Abstract
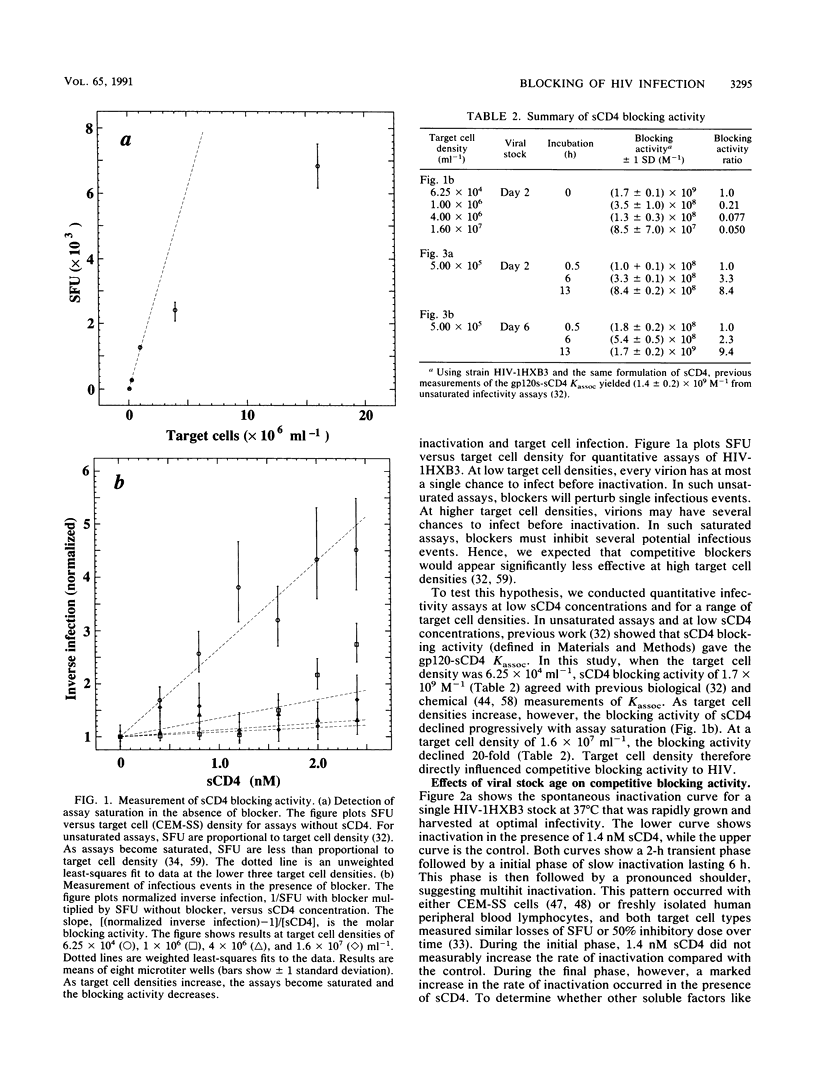
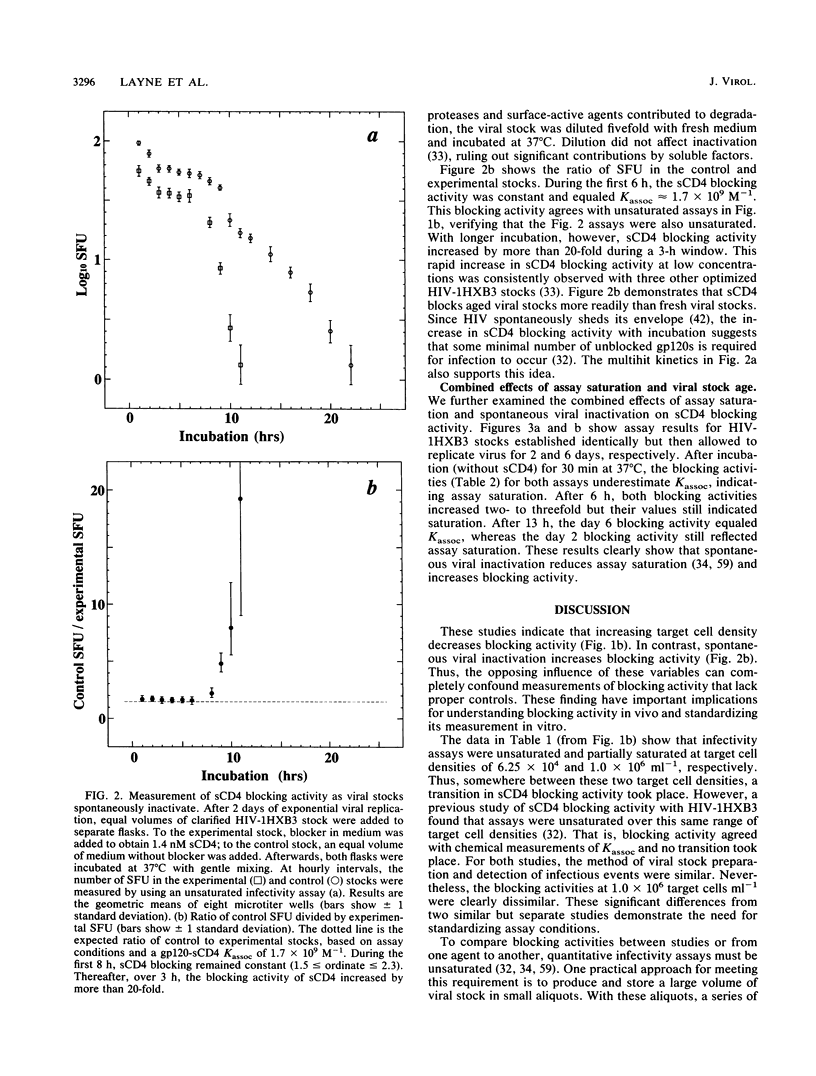
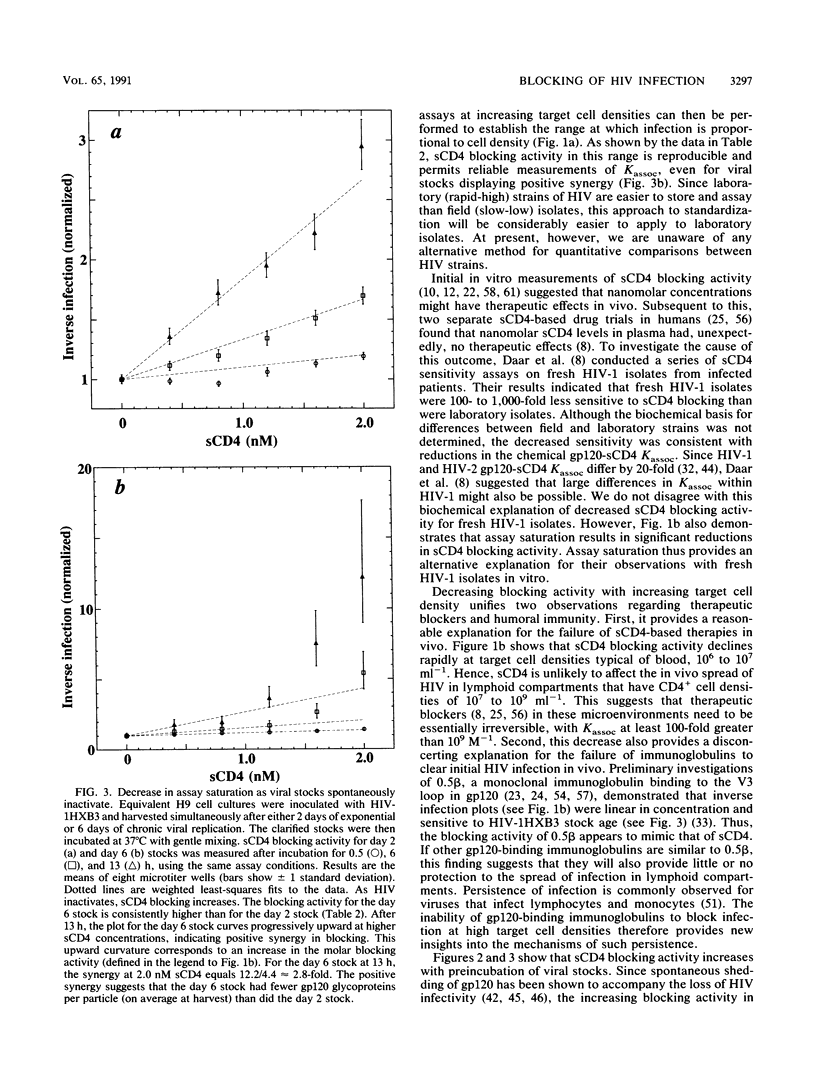
Quantitative infectivity assays were used to study how the blocking activity of soluble CD4 (sCD4) is affected by sCD4 concentration, target cell density, and viral stock age. During incubation with 20 nM sCD4, human immunodeficiency virus type 1 (HIV-1) stocks underwent irreversible inactivation. In contrast, inactivation with 2 nM sCD4 was almost entirely reversible. At lower sCD4 concentrations (less than or equal to 2 nM) and target cell densities of 6.25 x 10(4) ml-1, sCD4 blocking activity for HIV-1 gave a gp120-sCD4 association constant (Kassoc) of 1.7 x 10(9) M-1, which agrees with chemical measurements. At the higher density of 1.6 x 10(7) cells ml-1, however, the blocking activity was 20-fold less. During incubation of HIV-1 stock optimized for infectivity by rapid harvest, sCD4 blocking activity increased 20-fold during a 3-h window. These results show that competitive blocking activity depends strongly on target cell density and virion age. Thus, unappreciated variations in HIV stocks and assay conditions may hinder comparisons of blockers from laboratory to laboratory, and the age of HIV challenge stocks may influence studies of drug and vaccine efficacy. The results also suggest that blocking of viral particles in lymphoid compartments will require very high competitive blocker concentrations, which may explain the refractory outcomes from sCD4-based drug trials in humans.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berman P. W., Gregory T. J., Riddle L., Nakamura G. R., Champe M. A., Porter J. P., Wurm F. M., Hershberg R. D., Cobb E. K., Eichberg J. W. Protection of chimpanzees from infection by HIV-1 after vaccination with recombinant glycoprotein gp120 but not gp160. Nature. 1990 Jun 14;345(6276):622–625. doi: 10.1038/345622a0. [DOI] [PubMed] [Google Scholar]
- Byrn R. A., Mordenti J., Lucas C., Smith D., Marsters S. A., Johnson J. S., Cossum P., Chamow S. M., Wurm F. M., Gregory T. Biological properties of a CD4 immunoadhesin. Nature. 1990 Apr 12;344(6267):667–670. doi: 10.1038/344667a0. [DOI] [PubMed] [Google Scholar]
- Byrn R. A., Sekigawa I., Chamow S. M., Johnson J. S., Gregory T. J., Capon D. J., Groopman J. E. Characterization of in vitro inhibition of human immunodeficiency virus by purified recombinant CD4. J Virol. 1989 Oct;63(10):4370–4375. doi: 10.1128/jvi.63.10.4370-4375.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capon D. J., Chamow S. M., Mordenti J., Marsters S. A., Gregory T., Mitsuya H., Byrn R. A., Lucas C., Wurm F. M., Groopman J. E. Designing CD4 immunoadhesins for AIDS therapy. Nature. 1989 Feb 9;337(6207):525–531. doi: 10.1038/337525a0. [DOI] [PubMed] [Google Scholar]
- Chanh T. C., Dreesman G. R., Kanda P., Linette G. P., Sparrow J. T., Ho D. D., Kennedy R. C. Induction of anti-HIV neutralizing antibodies by synthetic peptides. EMBO J. 1986 Nov;5(11):3065–3071. doi: 10.1002/j.1460-2075.1986.tb04607.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clapham P. R., Weber J. N., Whitby D., McIntosh K., Dalgleish A. G., Maddon P. J., Deen K. C., Sweet R. W., Weiss R. A. Soluble CD4 blocks the infectivity of diverse strains of HIV and SIV for T cells and monocytes but not for brain and muscle cells. Nature. 1989 Jan 26;337(6205):368–370. doi: 10.1038/337368a0. [DOI] [PubMed] [Google Scholar]
- Clayton L. K., Hussey R. E., Steinbrich R., Ramachandran H., Husain Y., Reinherz E. L. Substitution of murine for human CD4 residues identifies amino acids critical for HIV-gp120 binding. Nature. 1988 Sep 22;335(6188):363–366. doi: 10.1038/335363a0. [DOI] [PubMed] [Google Scholar]
- Daar E. S., Li X. L., Moudgil T., Ho D. D. High concentrations of recombinant soluble CD4 are required to neutralize primary human immunodeficiency virus type 1 isolates. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6574–6578. doi: 10.1073/pnas.87.17.6574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalgleish A. G., Beverley P. C., Clapham P. R., Crawford D. H., Greaves M. F., Weiss R. A. The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature. 1984 Dec 20;312(5996):763–767. doi: 10.1038/312763a0. [DOI] [PubMed] [Google Scholar]
- Deen K. C., McDougal J. S., Inacker R., Folena-Wasserman G., Arthos J., Rosenberg J., Maddon P. J., Axel R., Sweet R. W. A soluble form of CD4 (T4) protein inhibits AIDS virus infection. Nature. 1988 Jan 7;331(6151):82–84. doi: 10.1038/331082a0. [DOI] [PubMed] [Google Scholar]
- Fisher R. A., Bertonis J. M., Meier W., Johnson V. A., Costopoulos D. S., Liu T., Tizard R., Walker B. D., Hirsch M. S., Schooley R. T. HIV infection is blocked in vitro by recombinant soluble CD4. Nature. 1988 Jan 7;331(6151):76–78. doi: 10.1038/331076a0. [DOI] [PubMed] [Google Scholar]
- Gelderblom H. R., Hausmann E. H., Ozel M., Pauli G., Koch M. A. Fine structure of human immunodeficiency virus (HIV) and immunolocalization of structural proteins. Virology. 1987 Jan;156(1):171–176. doi: 10.1016/0042-6822(87)90449-1. [DOI] [PubMed] [Google Scholar]
- Gelderblom H. R., Reupke H., Pauli G. Loss of envelope antigens of HTLV-III/LAV, a factor in AIDS pathogenesis? Lancet. 1985 Nov 2;2(8462):1016–1017. doi: 10.1016/s0140-6736(85)90570-7. [DOI] [PubMed] [Google Scholar]
- Goudsmit J., Debouck C., Meloen R. H., Smit L., Bakker M., Asher D. M., Wolff A. V., Gibbs C. J., Jr, Gajdusek D. C. Human immunodeficiency virus type 1 neutralization epitope with conserved architecture elicits early type-specific antibodies in experimentally infected chimpanzees. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4478–4482. doi: 10.1073/pnas.85.12.4478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goudsmit J., Kuiken C. L., Nara P. L. Linear versus conformational variation of V3 neutralization domains of HIV-1 during experimental and natural infection. AIDS. 1989;3 (Suppl 1):S119–S123. doi: 10.1097/00002030-198901001-00017. [DOI] [PubMed] [Google Scholar]
- Hayashi Y., Ikuta K., Fujii N., Ezawa K., Kato S. Inhibition of HIV-1 replication and syncytium formation by synthetic CD4 peptides. Arch Virol. 1989;105(1-2):129–135. doi: 10.1007/BF01311123. [DOI] [PubMed] [Google Scholar]
- Ho D. D., Kaplan J. C., Rackauskas I. E., Gurney M. E. Second conserved domain of gp120 is important for HIV infectivity and antibody neutralization. Science. 1988 Feb 26;239(4843):1021–1023. doi: 10.1126/science.2830667. [DOI] [PubMed] [Google Scholar]
- Ho D. D., McKeating J. A., Li X. L., Moudgil T., Daar E. S., Sun N. C., Robinson J. E. Conformational epitope on gp120 important in CD4 binding and human immunodeficiency virus type 1 neutralization identified by a human monoclonal antibody. J Virol. 1991 Jan;65(1):489–493. doi: 10.1128/jvi.65.1.489-493.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho D. D., Sarngadharan M. G., Hirsch M. S., Schooley R. T., Rota T. R., Kennedy R. C., Chanh T. C., Sato V. L. Human immunodeficiency virus neutralizing antibodies recognize several conserved domains on the envelope glycoproteins. J Virol. 1987 Jun;61(6):2024–2028. doi: 10.1128/jvi.61.6.2024-2028.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussey R. E., Richardson N. E., Kowalski M., Brown N. R., Chang H. C., Siliciano R. F., Dorfman T., Walker B., Sodroski J., Reinherz E. L. A soluble CD4 protein selectively inhibits HIV replication and syncytium formation. Nature. 1988 Jan 7;331(6151):78–81. doi: 10.1038/331078a0. [DOI] [PubMed] [Google Scholar]
- Javaherian K., Langlois A. J., LaRosa G. J., Profy A. T., Bolognesi D. P., Herlihy W. C., Putney S. D., Matthews T. J. Broadly neutralizing antibodies elicited by the hypervariable neutralizing determinant of HIV-1. Science. 1990 Dec 14;250(4987):1590–1593. doi: 10.1126/science.1703322. [DOI] [PubMed] [Google Scholar]
- Javaherian K., Langlois A. J., McDanal C., Ross K. L., Eckler L. I., Jellis C. L., Profy A. T., Rusche J. R., Bolognesi D. P., Putney S. D. Principal neutralizing domain of the human immunodeficiency virus type 1 envelope protein. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6768–6772. doi: 10.1073/pnas.86.17.6768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn J. O., Allan J. D., Hodges T. L., Kaplan L. D., Arri C. J., Fitch H. F., Izu A. E., Mordenti J., Sherwin J. E., Groopman J. E. The safety and pharmacokinetics of recombinant soluble CD4 (rCD4) in subjects with the acquired immunodeficiency syndrome (AIDS) and AIDS-related complex. A phase 1 study. Ann Intern Med. 1990 Feb 15;112(4):254–261. doi: 10.7326/0003-4819-112-4-. [DOI] [PubMed] [Google Scholar]
- Kirsh R., Hart T. K., Ellens H., Miller J., Petteway S. A., Jr, Lambert D. M., Leary J., Bugelski P. J. Morphometric analysis of recombinant soluble CD4-mediated release of the envelope glycoprotein gp120 from HIV-1. AIDS Res Hum Retroviruses. 1990 Oct;6(10):1209–1212. doi: 10.1089/aid.1990.6.1209. [DOI] [PubMed] [Google Scholar]
- Klatzmann D., Champagne E., Chamaret S., Gruest J., Guetard D., Hercend T., Gluckman J. C., Montagnier L. T-lymphocyte T4 molecule behaves as the receptor for human retrovirus LAV. Nature. 1984 Dec 20;312(5996):767–768. doi: 10.1038/312767a0. [DOI] [PubMed] [Google Scholar]
- Kowalski M., Potz J., Basiripour L., Dorfman T., Goh W. C., Terwilliger E., Dayton A., Rosen C., Haseltine W., Sodroski J. Functional regions of the envelope glycoprotein of human immunodeficiency virus type 1. Science. 1987 Sep 11;237(4820):1351–1355. doi: 10.1126/science.3629244. [DOI] [PubMed] [Google Scholar]
- Landau N. R., Warton M., Littman D. R. The envelope glycoprotein of the human immunodeficiency virus binds to the immunoglobulin-like domain of CD4. Nature. 1988 Jul 14;334(6178):159–162. doi: 10.1038/334159a0. [DOI] [PubMed] [Google Scholar]
- Layne S. P., Merges M. J., Dembo M., Spouge J. L., Nara P. L. HIV requires multiple gp120 molecules for CD4-mediated infection. Nature. 1990 Jul 19;346(6281):277–279. doi: 10.1038/346277a0. [DOI] [PubMed] [Google Scholar]
- Layne S. P., Spouge J. L., Dembo M. Quantifying the infectivity of human immunodeficiency virus. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4644–4648. doi: 10.1073/pnas.86.12.4644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lifson J. D., Hwang K. M., Nara P. L., Fraser B., Padgett M., Dunlop N. M., Eiden L. E. Synthetic CD4 peptide derivatives that inhibit HIV infection and cytopathicity. Science. 1988 Aug 5;241(4866):712–716. doi: 10.1126/science.2969619. [DOI] [PubMed] [Google Scholar]
- Looney D. J., Hayashi S., Nicklas M., Redfield R. R., Broder S., Wong-Staal F., Mitsuya H. Differences in the interaction of HIV-1 and HIV-2 with CD4. J Acquir Immune Defic Syndr. 1990;3(7):649–657. [PubMed] [Google Scholar]
- Maddon P. J., Dalgleish A. G., McDougal J. S., Clapham P. R., Weiss R. A., Axel R. The T4 gene encodes the AIDS virus receptor and is expressed in the immune system and the brain. Cell. 1986 Nov 7;47(3):333–348. doi: 10.1016/0092-8674(86)90590-8. [DOI] [PubMed] [Google Scholar]
- Matsushita S., Robert-Guroff M., Rusche J., Koito A., Hattori T., Hoshino H., Javaherian K., Takatsuki K., Putney S. Characterization of a human immunodeficiency virus neutralizing monoclonal antibody and mapping of the neutralizing epitope. J Virol. 1988 Jun;62(6):2107–2114. doi: 10.1128/jvi.62.6.2107-2114.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCune J. M., Rabin L. B., Feinberg M. B., Lieberman M., Kosek J. C., Reyes G. R., Weissman I. L. Endoproteolytic cleavage of gp160 is required for the activation of human immunodeficiency virus. Cell. 1988 Apr 8;53(1):55–67. doi: 10.1016/0092-8674(88)90487-4. [DOI] [PubMed] [Google Scholar]
- McDougal J. S., Martin L. S., Cort S. P., Mozen M., Heldebrant C. M., Evatt B. L. Thermal inactivation of the acquired immunodeficiency syndrome virus, human T lymphotropic virus-III/lymphadenopathy-associated virus, with special reference to antihemophilic factor. J Clin Invest. 1985 Aug;76(2):875–877. doi: 10.1172/JCI112045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDougal J. S., Mawle A., Cort S. P., Nicholson J. K., Cross G. D., Scheppler-Campbell J. A., Hicks D., Sligh J. Cellular tropism of the human retrovirus HTLV-III/LAV. I. Role of T cell activation and expression of the T4 antigen. J Immunol. 1985 Nov;135(5):3151–3162. [PubMed] [Google Scholar]
- McKeating J. A., McKnight A., Moore J. P. Differential loss of envelope glycoprotein gp120 from virions of human immunodeficiency virus type 1 isolates: effects on infectivity and neutralization. J Virol. 1991 Feb;65(2):852–860. doi: 10.1128/jvi.65.2.852-860.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizukami T., Fuerst T. R., Berger E. A., Moss B. Binding region for human immunodeficiency virus (HIV) and epitopes for HIV-blocking monoclonal antibodies of the CD4 molecule defined by site-directed mutagenesis. Proc Natl Acad Sci U S A. 1988 Dec;85(23):9273–9277. doi: 10.1073/pnas.85.23.9273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore J. P., McKeating J. A., Norton W. A., Sattentau Q. J. Direct measurement of soluble CD4 binding to human immunodeficiency virus type 1 virions: gp120 dissociation and its implications for virus-cell binding and fusion reactions and their neutralization by soluble CD4. J Virol. 1991 Mar;65(3):1133–1140. doi: 10.1128/jvi.65.3.1133-1140.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore J. P., McKeating J. A., Weiss R. A., Sattentau Q. J. Dissociation of gp120 from HIV-1 virions induced by soluble CD4. Science. 1990 Nov 23;250(4984):1139–1142. doi: 10.1126/science.2251501. [DOI] [PubMed] [Google Scholar]
- Moore J. P. Simple methods for monitoring HIV-1 and HIV-2 gp120 binding to soluble CD4 by enzyme-linked immunosorbent assay: HIV-2 has a 25-fold lower affinity than HIV-1 for soluble CD4. AIDS. 1990 Apr;4(4):297–305. doi: 10.1097/00002030-199004000-00003. [DOI] [PubMed] [Google Scholar]
- Nara P. L., Fischinger P. J. Quantitative infectivity assay for HIV-1 and-2. Nature. 1988 Mar 31;332(6163):469–470. doi: 10.1038/332469a0. [DOI] [PubMed] [Google Scholar]
- Nara P. L., Hatch W. C., Dunlop N. M., Robey W. G., Arthur L. O., Gonda M. A., Fischinger P. J. Simple, rapid, quantitative, syncytium-forming microassay for the detection of human immunodeficiency virus neutralizing antibody. AIDS Res Hum Retroviruses. 1987 Fall;3(3):283–302. doi: 10.1089/aid.1987.3.283. [DOI] [PubMed] [Google Scholar]
- Nara P. L., Hwang K. M., Rausch D. M., Lifson J. D., Eiden L. E. CD4 antigen-based antireceptor peptides inhibit infectivity of human immunodeficiency virus in vitro at multiple stages of the viral life cycle. Proc Natl Acad Sci U S A. 1989 Sep;86(18):7139–7143. doi: 10.1073/pnas.86.18.7139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nara P. L., Smit L., Dunlop N., Hatch W., Merges M., Waters D., Kelliher J., Gallo R. C., Fischinger P. J., Goudsmit J. Emergence of viruses resistant to neutralization by V3-specific antibodies in experimental human immunodeficiency virus type 1 IIIB infection of chimpanzees. J Virol. 1990 Aug;64(8):3779–3791. doi: 10.1128/jvi.64.8.3779-3791.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldstone M. B. Viral persistence. Cell. 1989 Feb 24;56(4):517–520. doi: 10.1016/0092-8674(89)90573-4. [DOI] [PubMed] [Google Scholar]
- Palker T. J., Clark M. E., Langlois A. J., Matthews T. J., Weinhold K. J., Randall R. R., Bolognesi D. P., Haynes B. F. Type-specific neutralization of the human immunodeficiency virus with antibodies to env-encoded synthetic peptides. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1932–1936. doi: 10.1073/pnas.85.6.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putney S. D., Matthews T. J., Robey W. G., Lynn D. L., Robert-Guroff M., Mueller W. T., Langlois A. J., Ghrayeb J., Petteway S. R., Jr, Weinhold K. J. HTLV-III/LAV-neutralizing antibodies to an E. coli-produced fragment of the virus envelope. Science. 1986 Dec 12;234(4782):1392–1395. doi: 10.1126/science.2431482. [DOI] [PubMed] [Google Scholar]
- Rusche J. R., Javaherian K., McDanal C., Petro J., Lynn D. L., Grimaila R., Langlois A., Gallo R. C., Arthur L. O., Fischinger P. J. Antibodies that inhibit fusion of human immunodeficiency virus-infected cells bind a 24-amino acid sequence of the viral envelope, gp120. Proc Natl Acad Sci U S A. 1988 May;85(9):3198–3202. doi: 10.1073/pnas.85.9.3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schooley R. T., Merigan T. C., Gaut P., Hirsch M. S., Holodniy M., Flynn T., Liu S., Byington R. E., Henochowicz S., Gubish E. Recombinant soluble CD4 therapy in patients with the acquired immunodeficiency syndrome (AIDS) and AIDS-related complex. A phase I-II escalating dosage trial. Ann Intern Med. 1990 Feb 15;112(4):247–253. doi: 10.7326/0003-4819-112-4-247. [DOI] [PubMed] [Google Scholar]
- Skinner M. A., Ting R., Langlois A. J., Weinhold K. J., Lyerly H. K., Javaherian K., Matthews T. J. Characteristics of a neutralizing monoclonal antibody to the HIV envelope glycoprotein. AIDS Res Hum Retroviruses. 1988 Jun;4(3):187–197. doi: 10.1089/aid.1988.4.187. [DOI] [PubMed] [Google Scholar]
- Smith D. H., Byrn R. A., Marsters S. A., Gregory T., Groopman J. E., Capon D. J. Blocking of HIV-1 infectivity by a soluble, secreted form of the CD4 antigen. Science. 1987 Dec 18;238(4834):1704–1707. doi: 10.1126/science.3500514. [DOI] [PubMed] [Google Scholar]
- Spouge J. L., Layne S. P., Dembo M. Analytic results for quantifying HIV infectivity. Bull Math Biol. 1989;51(6):715–730. doi: 10.1007/BF02459657. [DOI] [PubMed] [Google Scholar]
- Sun N. C., Ho D. D., Sun C. R., Liou R. S., Gordon W., Fung M. S., Li X. L., Ting R. C., Lee T. H., Chang N. T. Generation and characterization of monoclonal antibodies to the putative CD4-binding domain of human immunodeficiency virus type 1 gp120. J Virol. 1989 Sep;63(9):3579–3585. doi: 10.1128/jvi.63.9.3579-3585.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas E. K., Weber J. N., McClure J., Clapham P. R., Singhal M. C., Shriver M. K., Weiss R. A. Neutralizing monoclonal antibodies to the AIDS virus. AIDS. 1988 Feb;2(1):25–29. doi: 10.1097/00002030-198802000-00004. [DOI] [PubMed] [Google Scholar]
- Traunecker A., Lüke W., Karjalainen K. Soluble CD4 molecules neutralize human immunodeficiency virus type 1. Nature. 1988 Jan 7;331(6151):84–86. doi: 10.1038/331084a0. [DOI] [PubMed] [Google Scholar]
- Traunecker A., Schneider J., Kiefer H., Karjalainen K. Highly efficient neutralization of HIV with recombinant CD4-immunoglobulin molecules. Nature. 1989 May 4;339(6219):68–70. doi: 10.1038/339068a0. [DOI] [PubMed] [Google Scholar]
- Wang J. H., Yan Y. W., Garrett T. P., Liu J. H., Rodgers D. W., Garlick R. L., Tarr G. E., Husain Y., Reinherz E. L., Harrison S. C. Atomic structure of a fragment of human CD4 containing two immunoglobulin-like domains. Nature. 1990 Nov 29;348(6300):411–418. doi: 10.1038/348411a0. [DOI] [PubMed] [Google Scholar]
- Watanabe M., Reimann K. A., DeLong P. A., Liu T., Fisher R. A., Letvin N. L. Effect of recombinant soluble CD4 in rhesus monkeys infected with simian immunodeficiency virus of macaques. Nature. 1989 Jan 19;337(6204):267–270. doi: 10.1038/337267a0. [DOI] [PubMed] [Google Scholar]



