Abstract
Endothelin-1 (ET-1) and its receptors (ETAR and ETBR), referred to as the endothelin (ET) axis, are overexpressed in breast carcinomas and appear to influence tumour growth and progression. The objective of this study was to determine the effect of expression of the ET axis in breast carcinomas on response to cytotoxic chemotherapy. The study included 44 patients with locally advanced breast cancer participating in a prospective phase III study evaluating high-dose neoadjuvant chemotherapy of epirubicin and cyclophosphamide. Expression of ET-1, ETAR and ETBR was determined by semiquantitative immunohistochemical analysis of breast cancer tissue from prechemotherapy tru-cut biopsies. Immunohistochemical staining was positive for ET-1 in 61.5%, for ETAR in 35% and for ETBR in 35.9% of breast carcinomas. Pathological response to chemotherapy was significantly decreased in ETAR-positive patients (P=0.002). In total, 50% of ETAR-positive patients as compared to 7.7% of ETAR-negative patients attained pathologically ‘no change’. Logistic regression confirmed ETAR as an independent predictive marker for pathological response (P=0.009). These data indicate that increased expression of ETAR in breast carcinomas is associated with resistance to chemotherapy. Determination of ETAR status may serve as a predictive marker for identifying patients less likely to be responsive to conventional chemotherapy.
Keywords: immunohistochemistry, preoperative, ETAR, ETBR, ET-1, chemoresistance
Breast cancer is the most common cancer in women worldwide and distant metastases are the leading cause of breast cancer related death (Ferlay et al, 2000). Thus, adjuvant systemic therapy has become the standard therapy to destroy residual or disseminated tumour cells. Neoadjuvant chemotherapy has been developed to improve breast-conserving operability in locally advanced breast cancers. Results from the NSABP B-18 study confirmed that neoadjuvant and adjuvant chemotherapy are equally effective on locoregional disease in women with operable breast cancer. Moreover, this study demonstrated that clinical and pathological response to neoadjuvant chemotherapy is predictive of patient outcome (Fisher et al, 1997, 1998). However, the failure of a number of tumours to respond to treatment and the appearance of resistant tumour cell populations upon relapse of an originally responsive malignancy are still major impediments to successful chemotherapy. Currently, no tumour marker is available for clinical use in predicting chemotherapy response in breast cancer. It is thus an important goal in oncologic research to identify molecular markers to facilitate risk-adapted individual therapy concepts.
The endothelins (ETs) comprise three 21 amino-acid peptides, ET-1, ET-2, and ET-3, which are multifunctional peptides with diverse activity (Yanagisawa et al, 1988). ET-1 is expressed primarily by endothelial, vascular smooth muscle and epithelial cells, whereas ET-2 and ET-3 are expressed in kidney epithelial cells and in gastrointestinal stromal cells (Levin, 1995; Nelson et al, 2003). Of the three ET isoforms, ET-1 has been the most extensively studied, and appears to be the most important isoform in cancer pathophysiology (Grant et al, 2003; Nelson et al, 2003). ET-1 expression is increased in several human malignancies including ovarian, prostate and colorectal cancer (Nelson et al, 1996, 2003; Salani et al, 2000; Asham et al 2001). Upregulation of ET-1 occurs in response to cell activation and is induced by various stimuli such as hypoxia, growth factors, and cytokines (Kourembanas et al, 1991). ET-1 effects are mediated through two subtypes of G protein-coupled receptors, ETAR and ETBR. ETAR binds ET-1 and ET-2 with high affinity and ET-3 with low affinity, whereas ETBR is nonselective with equal affinity for the three subtypes (Rubanji and Polokoff, 1994). It has been shown that ET-1 competitively binds to the ETA- and ETB-receptor, although ETAR is the dominant receptor (Bagnato et al, 1995, 1999). The complex of ET-peptides and ET receptors is referred to as the ‘ET axis’ (Nelson and Carducci, 1999). With respect to ET receptors, predominantly ETAR mediates tumour-associated functions, whereas there is less evidence for ETBR-dependent tumour-related functions (Grant et al, 2003; Nelson et al, 2003). Engagement of ETA-receptor by ET-1 triggers tumorigenesis and tumour progression by activation of tumour proliferation, invasion, angiogenesis, and inhibition of apoptosis (Pedram et al, 1997; Bagnato and Catt, 1998; Bagnato et al, 1999; Eberl et al, 2000b; Salani et al, 2000; Rosano et al, 2001; Del Bufalo et al, 2002). There is also evidence for an autocrine and/or paracrine mechanism of action of ET-1 including angiogenesis-promoting effects in malignant tissues. It has been shown that ET-1 induces endothelial cell growth through ETBR and exerts mitogenic effects on vascular smooth muscle cells and pericytes through ETAR (Bek and McMillen, 2000; Salani et al, 2000). Moreover, activation of ETAR by ET-1 stimulates the production of VEGF, which in turn induces endothelial cell proliferation and vascular permeability by increasing the levels of HIF-1α (Spinella et al, 2002). In addition to its mitogenic effects, ET-1 has also been found to contribute to tumour growth by protecting tumour cells from apoptosis (Eberl et al, 2000b). Increased ETAR expression has been demonstrated in malignant tissue in several cancer types including ovarian, prostate and colorectal cancer, whereas in normal tissue from these sites ETBR predominates (Nelson et al, 1996; Bagnato et al, 1999; Ali et al, 2000).
Studies have investigated expression of ET-1 in human breast carcinoma by applying radioimmunoassay (Yamashita et al, 1991; Yamashita et al, 1995), immunohistochemistry (Kojima and Nihei, 1995; Alanen et al, 2000), and quantitative RT–PCR (Alanen et al, 2000). Consistent with other reports on altered ET axis in breast cancer (Yamashita et al, 1991; Alanen et al, 2000), we have previously demonstrated an increased ET-1, ETAR, and ETBR expression in human breast carcinomas (Wülfing et al, 2003). In our series, overexpression of ET-1, ETBR and especially of ETAR was associated with clinicopathological parameters that characterise aggressive types of breast cancer and indicated a poor outcome, whereas other studies failed to confirm such correlations. In view of the above findings, the ET axis and especially ETAR has been proposed as a potential target for anticancer therapy (Bagnato et al, 2002; Pirtskhalaishvili and Nelson, 2002; Rosano et al, 2003).
Various biological markers are currently being investigated as predictors of chemotherapy response in breast cancer. Such factors may be used in the selection of neoadjuvant or adjuvant chemotherapeutic treatment. Indeed, biological markers with confirmed prognostic or predictive impact could also serve as targets for therapeutic compounds to improve current breast cancer therapies. Thus, the objective of the present study was to investigate the predictive value of the ET axis for response to neoadjuvant chemotherapy in patients treated for locally advanced breast cancer. As the vast majority of studies in the literature dealing with ET expression in malignancies showed the predominant role of the ET-1 isoform and the ETA-receptor, this study focused on the expression of these markers.
PATIENTS AND METHODS
Patients
A total of 44 patients diagnosed with locally advanced breast cancer (T2–4N0–2M0) participating in a prospective randomised phase III study evaluating a high-dose neoadjuvant chemotherapy consisting of epirubicin (Pharmacia GmbH, Erlangen, Germany) and cyclophosphamide (Baxter, Frankfurt, Germany) were included in the study (Euler et al, 2002). Patients were diagnosed and treated at the Department of Obstetrics and Gynecology, Klinikum Bayreuth, Germany, between August 1997 and March 2002. Their median age was 51 years (range, 29–66 years). None of the patients had objective skin inflammation or oedema. On first presentation, a tru-cut biopsy was performed to confirm the diagnosis histologically. Initial staging was comprised of clinical examination, bilateral mammography, bilateral breast sonography, sonography of the axillary region, chest X-ray, liver sonography, and bone scintigraphy. Table 1 lists the results of the clinically assessed pretherapeutic tumour size and lymph node status.
Table 1. Characteristics of patients and tumours at the time of diagnosis (prior to neoadjuvant chemotherapy).
| No. of patients | 44 |
| Age (years) | |
| Median (range) | 51 (29–66) |
| Menopausal status | |
| Premenopausal | 15 (34.9%) |
| Postmenopausal | 28 (65.1%) |
| Unknown | 1 |
| Tumour size | |
| T2 | 39 (90.7%) |
| T3 | 3 (7%) |
| T4 | 1 (2.3%) |
| Unknown | 1 |
| Lymph node status | |
| N0 | 24 (57.1%) |
| N1 | 18 (42.9%) |
| Unknown | 2 |
| Gradinga | |
| I | 1 (2.7%) |
| II | 12 (32.4%) |
| III | 24 (64.9%) |
| Unknown | 7 |
| Histology | |
| Ductal | 29 (65.9%) |
| Lobular | 11 (25%) |
| Other | 4 (9.1%) |
| Oestrogen receptor | |
| Negative | 9 (21.4%) |
| Positive | 33 (78.6%) |
| Unknown | 2 |
| Progesterone receptor | |
| Negative | 12 (28.6%) |
| Positive | 30 (71.4%) |
| Unknown | 2 |
| Her-2/neu | |
| Negative | 29 (70.7%) |
| Positive | 12 (29.3%) |
| Unknown | 3 |
| Ki67 | |
| <18% | 17 (41.5%) |
| ⩾18% | 24 (58.5%) |
| Unknown | 3 |
| p53 | |
| Normal | 28 (68.3%) |
| Mutation | 13 (31.7%) |
| Unknown | 3 |
Grading of surgical resection specimens postchemotherapy; not applicable in seven patients.
Tumour samples
Prechemotherapy tissue samples obtained by tru-cut biopsy were processed by formaldehyde fixation and paraffin embedding. Oestrogen receptor (ER) and progesterone receptor (PgR) status, p53, Her-2/neu and Ki67 were assessed immunohistochemically. Analyses were performed by Dianon Systems, Inc. (Stratford, CT, USA), and Molecular Oncology International P.S. (Seattle, WA, USA) using scoring criteria suggested by these laboratories. Oestrogen receptor and PgR status were considered positive if ⩾10% of tumour cells stained positive. High proliferative activity was defined as ⩾18% of tumour cell nuclei stained with monoclonal antibodies (AB) against the Ki-67 antigen. Her-2/neu expression and p53 mutation were considered positive if ⩾10% of infiltrating tumour cells stained positive with at least moderate intensity. Details are given in Table 1.
Treatment protocol
The study protocol was approved by the institutional review board (ethical committee, University of Nürnberg-Erlangen, Germany), and their informed consent was obtained. Neoadjuvant chemotherapy consisted of epirubicin (120 mg m−2) and cyclophosphamide (600 mg m−2) per cycle. Cycles were repeated every 2 weeks (arm A) or every 3 weeks (arm B) (21 out of 44 patients were randomised for arm A, 23 out of 44 for arm B). For patients in arm A, chemotherapy was followed by 5 μg kg−1 day−1 GM-CSF (AMGEN, München, Germany) s.c. or i.v. on days 2–12 after each cycle. For arm B, supportive GM-CSF was required if leucocytes were <2000 μl−1. The sizes of primary tumours and of axillary lymph nodes, when applicable, were measured every cycle by palpation and by the same clinician using a calliper. To assess the clinical response, changes in the product of the two largest diameters recorded as baseline and at the end of chemotherapy prior to surgery were measured. Additionally, tumour size was evaluated using sonography and mammography. Clinical response to chemotherapy was classified according to the criteria of the International Union Against Cancer (UICC) as follows: (i) complete response (CR), disappearance of all clinical signs of disease; (ii) partial response (PR), ⩾50% decrease in tumour size; (iii) no change (NC), clinically <50% decrease or <25% increase in tumour size; (iv) progressive disease (PD), ⩾25% increase in tumour size or appearance of new lesions (Hayward et al, 1977).
At 2 or 3 weeks after the third cycle of chemotherapy surgery was planned. Patients who showed any clinical response to the treatment (n=40) underwent breast-conserving surgery and irradiation of the residual breast (60 Gy delivered in 6 weeks). In nine of these patients, reconstruction of the breast had to be performed concomitantly (latissimus dorsi flap). Four patients without any regression of the tumour had a modified radical mastectomy.
Pathological response to preoperative chemotherapy was categorised semiquantitatively into four groups, according to the literature (Sinn et al, 1994): no effect (=grade 0); resorption and tumour sclerosis (=grade 1); minimal residual invasive tumour <0.5 cm (=grade 2); residual noninvasive tumour only (=grade 3); no tumour detectable (=grade 4). The final pathological response was designated as follows: grade 0, NC; grade 1–3, PR; grade 4, CR. Patients with pathological response to neoadjuvant chemotherapy (CR or PR) postoperatively received a fourth cycle of high-dose epirubicin and cyclophosphamide (EC) and subsequently two cycles of a cyclophosphamide-, methotrexate-, 5-fluorouracil (CMF)-containing regimen (methotrexate: medac, Hamburg, Germany; 5-fluorouracil: GRY-Pharma, Kirchzarten, Germany). In total, 12 cycles of 5-FU (2 g m−2, weekly) and four cycles of Taxol (Bristol-Meyers-Squibb, München, Germany) (Taxol=175 mg m−2, every 3 weeks) were given to pathological nonresponders (Klaassen et al, 1998).
Preparation of surgical resection specimens
The surgical resection specimens at breast-conserving therapy or mastectomy were examined and cut up fresh.
Appropriate tissue blocks were routinely processed, fixed in formalin, and embedded in paraffin. Serial 3-μm sections were cut and stained with haematoxylin and eosin. Tumour size (pT classification) was measured macroscopically on stained serial sections containing the largest tumour specimen, or estimated on the basis of a sequential series of slides. The cases were classified according to the TNM classification (UICC, 5th edition). Tumour grade was also assigned based on the UICC criteria, dividing tumours into grade I (well differentiated), grade II (moderately differentiated), and grade III (poorly differentiated). Pathological response was determined as described above. All cases were reviewed by two experienced pathologists.
Immunohistochemistry for ET-1, ETAR, and ETBR expression
Since we wanted to investigate whether the ET axis has a predictive value for chemotherapy response, immunohistochemical analysis was carried out on paraffin-embedded tru-cut prechemotherapy biopsies. In total, 3-μm sections from 44 breast cancer samples were mounted on Polysine microslides. For antigen retrieval, dewaxed and rehydrated sections were immersed in Reveal buffer (BioCarta, Hamburg, Germany) and boiled in a pressure cooker (103 kPa/15 psi for 5 min). After blocking nonspecific binding sites, sections were immunoreacted with primary AB over night at 4°C. Primary AB were directed at Endothelin-1 (Affinity BioReagents, Golden, USA, diluted 1 : 500), Endothelin-A-Receptor, ETAR, and Endothelin-B-Receptor, ETBR (both from Alexis, Grünberg, Germany, diluted 1 : 400). Sections were then treated for 10 min with methanol containing 0.6% H2O2 to quench endogenous peroxidase. Bound primary mouse AB to Endothelin-1 was detected using DAKO Mouse-EnVision-HRP. Bound primary sheep AB to ETAR and ETBR were detected using DAKO Rabbit-EnVision-HRP, via bridge AB rabbit-anti-sheep (Dianova, Hamburg, Germany, 1 : 50). HRP label was visualised with NovaRed substrate kit (Vector Laboratories, Burlingame, CA, USA). Prostate cancer tissue known to express ETAR and smooth muscle tissue with ETB receptor activity served as positive controls.
Negative controls with omission of primary AB were included. After nuclear counterstaining with haematoxylin, cytoplasmic immunostaining intensity was categorised semiquantitatively into four groups as described previously (Wülfing et al, 2003): negative (score 0): no staining at all; weakly positive (score 1+): faint/barely perceptible cytoplasmic staining in the majority of the tumour cells; moderately positive (score 2+): a moderate cytoplasmic staining in the majority of the tumour cells and strongly positive (score 3+): a strong cytoplasmic staining of the majority of the tumour cells. The final score was designated as negative or positive as follows: score of 0–1, negative; and score of 2–3, positive.
Data analysis
Staining results were evaluated semiquantitatively in a blind manner. Statistical analysis was performed using SPSS 10.0 statistical software. Correlations between ET expression in breast carcinomas and clinical or pathological response to neoadjuvant chemotherapy were tested by cross-tables applying χ2 test. Also, expression of ET-1, ETAR and ETBR was correlated to each other and to classic prognostic factors with use of χ2 test. For the analysis of response prediction, clinical response and pathological response were divided into two categories: ‘response’ [CR+PR] and ‘nonresponse’ [NC]. Then associations between expression of the ET axis and response to chemotherapy were tested with logistic regression model with the tested factors regarded as continuous variables. The level of significance was P⩽0.05.
RESULTS
Immunohistochemical ET-1-, ETAR-, and ETBR-expression
Immunolabelling for ET-1, ETAR, and ETBR presented as homogenous cytoplasmic staining. Intensity of ET-1, ETAR, and ETBR staining among different tumours varied from complete absence of staining to strong diffuse staining. Moderate or strong staining intensity defined as ‘positive’ immunoreaction was present for ET-1 in 24 out of 39 (61.5%), for ETAR in 14 out of 40 (35.0%), and for ETBR in 14 out of 39 (35.9%) evaluable breast carcinomas (Figure 1). Strong stromal immunostaining was frequently detected in ETR-positive but not in ETR-negative cases. We observed a close and significant concordance between expression of ETAR and ETBR (P=0.013) and between ETAR and ET-1 (P=0.021) in breast cancers. No significant association between expression of ET-1 and ETBR (P=0.173) was found, although ET-1-positive tumours showed a trend towards ETBR positivity (Table 2 ).
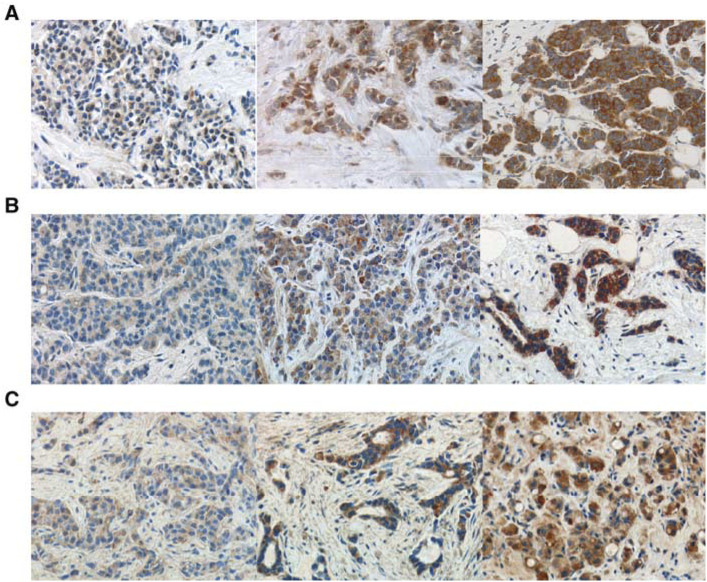
Figure 1.
Representative immunohistochemical staining patterns for ET-1 (A), ETAR (B) and ETBR (C) in breast carcinomas. For each marker, a sample with weak (left), moderate (middle), and strong (right) cytoplasmic immunostaining is shown.
Table 2. Significance of the correlations between various factors given as P's (χ2-test).
| ETARa | ETBRa | ERb,d | PgRc,d | Her-2/neue | Ki67d | p53e | |
|---|---|---|---|---|---|---|---|
| ET-1a | 0.021 | 0.173 | 0.031 | 0.006 | 0.630 | 0.258 | 0.630 |
| ETARa | 0.013 | 0.145 | 0.184 | 0.416 | 0.045 | 0.416 | |
| ETBRa | 0.219 | 0.270 | 0.459 | 0.415 | 0.351 | ||
| ERb,d | <0.001 | 0.245 | 0.008 | 0.695 | |||
| PgRc,d | 0.865 | 0.001 | 0.698 | ||||
| Her-2/neue | 0.169 | 0.553 | |||||
| Ki67d | 0.790 |
Immunohistochemistry given as staining intensity.
ER=oestrogen receptor status.
PgR=progesterone receptor status.
Immunohistochemistry given as percentage of positively stained cells (ER and PgR, ⩾10% of tumour cells stained positive; Ki67, ⩾18% of tumour cell nuclei stained).
Immunohistochemistry given as staining index (⩾10% of tumour cells stained positive with at least moderate intensity).
Correlation between expression of ET axis and clinical and tumorbiological factors
The relationship among the expression of the ET axis and clinical characteristics of the breast carcinomas prior to chemotherapy is depicted in Table 3 . We observed no significant correlation between expression patterns of the ET axis with tumour size, lymph node involvement, or histological grading. Comparison between ET axis and tumorbiological factors revealed a strong inverse relationship between ET-1 expression and the steroid hormone receptor status (ER, P=0.031; PgR, P=0.006) (Table 2). Both steroid hormone receptors correlated positively with each other (P<0.001). ETAR expression, ER- and PgR-status were inversely correlated with proliferation rate as assessed by Ki67.
Table 3. Relationship among ET-1, ETAR, and ETBR positive tumours and clinical/biological characteristics of breast carcinomas prior to neoadjuvant chemotherapy.
| ET-1 positive (n=39) | P | ETAR-positive (n=40) | P | ETBR-positive (n=39) | P | |
|---|---|---|---|---|---|---|
| T | ||||||
| T2 | 19/34 (55.9%) | 0.232 | 11/35 (31.4%) | 0.190 | 12/34 (35.3%) | 0.414 |
| T3 | 3/3 (100%) | 2/3 (66.7%) | 1/3 (33.3%) | |||
| T4 | 1/1 (100%) | 1/1 (100%) | 1/1 (100%) | |||
| Missing | 1 | 1 | 1 | |||
| N | ||||||
| N0 | 12/24 (50%) | 0.082 | 7/24 (29.2%) | 0.391 | 7/22 (31.8%) | 0.715 |
| N+ | 11/14 (78.6%) | 6/14 (42.9%) | 6/16 (37.5%) | |||
| Missing | 1 | 2 | 1 | |||
| Ga | ||||||
| G1 | — | 0.281 | — | 0.436 | 1/1 (100%) | 0.157 |
| G2 | 5/10 (50%) | 2/10 (20%) | 2/11 (18.2%) | |||
| G3 | 15/22 (68.2%) | 9/23 (39.1%) | 9/21 (42.9%) | |||
| Missing | 7 | 7 | 6 | |||
Grading of surgical resection specimens postchemotherapy.
Response to neoadjuvant chemotherapy
Treatment activity
All 44 investigated patients completed treatment according to the study protocol. The clinical response could not be determined in one patient (2.3%). At the end of the chemotherapy administration, eight patients (18.6%) attained a clinical CR (cCR) and 17 patients (39.5%) attained a clinical PR (cPR), for an overall clinical response rate of 58.1%. In total, 18 patients (41.9%) showed clinically NC (cNC), and no patient progressed. Information on pathological response was available for all patients. One patient (2.3%) was found to have a pathological CR (pCR) and 31 patients (70.5%) had a pathological PR (pPR). Thus, the overall pathological response rate was 72.7%. In all, 12 patients (27.3%) showed pathological NC (pNC).
Relationship between expression of ET axis and clinical response
Table 4 shows the clinical response to chemotherapy stratified with respect to the ET-1-, ETAR-, and ETBR-status. In total, 53.8% of ETAR-positive patients showed cNC as compared to 26.9% of ETAR-negative patients. Similarly, overall response to chemotherapy was more common in ETAR-negative than in ETAR-positive patients (73.1 vs 46.2%, respectively), although this difference did not reach statistical significance (P=0.098). No correlation was observed between ET-1 and ETBR-status and clinical response rate.
Table 4. Clinical response to neoadjuvant chemotherapy with respect to expression of ET-1, ETAR, and ETBR.
|
Clinical response |
||||||
|---|---|---|---|---|---|---|
| na | CRb | PR | NC | P1 | P2 | |
| ET-1 | ||||||
| Negative | 15 | 20% (3/15) | 46.7% (7/15) | 33.3% (5/15) | ||
| Positive | 23 | 21.7% (5/23) | 39.1% (9/23) | 39.1% (9/23) | 0.897 | 0.717 |
| ETAR | ||||||
| Negative | 26 | 23.1% (6/26) | 50% (13/26) | 26.9% (7/26) | ||
| Positive | 13 | 15.4% (2/13) | 30.8% (4/13) | 53.8% (7/13) | 0.255 | 0.098 |
| ETBR | ||||||
| Negative | 25 | 20% (5/25) | 40% (10/25) | 40% (10/25) | ||
| Positive | 13 | 23.1% (3/13) | 38.5% (5/13) | 38.5% (5/13) | 0.976 | 0.604 |
One patient missing in evaluation for clinical response.
CR=complete response; PR=partial response; NC=no change. P1=CR vs PR vs NC (χ2 test). P2=response (CR+PR) vs nonresponse (NC) (χ2 test).
Relationship between expression of ET axis and pathological response
Differential expression of the ET axis with respect to pathological response is summarised in Table 5 . Pathological response to chemotherapy was significantly decreased in ETAR-positive patients (P=0.002). Pathological NC was obtained in 50% of ETAR-positive patients as compared to 7.7% of ETAR-negative patients. None of the ETAR-positive patients had a pCR. Similar to ETAR, ETBR expression correlated with nonresponse (42.9%), although statistical significance was not reached (P=0.065). No significant correlation was observed for ET-1 expression and pathological response
Table 5. Pathological response to neoadjuvant chemotherapy stratified for ET-1, ETAR, and ETBR expression.
|
Pathological response |
||||||
|---|---|---|---|---|---|---|
| n | CRa | PR | NC | P1 | P2 | |
| ET-1 | ||||||
| Negative | 15 | — | 86.7% (13/15) | 13.3% (2/15) | ||
| Positive | 24 | 4.2% (1/24) | 62.5% (15/24) | 33.3% (8/24) | 0.245 | 0.164 |
| ETAR | ||||||
| Negative | 26 | 3.8% (1/26) | 88.5% (23/26) | 7.7% (2/26) | ||
| Positive | 14 | — | 50% (7/14) | 50% (7/14) | 0.008 | 0.002 |
| ETBR | ||||||
| Negative | 25 | — | 84% (21/25) | 16% (4/25) | ||
| Positive | 14 | 7.1% (1/14) | 50% (7/14) | 42.9% (6/14) | 0.056 | 0.065 |
CR=complete response; PR=partial response; NC=no change. P1=CR vs PR vs NC (χ2 test). P2=response (CR+PR) vs nonresponse (NC) (χ2 test).
Predictive value of ET axis expression for response
Multivariate analysis was performed by logistic regression to evaluate the predictive role of ET-1, ETAR, and ETBR status for clinical and pathological response. Clinicopathological factors associated with prognosis such as tumour size, lymph node status, grading, and steroid hormone receptor status were included for analysis. None of the investigated factors, ET-1, ETAR, or ETBR, had a predictive value for clinical response. Conversely, ETAR expression retained independence in predicting response, showing a significant inverse association with pathological response to treatment (P=0.009). Neither ET-1 (P=0.403) nor ETBR (P=0.056) expression was a significant obstacle to pathological response.
Treatment arms
Statistical analysis stratified for both treatment arms showed no differences between arm A and arm B concerning distribution of histopathological characteristics, expression of ET axis and response rates to chemotherapy.
DISCUSSION
The indication for treating breast cancer with chemotherapy is generally based on clinical and tumorbiological characteristics such as lymph node involvement, hormone receptor status, and Her-2/neu expression, which are indicators of prognosis but do not necessarily indicate likelihood of response. To date, there is no reliable marker for the prediction of chemotherapy response in breast cancer patients to facilitate individualised treatment. This study highlights the potential clinical role of ETBR and especially of ETAR as predictors of response to neoadjuvant chemotherapy in patients with locally advanced breast cancer.
Previously, we have demonstrated that elevated ET-1, ETAR, and ETBR expression is more common in breast cancers of patients with diminished disease-free and overall survival. In that study we also observed a close correlation between ETAR-positive tumours and clinicopathological markers for poor prognosis (Wülfing et al, 2003). To assess whether the ET axis may influence breast cancer response to chemotherapy treatment, a series of patients with locally advanced breast cancer was evaluated.
In this study, we found that overexpression of the ET receptors was correlated with pathological NC following chemotherapy, thus providing evidence that overexpression of the ET receptors may adversely affect response to chemotherapy treatment. Higher pathological response rates in ETBR- and especially in ETAR-negative than in ET receptor-positive patients further support the hypothesis that ET receptor expression is associated with resistance to chemotherapy. Our data also suggest a potential ability of ET receptor expression to predict breast cancer susceptibility to chemotherapy. The independent predictive value of ETAR expression for pathological response was confirmed applying logistic regression. Similar to these findings, analysis of clinical response to neoadjuvant chemotherapy with respect to ET expression revealed a reduced effect in ETAR-positive patients as compared to ETAR-negative patients although differences between these groups were less obvious. To determine whether our results were merely a reflection of differences between distinct groups, we evaluated whether tumorbiological factors with well-established prognostic relevance were related to ET-1, ETAR, and ETBR expression. Except for a statistically significant inverse relationship between ET-1 expression and steroid hormone receptor status as well as between ETAR expression and proliferation index (Ki67), with respect to conventional prognostic markers no further significant correlations were found.
Accumulating data have shown that ET-1 acting through ETAR functions as a survival factor for carcinoma cells. In colon carcinoma cells, ET-1 inhibits apoptosis mediated by Fas-ligand (FasL), which induces cell death via caspase activation, or Paclitaxel (Eberl et al, 2000a, 2000b). Similarly, in ovarian carcinoma cells, paclitaxel-induced apoptosis is inhibited by ET-1 (via ETAR), triggering antiapoptotic signalling through bcl-2-dependent and phosphatidylinositol 3-kinase-mediated AKT pathways (Vacca et al, 2000). Conversely, cervical cancer cell growth in vivo could be stopped by ETAR blockade with Atrasentan (ABT-627), a selective ETAR-inhibitor, alone, and in that study Atrasentan displayed additive antitumour effects when administered in combination with the cytotoxic agent Paclitaxel (Bagnato et al, 2002). Also, in ovarian carcinoma xenograft models, inhibition of tumour growth by Atrasentan was found to be as effective as Paclitaxel (Rosano et al, 2003). Since it is conceivable that antiapoptotic factors may contribute to resistance to antitumoral therapy, the observed chemotherapy resistance in ET receptor overexpressing tumours may be attributed to the autocrine influence of ET-1 acting via ETAR.
From the evidence to date, it appears that ET-1 and ETAR play the predominant role in malignancies. Also, selective ETAR antagonism provides the most likely method of ET axis inhibition in cancer (Grant et al, 2003; Nelson et al, 2003). Our findings may reflect the pivotal role of ETAR alteration in breast cancer. The observed higher influence of ETAR rather than ETBR expression on chemotherapy response can be explained in terms of data that ET-1 signalling through ETAR imparts a survival benefit of carcinoma cells by inhibiting chemotherapy induced apoptosis. In contrast, so far, there is no evidence from the literature that ETBR signalling is also associated with apoptosis inhibition.
We suggest that in breast cancer ET-1 produced by the tumour cells acts in an autocrine mechanism via ET receptors. The lack of correlation between ET-1 expression and chemotherapy response could be explained by the fact that ET-1 exerts its effects through ET receptor binding. ET-1 levels and expression may, however, not be an appropriate correlate for the ET-1 activity at the microenvironment level. ET-1 expression may therefore not be an adequate indicator of the activity of the ETAR signalling. It is the expression of the ETAR that signifies the potential antiapoptotic effects of ET-1. Also in view of the literature, ETAR activation rather than ET-1 itself promotes tumour progression by means of various mechanisms (Nelson et al, 2003).
Our data support the predictive role of ETAR overexpression as a marker of adverse pathological response to chemotherapy treatment in breast cancer. However, generalisation of our results may be limited by the relatively small sample size. This limitation notwithstanding, our data could offer a valid background for further confirmatory research.
In conclusion, our findings suggest that increased expression of the ET receptors and especially of ETAR in breast carcinomas is associated with resistance to chemotherapy. Furthermore, in this cohort of patients with advanced breast cancer, the ETAR status was an independent predictor of response to chemotherapy. Thus, immunohistochemical analysis of ETAR expression may serve as a convenient, low-cost technique for identifying breast cancer patients with a poor chance of response to chemotherapy and, therefore, potential candidates for more individualised treatments. Since our findings can possibly be explained by an ETAR-mediated chemotherapy resistance, combining ETAR antagonism with conventional chemotherapy may improve the outcome of breast cancer patients.
Acknowledgments
We thank Vera Samoilova for excellent technical assistance. We also thank Robert Hansen and Darryl Sleep who made many contributions to the manuscript.
References
- Alanen K, Deng DX, Chakrabarti S (2000) Augmented expression of endothelin-1, endothelin-3 and the endothelin-B receptor in breast carcinoma. Histopathology 36: 161–167 [DOI] [PubMed] [Google Scholar]
- Ali H, Dashwood M, Dawas K, Loizidou M, Savage F, Taylor I (2000) Endothelin receptor expression in colorectal cancer. J Cardiovasc Pharm 36: S69–S71 [DOI] [PubMed] [Google Scholar]
- Asham E, Shankar A, Loizidou M, Fredericks S, Miller K, Boulos PB, Burnstock G, Taylor I (2001) Increased endothelin-1 in colorectal cancer and reduction of tumor growth by ET(A)receptor antagonism. Br J Cancer 30: 1759–1763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagnato A, Catt KJ (1998) Endothelin as autocrine regulator of tumor cell growth. Trends Endocrinol Metab 9: 378–383 [DOI] [PubMed] [Google Scholar]
- Bagnato A, Cirilli A, Salani D, Simeone P, Muller A, Nicotra MR, Natali PG, Venuti A (2002) Growth inhibition of cervix carcinoma cells in vivo by Endothelin A Receptor blockade. Cancer Res 62: 6381–6384 [PubMed] [Google Scholar]
- Bagnato A, Salani D, Di Castro V, Wu-Wong JR, Tecce R, Nicotra MR, Venuti A, Natali PG (1999) Expression of endothelin-1 and endothelin A receptor in ovarian carcinoma: evidence for an autocrine role in tumor growth. Cancer Res 59: 1–8 [PubMed] [Google Scholar]
- Bagnato A, Tecce R, Moretti C, DiCastro V, Spergel D, Catt KG (1995) Autocrine actions of ET-1 as a growth factor in human ovarian carcinoma cells. Clin Cancer Res 1: 1059–1066 [PubMed] [Google Scholar]
- Bek EL, McMillen MA (2000) Endothelins are angiogenic. J Cardiovasc Pharmacol 36: S135–S139 [DOI] [PubMed] [Google Scholar]
- Del Bufalo D, Di Castro V, Biroccio A, Varmi M, Salani D, Rosano L, Trisciuglio D, Spinella F, Bagnato A (2002) Endothelin-1 protects ovarian carcinoma cells against paclitaxel-induced apoptosis: requirement for Akt activation. Mol Pharmacol 61: 524–532 [DOI] [PubMed] [Google Scholar]
- Eberl LP, Egidy G, Pinet F, Juillerat-Jeanneret L (2000a) Endothelin receptor blockade potentiates FaslL-induced apoptosis in colon carcinoma cells via the protein kinase C-pathway. J Cardiovasc Pharmacol 36: S354–S356 [DOI] [PubMed] [Google Scholar]
- Eberl LP, Valdenaire O, Saintgiorgio V, Jeannin JF, Juillerat-Jeanneret L (2000b) Endothelin receptor blockade potentiates FasL-induced apoptosis in rat colon carcinoma cells. Int J Cancer 86: 182–187 [DOI] [PubMed] [Google Scholar]
- Euler U, Dresel V, Bühner M, Volkholz H, Tulusan AH (2002) Dose and time intensified epirubicin/cyclophosphamide (EC) as preoperative treatment in locally advanced breast cancer. Breast Cancer Res Treat 76(Suppl 1): S51 [Google Scholar]
- Ferlay J, Bray F, Pisani P, Parkin D, Globocan 2000. Cancer incidence, mortality and prevalence worldwide, Version 1.0. IARC Cancer BASE No. 5. IARC Press [monography] 2001. Available from http://www-dep.iarc.fr/globocan/globocan.html [accessed Dec 23, 2003]
- Fisher B, Brown A, Mamounas E, Wieand S, Robidoux A, Margolese RG, Cruz Jr AB, Fisher ER, Wickerham DL, Wolmark N, DeCillis A, Hoehn JL, Lees AW, Dimitrov NV (1997) Effect of preoperative chemotherapy on loco-regional disease in women with operable breast cancer: findings from the National Surgical Adjuvant Breast and Bowel Project B-18. J Clin Oncol 15: 2483–2493 [DOI] [PubMed] [Google Scholar]
- Fisher B, Bryant J, Wolmark N, Mamounas E, Brown A, Fisher ER, Wickerham DL, Begovic M, DeCillis A, Robidoux A, Margolese RG, Cruz Jr AB, Hoehn JL, Lees AW, Dimitrov NV, Bear HD (1998) Effect of preoperative chemotherapy on the outcome of women with operable breast cancer. J Clin Oncol 16: 2672–2685 [DOI] [PubMed] [Google Scholar]
- Grant K, Loizidou M, Taylor I (2003) Endothelin-1: a multifunctional molecule in cancer. Br J Cancer 88: 163–166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward JL, Rubens RD, Carbone PP, Heuson JC, Kumaoka S, Segaloff A (1977) Assessment of response to therapy in advanced breast cancer. Br J Cancer 35: 292–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klaassen U, Wilke H, Weyhofen R, Harstrick A, Eberhardt W, Muller C, Korn M, Hanske M, Diergarten K, Seeber S (1998) Phase II study with cisplatin and paclitaxel in combination with weekly high-dose 24 h infusional 5-fluorouracil/leucovorin for first-line treatment of metastatic breast cancer. Anticancer Drugs 9: 203–207 [DOI] [PubMed] [Google Scholar]
- Kojima K, Nihei Z (1995) Expression of endothelin-1 immunoreactivity in breast cancer. Surg Oncol 4: 309–315 [DOI] [PubMed] [Google Scholar]
- Kourembanas S, Marsden PA, McQuillan LP, Faller DV (1991) Hypoxia induces endothelin gene expression and secretion in cultured endothelium. J Clin Invest 88: 1054–1057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin ER (1995) Endothelins. N Engl J Med 333: 356–363 [DOI] [PubMed] [Google Scholar]
- Nelson BJ, Chan-Tack K, Hedican SP, Magnuson SR, Opgenorth TJ, Bova GS, Simons JW (1996) Endothelin-1 production and decreased Endothelin B Receptor expression in advanced prostate cancer. Cancer Res 56: 663–668 [PubMed] [Google Scholar]
- Nelson JB, Carducci MA (1999) The role of the endothelin axis in prostate cancer. Prostate J 1: 126–130 [Google Scholar]
- Nelson J, Bagnato A, Battistini B, Nisen P (2003) The endothelin axis: emerging role in cancer. Nature Rev 3: 110–116 [DOI] [PubMed] [Google Scholar]
- Pedram A, Rasandi M, Hu RM, Levin ER (1997) Vasoactive peptides modulate vascular endothelial cell growth factor production and endothelin cell proliferation and invasion. J Biol Chem 272: 17097–17103 [DOI] [PubMed] [Google Scholar]
- Pirtskhalaishvili G, Nelson JB (2002) The endothelin receptor: a novel target for anticancer therapy. Am J Cancer 1: 1–7 [Google Scholar]
- Rosano L, Spinella F, Salani D, Di Castro V, Venuti A, Nicotra MR, Natali PG, Bagnato A (2003) Therapeutic targeting of the Endothelin A Receptor in Human Ovarian Carcinoma. Cancer Res 63: 2447–2453 [PubMed] [Google Scholar]
- Rosano L, Varmi M, Salani D, Di Castro V, Spinella F, Natali PG, Bagnato A (2001) Endothelin-1 induces tumor proteinase activation and invasiveness of ovarian carcinoma cells. Cancer Res 61: 8340–8346 [PubMed] [Google Scholar]
- Rubanji GM, Polokoff MA (1994) Endothelins: molecular biology, biochemistry, pharmacology, physiology and pathophysiology. Pharmacol Rev 46: 325–415 [PubMed] [Google Scholar]
- Salani D, Di Castro V, Nicotra MR, Rosano L, Tecce R, Venuti A, Natali PG, Bagnato A (2000) Role of Endothelin-1 in neovascularization of ovarian carcinoma. Am J Pathol 157: 1537–1547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinn HP, Schmid H, Junkermann H, Huober J, Leppien G, Kaufmann M, Bastert G, Otto HF (1994) Histologic regression of breast cancer after primary (neoadjuvant) chemotherapy. Geburtshilfe Frauenheilkd 54: 552–558 [DOI] [PubMed] [Google Scholar]
- Spinella F, Rosano L, DiCastro V, Natali PG, Bagnato A (2002) Endothelin-1 induces vascular endothelial growth factor by increasing hypoxia-inducible factor 1α in ovarian cancer cells. J Biol Chem 277: 27850–27855 [DOI] [PubMed] [Google Scholar]
- Vacca F, Bagnato A, Catt KJ, Tecce R (2000) Transactivation of the epidermal growth factor receptor in endothelin-1-induced mitogenic signaling in human ovarian carcinoma cells. Cancer Res 60: 5310–5317 [PubMed] [Google Scholar]
- Wülfing P, Diallo R, Kersting C, Wülfing C, Poremba C, Rody A, Greb RR, Böcker W, Kiesel L (2003) Expression of Endothelin-1, Endothelin-A and Endothelin-B Receptor in human breast cancer and correlation with long-term follow-up. Clin Cancer Res 9: 4125–4131 [PubMed] [Google Scholar]
- Yamashita J, Ogawa M, Inada K, Yamashita S, Matsuo S, Takano S (1991) A large amount of endothelin-1 is present in human breast cancer tissues. Res Commun Chem Pathol Pharmacol 74: 363–369 [PubMed] [Google Scholar]
- Yamashita J, Ogawa M, Sakai K (1995) Prognostic significance of three novel biologic factors in a clinical trial of adjuvant therapy for node-negative breast cancer. Surgery 117: 601–608 [DOI] [PubMed] [Google Scholar]
- Yanagisawa M, Kurihara H, Kimura S, Tomobe Y, Kabayashi M, Mitsui Y, Yazaki Y, Goto K, Masaki Y (1988) A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature 332: 411–415 [DOI] [PubMed] [Google Scholar]