Abstract
Elderly patients are recommended to have a reduced starting dose (300 mg m−2 once every 3 weeks) of irinotecan monotherapy. The aims of this analysis are to compare toxicity and survival according to age, performance status (PS), gender and prior radical pelvic radiotherapy (RT). The primary end points were overall survival and an irinotecan-specific toxicity composite end point (TCE) defined as the occurrence of grade 3 or 4 diarrhoea, neutropenia, febrile neutropenia, fever, infection or nausea and vomiting. Between 1997 and 2003, 339 eligible patients with advanced colorectal cancer (CRC) progressing on or within 24 weeks of completing fluoropyrimidine-based chemotherapy were prospectively registered in a multicentre randomised trial. All patients commenced irinotecan at 350 mg m−2 once every 3 weeks. There were no differences in proportions of patients developing TCE by age (<70 vs ⩾70 : 37.8 vs 45.8%; P=0.218), PS (0–1 vs 2 : 39.3 vs 41.5%; P=0.793) or prior RT (RT vs no RT : 45.1 vs 38.5%; P=0.377). Males experienced more toxicity than females (44.3 vs 32.6%; P=0.031), but this was not significant after controlling for other co-variates (P=0.06). Patients aged ⩾70 had similar objective responses (11.1 vs 9%; P=0.585) and survival (median 9.4 vs 9 months; log rank P=0.74) compared to younger patients. Elderly patients derive the same benefit without experiencing more toxicity with second-line irinotecan treatment for advanced CRC. Our data do not support the recommendation to reduce the starting dose for the elderly patients.
Keywords: irinotecan, colorectal cancer, elderly, performance status, toxicity, survival
Elderly patients with colorectal cancer (CRC) have the same benefit from fluorouracil-based chemotherapy as younger patients in both adjuvant (Sargent et al, 2001; Sundararajan et al, 2002) and advanced disease (Popescu et al, 1999) settings. The role of irinotecan is now established in patients with fluoropyrimidine-refractory CRC based on two randomised studies demonstrating survival benefit over best supportive care or alternative schedules of infused fluorouracil (Cunningham et al, 1998; Rougier et al, 1998). Despite its efficacy, irinotecan produces toxicities that could be potentially life-threatening, especially when given with bolus 5-FU/leucovorin (LV) (Rothenberg et al, 2001). It is currently unclear whether elderly patients tolerate irinotecan poorly and whether a reduced starting dose for these patients is preferable. Moreover, the potential risks need to be weighed against the expected benefits of receiving irinotecan, especially in this older age group.
The dose adjustment guideline for irinotecan monotherapy, produced by the manufacturer which is in general circulation with clinicians, recommends a reduced starting dose of 300 mg m−2 once every 3 weeks for patients aged >70 years with World Health Organisation (WHO) performance status (PS) 2. This guideline is based on the two pivotal second-line studies – both recommended 300 mg m−2 for patients aged ⩾70 or PS 2 as these factors were previously recognised risk factors for developing toxicity (Cunningham et al, 1998; Rougier et al, 1998). One further phase III study comparing two irinotecan dose regimens in second-line therapy of metastatic CRC also made the same recommendation (Fuchs et al, 2003). However, none of these studies reported any data that prompted this recommendation of a reduced starting dose (300 mg m−2) for these particular patient populations. In addition, it is generally accepted that patients who had previous radical pelvic radiotherapy (RT) are also at risk of developing severe toxicity with irinotecan, especially diarrhoea. The aims of our analysis are to compare toxicity and survival according to age, PS, gender and prior radical pelvic RT in a group of patients treated within a prospective randomised controlled trial, all of whom commenced irinotecan at a dose of 350 mg m−2 given every 3 weeks.
PATIENTS AND METHODS
We performed a phase III multicentre prospective randomised controlled trial recruiting patients from six oncology centres in the United Kingdom. The primary efficacy end point of this study has been reported previously (Lal et al, 2004). The eligibility criteria included: locally advanced or metastatic histologically proven colorectal cancer that progressed on or within 24 weeks of fluorouracil, raltitrexed or oral fluoropyrimidine-based chemotherapy; WHO PS ⩽2; bidimensionally measurable disease assessed by chest X-ray or computed tomography (CT) and satisfactory haematological, renal and liver functions. Patients who had received previous adjuvant chemotherapy and up to a maximum of three lines of palliative chemotherapy as well as those with no measurable disease were permitted into the study.
All patients fulfilling the eligibility criteria were prospectively registered for the trial. Patients who achieved a radiological objective response or disease stabilisation after 24 weeks of irinotecan were then randomly assigned to either stop irinotecan or continue irinotecan on a 1 : 1 basis using random permuted blocks. Patients were stratified according to number of previous lines of treatment. The protocol was approved by the Scientific and Research Ethics Committee of the participating institutions as well as the London Multicentre Research Ethics Committee. Written informed consent was obtained from each patient at registration.
Patients were treated with irinotecan 350 mg m−2 intravenously over 30 min every 3 weeks for eight cycles. No reduced starting dose was recommended in the protocol for patients aged ⩾70 years, WHO PS 2 or previous radical pelvic RT. Toxicity was measured using National Cancer Institute-Common Toxicity Criteria version 2. Radiological assessment with CT scan was made after every four cycles of irinotecan. Radiological tumour response was evaluated according to WHO Criteria (Miller et al, 1981). Complete response (CR) was defined as the complete disappearance of all measurable lesions, without the appearance of new lesion(s). Partial response (PR) was defined as a reduction of bi-dimensional lesions by ⩾50% of the sum of the products of the largest perpendicular diameters of each measurable lesion and no progression in other lesions or the appearance of any new lesions. Stable disease (SD) was defined as a <50% reduction of tumour volume or a <25% increase of the volume of one or more measurable lesions, with no new lesions. Progressive disease (PD) was defined as an increase of ⩾25% of the size of at least one bi-dimensionally measurable lesion, the appearance of new lesion(s), and/or the onset of ascites or pleural effusion with cytological confirmation.
STATISTICAL CONSIDERATIONS
In this secondary analysis of the trial, the primary objectives were to compare toxicity and survival in the whole cohort of registered patients with the following subgroups which were set a priori: (i) aged <70 vs ⩾70 years; (ii) PS 0–1 vs 2; (iii) male vs female and (iv) previous pelvic RT (⩾45 Gy total dose) vs no pelvic RT. The primary end points were irinotecan-specific toxicity composite end point (TCE) and overall survival (OS). TCE was defined as the occurrence of either grade 3 or 4 diarrhoea, neutropenia, febrile neutropenia, fever, infection or nausea and vomiting. These toxicities were components of the gastrointestinal syndrome that caused early 60-day mortality related to irinotecan therapy (Rothenberg et al, 2001). Logistic regression modelling was used to compare different groups in the frequency of developing TCE. Overall survival was calculated from the date of registration until death from any cause or censored at last follow-up. Both time to developing first TCE and OS were calculated using the Kaplan–Meier method (Kaplan and Meier, 1958) and were compared between the groups using the log-rank test (Peto and Peto, 1972). Univariate analysis was performed using logistic regression and the log-rank test to identify characteristics predictive for occurrence of TCE and survival, respectively. The predictive factors analysed for effect were age (<70 vs ⩾70), PS (0–1 vs 2), gender (male vs female), previous pelvic RT (yes vs no), number of metastatic sites (0 or 1 vs >1), baseline serum alkaline phosphatase (⩽upper limit of normal range (ULN) vs >ULN), bilirubin (as a continuous variable as few patients had elevated bilirubin level due to trial eligibility), haemoglobin (⩽11 g/dl vs >11g dl−1) and white blood cell (⩽11 × 109 l vs >11 × 109 l−1) levels. Apart from the a priori defined comparison groups, other factors were chosen because of their prognostic value in patients treated with 5-FU based chemotherapy for metastatic CRC (Kohne et al, 2002). Multivariate survival analysis was performed using Cox's proportional hazards model (Cox, 1972) and corrected for all the significant prognostic factors. All end points were updated in December 2003. Analyses were performed using SPSS package version 12 (SPSS Inc., Chicago, IL, USA) and two-sided P-values <0.05 were considered statistically significant.
RESULTS
Between November 1997 and May 2003, 348 patients were prospectively registered into this study. A total of 55 patients with responding or SD after eight cycles of irinotecan were randomised to stop irinotecan (n=30) or continue until disease progression (n=25). The efficacy of the randomisation part of the study is the subject of a separate publication. In this current analysis, nine (2.6%) patients were excluded due to reduced starting dose (300 mg m−2, n=5), ineligible patient (n=1), death before starting treatment (n=1) and no clinical data (n=2); therefore, 339 patients treated with irinotecan 350 mg m−2 once every 3 weeks were analysed.
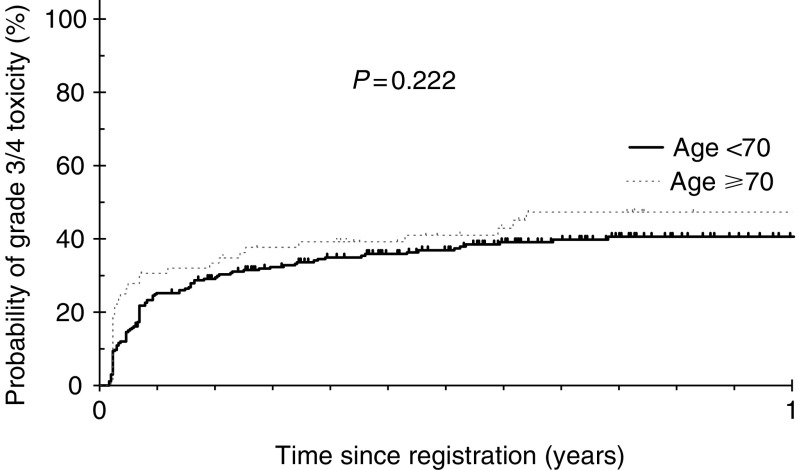
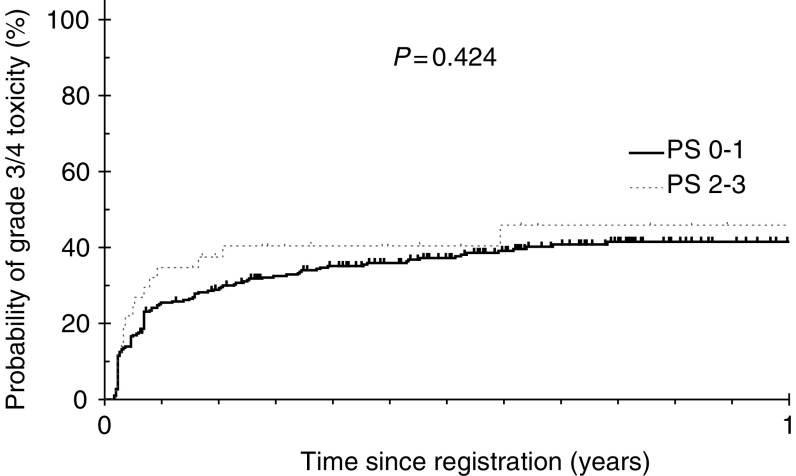
Table 1 shows the baseline characteristics at registration for the whole group. The median age for patients in the <70 years age group was 58 (range=29–69) whereas the median age for those in the ⩾70 years age group was 72 (range=70–80). Only one patient had PS 3. Table 2 shows the incidences of maximum grade adverse events occurring during any cycle in the whole group. Although the elderly had a higher incidence of neutropenia (P=0.0228), the incidences of infection, fever and febrile neutropenia were not significantly increased. Patients who had prior RT did not have a significantly higher incidence of diarrhoea compared to those who did not receive prior RT (P=0.0921). Table 3 shows the number of patients developing TCE according to age, PS, gender and prior radical pelvic RT. There were no significant differences in the proportions of patients developing TCE by age, PS or prior RT (P>0.05). Time to occurrence of TCE was also similar by age (log rank P=0.222; Figure 1) and PS (P=0.424; Figure 2). Males experienced more toxicity than females (44.3 vs 32.6%; P=0.031), but this was not significant after controlling for other co-variates (P=0.06) in multivariate logistic regression modelling. Baseline bilirubin level was not significantly associated with TCE (P=0.149).
Table 1. Baseline characteristics at registration for the whole group.
| Characteristics | Number of patients |
|---|---|
| Total number of patients | 339 |
| Median age (years) | 62 |
| Range | 29–80 |
| <70 years old | 267 (78.8%) |
| ⩾70 years old | 72 (21.2%) |
| Gender | |
| Male | 201 (59.3%) |
| Female | 138 (40.7%) |
| Performance status | |
| 0 | 90 (26.6%) |
| 1 | 205 (60.5%) |
| 2 | 40 (11.8%) |
| 3 | 1 (0.3%) |
| Unknown | 3 (0.9%) |
| Primary tumour sites | |
| Colon | 209 (61.7%) |
| Rectum | 88 (26%) |
| Rectosigmoid junction | 20 (5.9%) |
| Synchrounous | 13 (3.8%) |
| Others | 4 (1.2%) |
| Unknown | 5 (1.5%) |
| Involved disease sites | |
| Liver | 246 (72.6%) |
| Lung | 143 (42.2%) |
| Peritoneum | 55 (16.2%) |
| Primary tumour in situ or local recurrence | 107 (31.6%) |
| Previous radical pelvic radiotherapy | |
| Yes | 51 (15%) |
| No | 288 (85%) |
Table 2. Incidences of grade 3 or 4 toxicities.
| Toxicities | Whole group (n=339) | <70 years (n=267) | ⩾70 years (n=72) | Male (n=201) | Female (n=138) | PS 0–1 (n=295) | PS 2–3 (n=41) | Previous RT (n=51) | No previous RT (n=288) |
|---|---|---|---|---|---|---|---|---|---|
| Anaemia | 17 (5%) | 15 (6%) | 2 (3%) | 10 (5%) | 7 (5%) | 16 (5%) | 1 (2%) | 3 (6%) | 14 (5%) |
| Neutropenia | 83 (24%) | 58 (22%) | 25 (35%) | 61 (30%) | 22 (16%) | 72 (24%) | 11 (27%) | 12 (24%) | 71 (25%) |
| Thrombocytopenia | 12 (4%) | 8 (3%) | 4 (6%) | 7 (3%) | 5 (4%) | 10 (3%) | 2 (5%) | 3 (6%) | 9 (3%) |
| Febrile neutropenia | 4 (1%) | 2 (0.7%) | 2 (3%) | 3 (1%) | 1 (0.7%) | 3 (1%) | 1 (2%) | 0 (0%) | 4 (1%) |
| Diarrhoea | 53 (16%) | 42 (16%) | 11 (15%) | 35 (17%) | 18 (13%) | 43 (15%) | 9a (22%) | 12 (24%) | 41 (14%) |
| Nausea and vomiting | 18 (5%) | 15 (6%) | 3 (4%) | 10 (5%) | 8 (6%) | 17 (6%) | 1 (2%) | 2 (4%) | 16 (6%) |
| Infection | 20 (6%) | 18 (7%) | 2 (3%) | 14 (7%) | 6 (4%) | 17 (6%) | 3 (7%) | 4 (8%) | 16 (6%) |
| Fever | 13 (4%) | 12 (5%) | 1 (1%) | 9 (5%) | 4 (3%) | 11 (4%) | 2 (5%) | 1 (2%) | 12 (4%) |
| Lethargy | 72 (21%) | 57 (21%) | 15 (21%) | 45 (22%) | 27 (20%) | 60 (20%) | 11 (27%) | 10 (20%) | 62 (22%) |
PS: performance status. RT: radiotherapy.
One patient with grade 3–4 diarrhoea had unknown baseline performance status.
Table 3. Number of patients developing toxicity composite endpoint according to age, performance, sex and previous radical pelvic radiotherapy.
| Comparison groups | Number of patients reaching TCE | χ2-test P |
|---|---|---|
| Age<70 | 101/267 (37.8%) | 0.218 |
| Age⩾70 | 33/72 (45.8%) | |
| Performance status 0–1 | 116/295 (39.3%) | 0.793 |
| Performance status 2–3 | 17/41 (41.5%) | |
| Male | 89/201 (44.3%) | 0.031 |
| Female | 45/138 (32.6%) | |
| Pelvic radiotherapy | 23/51 (45.1%) | 0.377 |
| No pelvic radiotherapy | 111/288 (38.5%) |
TCE: toxicity composite end point.
Figure 1.
Time of occurrence of toxicity composite end point by age groups.
Figure 2.
Time to occurrence of toxicity composite end point by performance status groups.
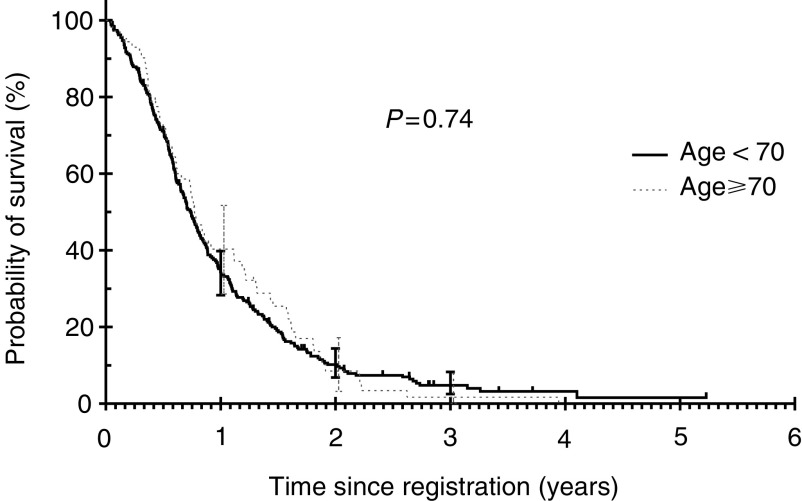
For the whole group, the objective response rate was 9.4% (95% confidence interval (CI): 6.3–12.6%). Table 4 shows the objective responses in patients aged <70 and ⩾70 years with no differences between the two age groups. For the whole group, the median survival was 9.1 months and 1-year survival was 35.3% (95% CI: 30.1–40.5%). There was no difference in survival between patients aged <70 years and ⩾70 years (log-rank test P=0.74; Figure 3). Table 5 shows the multivariate survival analysis. Number of metastatic sites, serum alkaline phosphatase, haemoglobin, white blood cell count and prior pelvic RT were significant prognostic factors. Performance status (P=0.092) and gender (P=0.512) were not significant prognostic factors. There was no difference in survival between patients who developed the TCE and those who did not (P=0.317).
Table 4. Objective responses for patients aged <70 compared to those aged ⩾70.
| <70 years old (n=267) | ⩾70 years old (n=72) | P | |
|---|---|---|---|
| Complete response | 0 (0%) | 1 (1.4%) | |
| Partial response | 24 (9%) | 7 (9.7%) | |
| Stable disease | 84 (31.5%) | 28 (38.9%) | |
| Progressive disease | 159 (59.6%) | 36 (50%) | |
| Objective response rate (95% confidence interval) | 9% (5.6–12.4%) | 11.1% (4.9–20.7%) | 0.585 |
Figure 3.
Overall survival by age groups.
Table 5. Multivariate analysis of prognostic factors on overall survival.
| Factors | Hazard ratio | 95% confidence interval | P |
|---|---|---|---|
| >1 metastatic sites | 1.275 | 1.01–1.61 | 0.041 |
| Alkaline phosphatase >ULN | 1.951 | 1.519–2.506 | <0.001 |
| Haemoglobin ⩽11g l−1 | 1.65 | 1.247–2.188 | <0.001 |
| White blood cell >11 × 109 l−1 | 1.662 | 1.248–2.212 | 0.001 |
| Previous pelvic radiotherapy | 1.684 | 1.213–2.339 | 0.002 |
| Performance status | 0.092 | ||
| Agea | 0.734 | ||
| Sexa | 0.512 |
Univariate analyses. ULN: upper limit of normal.
DISCUSSION
Our study included 339 fluoropyrimidine and thymidylate synthase inhibitor-resistant CRC patients, all treated uniformly at 350 mg m−2 of irinotecan once every 3 weeks and this represents the largest single study with second-line irinotecan monotherapy reported to our knowledge. In this study we have shown that patients aged 70 or over had similar benefit and toxicity to irinotecan as younger patients. Poor PS and previous pelvic RT did not influence the incidence of irinotecan-related severe toxicity in these patients.
Although survival is the most important end point in evaluating new agents, insistence on its use as the only end point in clinical trials can result in the need for thousands of patients to be studied. Accordingly, composite end points have been increasingly used to increase the overall event rate and thereby reduce the number of patients needed to test specific hypotheses. The use of composite end points has resulted in widespread acceptance of therapies in heart failures and acute coronary syndrome (Cannon, 1997), although this approach is less utilised in oncological studies. To be used as part of composite end point, nonfatal end points should be clinically meaningful and related to an adverse outcome (Cannon, 1997). In our analysis, we were interested in evaluating irinotecan-related toxicity in predefined patient populations. The incidences of individual grade 3 & 4 toxicity were low (Table 2), despite our study being one of the largest conducted to date in this setting and this prevented us from comparing individual toxicities by specific patient groups. The gastrointestinal syndrome, comprised of diarrhoea, neutropenia, infection and nausea and vomiting, has been shown to be associated with early treatment-related or exacerbated deaths with irinotecan when used with bolus 5-FU/leucovorin (Rothenberg et al, 2001). These toxicities are well recognised as serious toxicities associated with irinotecan treatment and thus justified their use as components of our TCE.
The dose adjustment guidelines for irinotecan, produced by the manufacturer, recommend a reduced starting dose of 300 mg m−2 in patients aged ⩾70 years with PS 2. These guidelines have not, however, been incorporated into the Summaries of Product Characteristics. We have sought to use our independent data set to validate or refute these recommendations. Elderly patients are perceived to tolerate treatment more poorly and the benefits are less certain in elderly patients. In a systematic review of managing CRC in elderly patients, it was concluded that there is good evidence to support patients ⩽80 years of age having similar OS benefits with adjuvant 5-FU-based chemotherapy for colon cancer and with palliative first-line monotherapy for colorectal cancer, to younger patients (Au et al, 2003). Moreover, advancing age was not found to be related to the incidences of grade 3–4 nausea or vomiting, stomatitis or diarrhoea in patients treated with 5-FU-based adjuvant chemotherapy, although more leucopenia occurred with increasing age (Sargent et al, 2001). In the advanced disease setting, there was also no increase in toxicity in patients >70 years of age compared with younger patients (Popescu et al, 1999). However, the previously mentioned systematic review only included studies evaluating first-line palliative chemotherapy (Au et al, 2003). The effect of age in the second-line treatment of advanced CRC is much less evaluated. Our study showed that patients aged 70 or over had a similar survival and radiological response rate compared to younger patients without any increase in toxicity. However, the maximum age of patients recruited into our study was 80; therefore, our findings may not extend to octogenarians and nonagenarians.
In a pooled analysis of five phase II trials, 455 patients with metastatic CRC progressing on 5-FU were assessed for clinical efficacy and/or tolerance to irinotecan given at 350 mg m−2 every 3 weeks (Freyer et al, 2000). However, in three of these studies, treatment included an enkephalinase inhibitor against diarrhoea, racecadotril, which was assessed as the primary therapeutic intervention. In this pooled analysis, age was not significantly associated with disease response or stabilisation, although patients younger than 58 years old had worse progression-free survival compared to older patients. Overall survival was not assessed in this study (Freyer et al, 2000). Age was also not related to the occurrence of grade 3–4 neutropenia or diarrhoea (Freyer et al, 2000), consistent with our data. In a retrospective analysis of a randomised study evaluating a biweekly bolus irinotecan/5-FU/LV regimen, patients aged ⩾70 (n=17) did not suffer higher frequency of grades 3–4 toxicity compared to those aged under 70 (n=101) (Comella et al, 2003). Survival was also unaffected by age of patients. In another multicentre phase II study, it has been shown that chemotherapy with irinotecan or oxaliplatin-based treatment was feasible with manageable toxicity in the elderly (Aparicio et al, 2003). Similar data have also been found in first-line settings (Mitry et al, 2003; Rougier et al, 2003).
A meta-analysis of 2448 patients in five NCCTG clinical trials, using bolus schedules of 5-FU and LV, reported significantly more stomatits, diarrhoea, alopecia and leucopenia in women compared to men (Sloan et al, 2002). In addition, women experienced more toxicity than men consistently across all cycles of treatment and for all toxicities despite dose reduction after first cycles. These results suggested that women might be intrinsically more sensitive to 5-FU. However, this gender difference in toxicity is not limited to bolus 5-FU/LV schedules, but also extends to infused 5-FU (Tebbutt et al, 2003). In our analysis, we evaluated whether there were gender differences in efficacy and developing toxicity to irinotecan. Male sex was associated with a greater incidence of TCE, although this was not significant after controlling for other co-variates. No survival differences were seen between males and females. However, few other published data are available evaluating gender difference to irinotecan therapy (Innocenti et al, 2004). It is commonly accepted that abdomino-pelvic RT is associated with a greater incidence of irinotecan toxicities and many clinicians would elect to give a reduced starting dose. Previous RT has been shown to result in a greater incidence of grades 3–4 diarrhoea with irinotecan (P=0.046) (Freyer et al, 2000), although this observation was only of borderline significance. Our data and others did not support such a notion (Venook et al, 2003).
In our study, baseline serum bilirubin level did not influence the occurrence of TCE (P=0.149), although one has to note that our eligibility criteria would exclude patients with bilirubin level above 1.25 and 1.5 times the ULN in the absence and presence of liver metastasis respectively. A recent study has also found that baseline serum bilirubin did not reliably predict overall irinotecan-related toxicity in patients treated with irinotecan monotherapy within a phase III trial (Meyerhardt et al, 2004). Significant elevation of bilirubin is however associated with higher incidences of irinotecan-related toxicity (Raymond et al, 2002; Venook et al, 2003) and precludes normal starting dose of irinotecan to be used. Most recent research effort has focussed on UGT1A1 polymorphism as a determinant of irinotecan toxicity. Irinotecan is converted by carboxyl-esterase to its active metabolite, SN-38, which in turn undergoes glucuronidation by UDP-glucuronosyltransferase (UGT). UGT1A1 is the enzyme responsible for bilirubin glucuronidation of SN-38. UGT1A1 polymorphisms result in reduced UGT1A1 activity giving rise to genetic hyperbilirubinaemic syndromes such as Crigler–Najjar types I & II and Gilbert's syndrome and can lead to reduced gluronidation of SN-38. It has been found that patients either heterozygous or homozygous for UGT1A1*28, a variant sequence in the promoter region experienced more severe toxicity to irinotecan and had higher area under curve (AUC) SN-38 ratio compared to SN-38 glucuronide ratio (Ando et al, 2000, 2002; Iyer et al, 2002; Innocenti et al, 2004) Thus, interindividual differences in susceptibility to irinotecan toxicity can be partly explained by UGT1A1 mutation. However, whether starting with a reduced dose of irinotecan based on UGT1A1 polymorphism is an appropriate strategy requires prospective evaluation.
In our study, we have confirmed that the prognostic importances of some clinical and biological factors found in 5-FU based chemotherapy (Kohne et al, 2002) were also valid in irinotecan chemotherapy, that is, elevated alkaline phosphatase, low haemoglobin, elevated white blood cell count and >1 metastatic sites. Performance status 2 was not significantly associated with worse survival. We are unable to explain the reason why previous radical RT was a poor prognostic factor and this could be a chance finding that requires confirmation in an independent data set.
In conclusion, elderly and PS 2 patients derive the same benefit without experiencing more toxicity with second-line irinotecan treatment for advanced colorectal cancer. Pelvic RT did not result in additional toxicity. Our data do not support the recommendations to give a reduced starting dose to elderly and PS 2 patients.
References
- Ando Y, Saka H, Ando M, Sawa T, Muro K, Ueoka H, Yokoyama A, Saitoh S, Shimokata K, Hasegawa Y (2000) Polymorphisms of UDP-glucuronosyltransferase gene and irinotecan toxicity: a pharmacogenetic analysis. Cancer Res 60: 6921–6926 [PubMed] [Google Scholar]
- Ando Y, Ueoka H, Sugiyama T, Ichiki M, Shimokata K, Hasegawa Y (2002) Polymorphisms of UDP-glucuronosyltransferase and pharmacokinetics of irinotecan. Ther Drug Monit 24: 111–116 [DOI] [PubMed] [Google Scholar]
- Aparicio T, Desrame J, Lecomte T, Mitry E, Belloc J, Etienney I, Montembault S, Vayre L, Locher C, Ezenfis J, Artru P, Mabro M, Dominguez S (2003) Oxaliplatin- or irinotecan-based chemotherapy for metastatic colorectal cancer in the elderly. Br J Cancer 89: 1439–1444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Au HJ, Mulder KE, Fields AL (2003) Systematic review of management of colorectal cancer in elderly patients. Clin Colorectal Cancer 3: 165–171 [DOI] [PubMed] [Google Scholar]
- Cannon CP (1997) Clinical perspectives on the use of composite endpoints. Control Clin Trials 18: 517–529 [DOI] [PubMed] [Google Scholar]
- Comella P, Farris A, Lorusso V, Palmeri S, Maiorino L, De Lucia L, Buzzi F, Mancarella S, De Vita F, Gambardella A (2003) Irinotecan plus leucovorin-modulated 5-fluorouracil I.V. bolus every other week may be a suitable therapeutic option also for elderly patients with metastatic colorectal carcinoma. Br J Cancer 89: 992–996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox DR (1972) Regression models and life tables. J Roy Stat Soc A 29: 187–220 [Google Scholar]
- Cunningham D, Pyrhonen S, James RD, Punt CJ, Hickish TF, Heikkila R, Johannesen TB, Starkhammar H, Topham CA, Awad L, Jacques C, Herait P (1998) Randomised trial of irinotecan plus supportive care versus supportive care alone after fluorouracil failure for patients with metastatic colorectal cancer. Lancet 352: 1413–1418 [DOI] [PubMed] [Google Scholar]
- Freyer G, Rougier P, Bugat R, Droz JP, Marty M, Bleiberg H, Mignard D, Awad L, Herait P, Culine S, Trillet-Lenoir V (2000) Prognostic factors for tumour response, progression-free survival and toxicity in metastatic colorectal cancer patients given irinotecan (CPT-11) as second-line chemotherapy after 5FU failure. CPT-11 F205, F220, F221 and V222 study groups. Br J Cancer 83: 431–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuchs CS, Moore MR, Harker G, Villa L, Rinaldi D, Hecht JR (2003) Phase III comparison of two irinotecan dosing regimens in second-line therapy of metastatic colorectal cancer. J Clin Oncol 21: 807–814 [DOI] [PubMed] [Google Scholar]
- Innocenti F, Undevia SD, Iyer L, Xian CP, Das S, Kocherginsky M, Karrison T, Janisch L, Ramirez J, Rudin CM, Vokes EE, Ratain MJ (2004) Genetic variants in the UDP-glucuronosyltransferase 1A1 gene predict the risk of severe neutropenia of irinotecan. J Clin Oncol 22: 1382–1388 [DOI] [PubMed] [Google Scholar]
- Iyer L, Das S, Janisch L, Wen M, Ramirez J, Karrison T, Fleming GF, Vokes EE, Schilsky RL, Ratain MJ (2002) UGT1A1*28 polymorphism as a determinant of irinotecan disposition and toxicity. Pharmacogenom J 2: 43–47 [DOI] [PubMed] [Google Scholar]
- Kaplan EL, Meier P (1958) Non parametric estimation from incomplete observations. J Am Stat Assoc 53: 457–481 [Google Scholar]
- Kohne CH, Cunningham D, Di CF, Glimelius B, Blijham G, Aranda E, Scheithauer W, Rougier P, Palmer M, Wils J, Baron B, Pignatti F, Schoffski P, Micheel S, Hecker H (2002) Clinical determinants of survival in patients with 5-fluorouracil-based treatment for metastatic colorectal cancer: results of a multivariate analysis of 3825 patients. Ann Oncol 13: 308–317 [DOI] [PubMed] [Google Scholar]
- Lal R, Dickson J, Cunningham D, Chau I, Norman AR, Ross PJ, Topham C, Middleton G, Hill M, Oates J (2004) A randomized trial comparing defined-duration with continuous irinotecan until disease progression in fluoropyrimidine and thymidylate synthase inhibitor-resistant advanced colorectal cancer. J Clin Oncol 22: 3023–3031 [DOI] [PubMed] [Google Scholar]
- Meyerhardt JA, Kwok A, Ratain MJ, McGovren JP, Fuchs CS (2004) Relationship of baseline serum bilirubin to efficacy and toxicity of single-agent irinotecan in patients with metastatic colorectal cancer. J Clin Oncol 22: 1439–1446 [DOI] [PubMed] [Google Scholar]
- Miller AB, Hoogstraten B, Staquet M, Winkler A (1981) Reporting results of cancer treatment. Cancer 47: 207–214 [DOI] [PubMed] [Google Scholar]
- Mitry E, Douillard J-Y, Van Cutsem E (2003) Predictive factors of survival in patients with advanced colorectal cancer: an individual data analysis of 602 patients included in CPT11 phase III trials (V302 and V303). Proc Am Soc Clin Oncol 22: 295. [DOI] [PubMed] [Google Scholar]
- Peto R, Peto J (1972) Asymptotically efficient invariant procedures. J Roy Stat Soc A 135: 185–206 [Google Scholar]
- Popescu RA, Norman A, Ross PJ, Parikh B, Cunningham D (1999) Adjuvant or palliative chemotherapy for colorectal cancer in patients 70 years or older. J Clin Oncol 17: 2412–2418 [DOI] [PubMed] [Google Scholar]
- Raymond E, Boige V, Faivre S, Sanderink GJ, Rixe O, Vernillet L, Jacques C, Gatineau M, Ducreux M, Armand JP (2002) Dosage adjustment and pharmacokinetic profile of irinotecan in cancer patients with hepatic dysfunction. J Clin Oncol 20: 4303–4312 [DOI] [PubMed] [Google Scholar]
- Rothenberg ML, Meropol NJ, Poplin EA, Van Cutsem E, Wadler S (2001) Mortality associated with irinotecan plus bolus fluorouracil/leucovorin: summary findings of an independent panel. J Clin Oncol 19: 3801–3807 [DOI] [PubMed] [Google Scholar]
- Rougier P, Van Cutsem E, Bajetta E, Niederle N, Possinger K, Labianca R, Navarro M, Morant R, Bleiberg H, Wils J, Awad L, Herait P, Jacques C (1998) Randomised trial of irinotecan versus fluorouracil by continuous infusion after fluorouracil failure in patients with metastatic colorectal cancer. Lancet 352: 1407–1412 [DOI] [PubMed] [Google Scholar]
- Rougier P, Mitry E, Cunningham D (2003) Is age a prognostic factor of toxicity and efficacy in patients with metastatic colorectal cancer receiving irintoecan in combination with 5-FU/folinic acid? Proc Am Soc Clin Oncol 22: 267 [Google Scholar]
- Sargent DJ, Goldberg RM, Jacobson SD, Macdonald JS, Labianca R, Haller DG, Shepherd LE, Seitz JF, Francini G (2001) A pooled analysis of adjuvant chemotherapy for resected colon cancer in elderly patients. N Engl J Med 345: 1091–1097 [DOI] [PubMed] [Google Scholar]
- Sloan JA, Goldberg RM, Sargent DJ, Vargas-Chanes D, Nair S, Cha SS, Novotny PJ, Poon MA, O'Connell MJ, Loprinzi CL (2002) Women experience greater toxicity with fluorouracil-based chemotherapy for colorectal cancer. J Clin Oncol 20: 1491–1498 [DOI] [PubMed] [Google Scholar]
- Sundararajan V, Mitra N, Jacobson JS, Grann VR, Heitjan DF, Neugut AI (2002) Survival associated with 5-Fluorouracil-based adjuvant chemotherapy among elderly patients with node-positive colon cancer. Ann Intern Med 136: 349–357 [DOI] [PubMed] [Google Scholar]
- Tebbutt NC, Norman AR, Cunningham D, Allen M, Chau I, Oates J, Hill M (2003) Analysis of the time course and prognostic factors determining toxicity due to infused fluorouracil. Br J Cancer 88: 1510–1515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venook AP, Enders KC, Fleming G, Hollis D, Leichman CG, Hohl R, Byrd J, Budman D, Villalona M, Marshall J, Rosner GL, Ramirez J, Kastrissios H, Ratain MJ (2003) A phase I and pharmacokinetic study of irinotecan in patients with hepatic or renal dysfunction or with prior pelvic radiation: CALGB 9863. Ann Oncol 14: 1783–1790 [DOI] [PubMed] [Google Scholar]