, suggesting involvement of a host protein(s) other than tubulin in the HPIV-3 transcription process. The transcription stimulatory factor was purified from uninfected cell extract by conventional chromatography and was found to contain a major 43-kDa polypeptide. In Western blot (immunoblot) analysis, this protein reacted with antiactin antibody, suggesting that the 43-kDa polypeptide is actin. This possibility was further supported by its polymerization activity and properties of binding to blue-Sepharose and heparin-Sepharose columns. Furthermore, when the cell extract was depleted of actin by immunoprecipitation by antiactin antibody, the stimulatory activity was abolished, indicating an involvement of actin in the stimulation of HPIV-3 transcription. After purification from RNAses, similar stimulatory activity associated with the 43-kDa protein was detected in other cell lines as well, including CV-1, HeLa, and BHK.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bohn W., Rutter G., Hohenberg H., Mannweiler K., Nobis P. Involvement of actin filaments in budding of measles virus: studies on cytoskeletons of infected cells. Virology. 1986 Feb;149(1):91–106. doi: 10.1016/0042-6822(86)90090-5. [DOI] [PubMed] [Google Scholar]
- CHANOCK R. M. Association of a new type of cytopathogenic myxovirus with infantile croup. J Exp Med. 1956 Oct 1;104(4):555–576. doi: 10.1084/jem.104.4.555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cattaneo R., Kaelin K., Baczko K., Billeter M. A. Measles virus editing provides an additional cysteine-rich protein. Cell. 1989 Mar 10;56(5):759–764. doi: 10.1016/0092-8674(89)90679-x. [DOI] [PubMed] [Google Scholar]
- Chattopadhyay D., Banerjee A. K. NH2-terminal acidic region of the phosphoprotein of vesicular stomatitis virus can be functionally replaced by tubulin. Proc Natl Acad Sci U S A. 1988 Nov;85(21):7977–7981. doi: 10.1073/pnas.85.21.7977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choppin P. W., Scheid A. The role of viral glycoproteins in adsorption, penetration, and pathogenicity of viruses. Rev Infect Dis. 1980 Jan-Feb;2(1):40–61. doi: 10.1093/clinids/2.1.40. [DOI] [PubMed] [Google Scholar]
- Côté M. J., Storey D. G., Kang C. Y., Dimock K. Nucleotide sequence of the coding and flanking regions of the human parainfluenza virus type 3 fusion glycoprotein gene. J Gen Virol. 1987 Apr;68(Pt 4):1003–1010. doi: 10.1099/0022-1317-68-4-1003. [DOI] [PubMed] [Google Scholar]
- De B. P., Banerjee A. K. Specific interactions of vesicular stomatitis virus L and NS proteins with heterologous genome ribonucleoprotein template lead to mRNA synthesis in vitro. J Virol. 1984 Sep;51(3):628–634. doi: 10.1128/jvi.51.3.628-634.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De B. P., Galinski M. S., Banerjee A. K. Characterization of an in vitro system for the synthesis of mRNA from human parainfluenza virus type 3. J Virol. 1990 Mar;64(3):1135–1142. doi: 10.1128/jvi.64.3.1135-1142.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimock K., Rud E. W., Kang C. Y. 3'-Terminal sequence of human parainfluenza virus 3 genomic RNA. Nucleic Acids Res. 1986 Jun 11;14(11):4694–4694. doi: 10.1093/nar/14.11.4694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egly J. M., Miyamoto N. G., Moncollin V., Chambon P. Is actin a transcription initiation factor for RNA polymerase B? EMBO J. 1984 Oct;3(10):2363–2371. doi: 10.1002/j.1460-2075.1984.tb02141.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elango N., Coligan J. E., Jambou R. C., Venkatesan S. Human parainfluenza type 3 virus hemagglutinin-neuraminidase glycoprotein: nucleotide sequence of mRNA and limited amino acid sequence of the purified protein. J Virol. 1986 Feb;57(2):481–489. doi: 10.1128/jvi.57.2.481-489.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott G. D., Yeo R. P., Afzal M. A., Simpson E. J., Curran J. A., Rima B. K. Strain-variable editing during transcription of the P gene of mumps virus may lead to the generation of non-structural proteins NS1 (V) and NS2. J Gen Virol. 1990 Jul;71(Pt 7):1555–1560. doi: 10.1099/0022-1317-71-7-1555. [DOI] [PubMed] [Google Scholar]
- Galinski M. S., Mink M. A., Lambert D. M., Wechsler S. L., Pons M. W. Molecular cloning and sequence analysis of the human parainfluenza 3 virus RNA encoding the nucleocapsid protein. Virology. 1986 Mar;149(2):139–151. doi: 10.1016/0042-6822(86)90116-9. [DOI] [PubMed] [Google Scholar]
- Galinski M. S., Mink M. A., Lambert D. M., Wechsler S. L., Pons M. W. Molecular cloning and sequence analysis of the human parainfluenza 3 virus gene encoding the matrix protein. Virology. 1987 Mar;157(1):24–30. doi: 10.1016/0042-6822(87)90309-6. [DOI] [PubMed] [Google Scholar]
- Galinski M. S., Mink M. A., Lambert D. M., Wechsler S. L., Pons M. W. Molecular cloning and sequence analysis of the human parainfluenza 3 virus mRNA encoding the P and C proteins. Virology. 1986 Nov;155(1):46–60. doi: 10.1016/0042-6822(86)90167-4. [DOI] [PubMed] [Google Scholar]
- Galinski M. S., Mink M. A., Pons M. W. Molecular cloning and sequence analysis of the human parainfluenza 3 virus gene encoding the L protein. Virology. 1988 Aug;165(2):499–510. doi: 10.1016/0042-6822(88)90594-6. [DOI] [PubMed] [Google Scholar]
- Giuffre R. M., Tovell D. R., Kay C. M., Tyrrell D. L. Evidence for an interaction between the membrane protein of a paramyxovirus and actin. J Virol. 1982 Jun;42(3):963–968. doi: 10.1128/jvi.42.3.963-968.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon D. J., Eisenberg E., Korn E. D. Characterization of cytoplasmic actin isolated from Acanthamoeba castellanii by a new method. J Biol Chem. 1976 Aug 10;251(15):4778–4786. [PubMed] [Google Scholar]
- Hamaguchi M., Nishikawa K., Toyoda T., Yoshida T., Hanaichi T., Nagai Y. Transcriptive complex of Newcastle disease virus. II. Structural and functional assembly associated with the cytoskeletal framework. Virology. 1985 Dec;147(2):295–308. doi: 10.1016/0042-6822(85)90132-1. [DOI] [PubMed] [Google Scholar]
- Hamaguchi M., Yoshida T., Nishikawa K., Naruse H., Nagai Y. Transcriptive complex of Newcastle disease virus. I. Both L and P proteins are required to constitute an active complex. Virology. 1983 Jul 15;128(1):105–117. doi: 10.1016/0042-6822(83)90322-7. [DOI] [PubMed] [Google Scholar]
- Herman R. C. Synthesis of respiratory syncytial virus RNA in cell-free extracts. J Gen Virol. 1989 Mar;70(Pt 3):755–761. doi: 10.1099/0022-1317-70-3-755. [DOI] [PubMed] [Google Scholar]
- Hill V. M., Summers D. F. A minor microtubule-associated protein is responsible for the stimulation of vesicular stomatitis virus transcription in vitro. J Gen Virol. 1990 Feb;71(Pt 2):289–298. doi: 10.1099/0022-1317-71-2-289. [DOI] [PubMed] [Google Scholar]
- Jambou R. C., Elango N., Venkatesan S., Collins P. L. Complete sequence of the major nucleocapsid protein gene of human parainfluenza type 3 virus: comparison with other negative strand viruses. J Gen Virol. 1986 Nov;67(Pt 11):2543–2548. doi: 10.1099/0022-1317-67-11-2543. [DOI] [PubMed] [Google Scholar]
- Jambou R. C., Elango N., Venkatesan S. Proteins associated with human parainfluenza virus type 3. J Virol. 1985 Oct;56(1):298–302. doi: 10.1128/jvi.56.1.298-302.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo K., Bando H., Tsurudome M., Kawano M., Nishio M., Ito Y. Sequence analysis of the phosphoprotein (P) genes of human parainfluenza type 4A and 4B viruses and RNA editing at transcript of the P genes: the number of G residues added is imprecise. Virology. 1990 Sep;178(1):321–326. doi: 10.1016/0042-6822(90)90413-l. [DOI] [PubMed] [Google Scholar]
- Korn E. D. Actin polymerization and its regulation by proteins from nonmuscle cells. Physiol Rev. 1982 Apr;62(2):672–737. doi: 10.1152/physrev.1982.62.2.672. [DOI] [PubMed] [Google Scholar]
- Kuziel W. A., Tucker P. W. Determination of vector: insert junctions in lambda gt10 cDNAs that do not recut with EcoRI. Nucleotide sequence of the lambda imm434 HindIII-EcoRI DNA fragment encoding part of the cI protein. Nucleic Acids Res. 1987 Apr 10;15(7):3181–3181. doi: 10.1093/nar/15.7.3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Luk D., Masters P. S., Sánchez A., Banerjee A. K. Complete nucleotide sequence of the matrix protein mRNA and three intergenic junctions of human parainfluenza virus type 3. Virology. 1987 Jan;156(1):189–192. doi: 10.1016/0042-6822(87)90453-3. [DOI] [PubMed] [Google Scholar]
- Luk D., Sánchez A., Banerjee A. K. Messenger RNA encoding the phosphoprotein (P) gene of human parainfluenza virus 3 is bicistronic. Virology. 1986 Sep;153(2):318–325. doi: 10.1016/0042-6822(86)90036-x. [DOI] [PubMed] [Google Scholar]
- Merril C. R., Goldman D., Sedman S. A., Ebert M. H. Ultrasensitive stain for proteins in polyacrylamide gels shows regional variation in cerebrospinal fluid proteins. Science. 1981 Mar 27;211(4489):1437–1438. doi: 10.1126/science.6162199. [DOI] [PubMed] [Google Scholar]
- Moyer S. A., Baker S. C., Horikami S. M. Host cell proteins required for measles virus reproduction. J Gen Virol. 1990 Apr;71(Pt 4):775–783. doi: 10.1099/0022-1317-71-4-775. [DOI] [PubMed] [Google Scholar]
- Moyer S. A., Baker S. C., Lessard J. L. Tubulin: a factor necessary for the synthesis of both Sendai virus and vesicular stomatitis virus RNAs. Proc Natl Acad Sci U S A. 1986 Aug;83(15):5405–5409. doi: 10.1073/pnas.83.15.5405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohgimoto S., Bando H., Kawano M., Okamoto K., Kondo K., Tsurudome M., Nishio M., Ito Y. Sequence analysis of P gene of human parainfluenza type 2 virus: P and cysteine-rich proteins are translated by two mRNAs that differ by two nontemplated G residues. Virology. 1990 Jul;177(1):116–123. doi: 10.1016/0042-6822(90)90465-4. [DOI] [PubMed] [Google Scholar]
- Paterson R. G., Lamb R. A. RNA editing by G-nucleotide insertion in mumps virus P-gene mRNA transcripts. J Virol. 1990 Sep;64(9):4137–4145. doi: 10.1128/jvi.64.9.4137-4145.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peluso R. W., Lamb R. A., Choppin P. W. Polypeptide synthesis in simian virus 5-infected cells. J Virol. 1977 Jul;23(1):177–187. doi: 10.1128/jvi.23.1.177-187.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollard T. D., Cooper J. A. Actin and actin-binding proteins. A critical evaluation of mechanisms and functions. Annu Rev Biochem. 1986;55:987–1035. doi: 10.1146/annurev.bi.55.070186.005011. [DOI] [PubMed] [Google Scholar]
- Portner A. Synthesis of message and genome RNAs in vitro by Sendai virus-infected cell nucleocapsids. J Gen Virol. 1982 May;60(Pt 1):67–75. doi: 10.1099/0022-1317-60-1-67. [DOI] [PubMed] [Google Scholar]
- Ray J., Fujinami R. S. Characterization of in vitro transcription and transcriptional products of measles virus. J Virol. 1987 Nov;61(11):3381–3387. doi: 10.1128/jvi.61.11.3381-3387.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray R., Galinski M. S., Compans R. W. Expression of the fusion glycoprotein of human parainfluenza type 3 virus in insect cells by a recombinant baculovirus and analysis of its immunogenic property. Virus Res. 1989 Feb;12(2):169–180. doi: 10.1016/0168-1702(89)90062-2. [DOI] [PubMed] [Google Scholar]
- Southern J. A., Precious B., Randall R. E. Two nontemplated nucleotide additions are required to generate the P mRNA of parainfluenza virus type 2 since the RNA genome encodes protein V. Virology. 1990 Jul;177(1):388–390. doi: 10.1016/0042-6822(90)90497-f. [DOI] [PubMed] [Google Scholar]
- Spriggs M. K., Collins P. L. Human parainfluenza virus type 3: messenger RNAs, polypeptide coding assignments, intergenic sequences, and genetic map. J Virol. 1986 Sep;59(3):646–654. doi: 10.1128/jvi.59.3.646-654.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spriggs M. K., Collins P. L. Sequence analysis of the P and C protein genes of human parainfluenza virus type 3: patterns of amino acid sequence homology among paramyxovirus proteins. J Gen Virol. 1986 Dec;67(Pt 12):2705–2719. doi: 10.1099/0022-1317-67-12-2705. [DOI] [PubMed] [Google Scholar]
- Spriggs M. K., Johnson P. R., Collins P. L. Sequence analysis of the matrix protein gene of human parainfluenza virus type 3: extensive sequence homology among paramyxoviruses. J Gen Virol. 1987 May;68(Pt 5):1491–1497. doi: 10.1099/0022-1317-68-5-1491. [DOI] [PubMed] [Google Scholar]
- Spriggs M. K., Olmsted R. A., Venkatesan S., Coligan J. E., Collins P. L. Fusion glycoprotein of human parainfluenza virus type 3: nucleotide sequence of the gene, direct identification of the cleavage-activation site, and comparison with other paramyxoviruses. Virology. 1986 Jul 15;152(1):241–251. doi: 10.1016/0042-6822(86)90388-0. [DOI] [PubMed] [Google Scholar]
- Stallcup K. C., Raine C. S., Fields B. N. Cytochalasin B inhibits the maturation of measles virus. Virology. 1983 Jan 15;124(1):59–74. doi: 10.1016/0042-6822(83)90290-8. [DOI] [PubMed] [Google Scholar]
- Storey D. G., Côté M. J., Dimock K., Kang C. Y. Nucleotide sequence of the coding and flanking regions of the human parainfluenza virus 3 hemagglutinin-neuraminidase gene: comparison with other paramyxoviruses. Intervirology. 1987;27(2):69–80. doi: 10.1159/000149722. [DOI] [PubMed] [Google Scholar]
- Storey D. G., Dimock K., Kang C. Y. Structural characterization of virion proteins and genomic RNA of human parainfluenza virus 3. J Virol. 1984 Dec;52(3):761–766. doi: 10.1128/jvi.52.3.761-766.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez A., Banerjee A. K. Cloning and gene assignment of mRNAs of human parainfluenza virus 3. Virology. 1985 Nov;147(1):177–186. doi: 10.1016/0042-6822(85)90237-5. [DOI] [PubMed] [Google Scholar]
- Sánchez A., Banerjee A. K., Furuichi Y., Richardson M. A. Conserved structures among the nucleocapsid proteins of the paramyxoviridae: complete nucleotide sequence of human parainfluenza virus type 3 NP mRNA. Virology. 1986 Jul 15;152(1):171–180. doi: 10.1016/0042-6822(86)90382-x. [DOI] [PubMed] [Google Scholar]
- Sánchez A., Banerjee A. K. Studies on human parainfluenza virus 3: characterization of the structural proteins and in vitro synthesized proteins coded by mRNAs isolated from infected cells. Virology. 1985 May;143(1):45–54. doi: 10.1016/0042-6822(85)90095-9. [DOI] [PubMed] [Google Scholar]
- Takeuchi K., Tanabayashi K., Hishiyama M., Yamada Y. K., Yamada A., Sugiura A. Detection and characterization of mumps virus V protein. Virology. 1990 Sep;178(1):247–253. doi: 10.1016/0042-6822(90)90400-l. [DOI] [PubMed] [Google Scholar]
- Thomas S. M., Lamb R. A., Paterson R. G. Two mRNAs that differ by two nontemplated nucleotides encode the amino coterminal proteins P and V of the paramyxovirus SV5. Cell. 1988 Sep 9;54(6):891–902. doi: 10.1016/S0092-8674(88)91285-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal S., Curran J., Kolakofsky D. Editing of the Sendai virus P/C mRNA by G insertion occurs during mRNA synthesis via a virus-encoded activity. J Virol. 1990 Jan;64(1):239–246. doi: 10.1128/jvi.64.1.239-246.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidal S., Kolakofsky D. Modified model for the switch from Sendai virus transcription to replication. J Virol. 1989 May;63(5):1951–1958. doi: 10.1128/jvi.63.5.1951-1958.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler S. L., Lambert D. M., Galinski M. S., Heineke B. E., Pons M. W. Human parainfluenza virus 3: purification and characterization of subviral components, viral proteins and viral RNA. Virus Res. 1985 Nov;3(4):339–351. doi: 10.1016/0168-1702(85)90434-4. [DOI] [PubMed] [Google Scholar]
- Wechsler S. L., Lambert D. M., Galinski M. S., Pons M. W. Intracellular synthesis of human parainfluenza type 3 virus-specified polypeptides. J Virol. 1985 Jun;54(3):661–664. doi: 10.1128/jvi.54.3.661-664.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]