Abstract
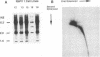
Rhesus Papillomavirus type 1 (RhPV-1) was recently cloned from a rhesus monkey lymph node metastasis of a penile squamous cell carcinoma. In this paper, we demonstrate that RhPV-1 cooperates with the activated ras oncogene to transform primary cells at a level comparable to human papillomavirus type 16. The viral DNAs were cloned such that their expression was under the control of their natural promoter elements. Unlike human papillomavirus type 16, RhPV-1 DNA cooperated with ras independently of the hormone dexamethasone. However, dexamethasone did have a positive influence on the ability of some RhPV-1 cotransformed cells to grow in soft-agar assays. The transformed cells are highly tumorigenic in vivo in nude mice.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker C. C., Phelps W. C., Lindgren V., Braun M. J., Gonda M. A., Howley P. M. Structural and transcriptional analysis of human papillomavirus type 16 sequences in cervical carcinoma cell lines. J Virol. 1987 Apr;61(4):962–971. doi: 10.1128/jvi.61.4.962-971.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cripe T. P., Haugen T. H., Turk J. P., Tabatabai F., Schmid P. G., 3rd, Dürst M., Gissmann L., Roman A., Turek L. P. Transcriptional regulation of the human papillomavirus-16 E6-E7 promoter by a keratinocyte-dependent enhancer, and by viral E2 trans-activator and repressor gene products: implications for cervical carcinogenesis. EMBO J. 1987 Dec 1;6(12):3745–3753. doi: 10.1002/j.1460-2075.1987.tb02709.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crook T., Storey A., Almond N., Osborn K., Crawford L. Human papillomavirus type 16 cooperates with activated ras and fos oncogenes in the hormone-dependent transformation of primary mouse cells. Proc Natl Acad Sci U S A. 1988 Dec;85(23):8820–8824. doi: 10.1073/pnas.85.23.8820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyson N., Howley P. M., Münger K., Harlow E. The human papilloma virus-16 E7 oncoprotein is able to bind to the retinoblastoma gene product. Science. 1989 Feb 17;243(4893):934–937. doi: 10.1126/science.2537532. [DOI] [PubMed] [Google Scholar]
- Dürst M., Gissmann L., Ikenberg H., zur Hausen H. A papillomavirus DNA from a cervical carcinoma and its prevalence in cancer biopsy samples from different geographic regions. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3812–3815. doi: 10.1073/pnas.80.12.3812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dürst M., Kleinheinz A., Hotz M., Gissmann L. The physical state of human papillomavirus type 16 DNA in benign and malignant genital tumours. J Gen Virol. 1985 Jul;66(Pt 7):1515–1522. doi: 10.1099/0022-1317-66-7-1515. [DOI] [PubMed] [Google Scholar]
- Gloss B., Bernard H. U., Seedorf K., Klock G. The upstream regulatory region of the human papilloma virus-16 contains an E2 protein-independent enhancer which is specific for cervical carcinoma cells and regulated by glucocorticoid hormones. EMBO J. 1987 Dec 1;6(12):3735–3743. doi: 10.1002/j.1460-2075.1987.tb02708.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaur P., McDougall J. K. Characterization of primary human keratinocytes transformed by human papillomavirus type 18. J Virol. 1988 Jun;62(6):1917–1924. doi: 10.1128/jvi.62.6.1917-1924.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloster B. E., Manias D. A., Ostrow R. S., Shaver M. K., McPherson S. W., Rangen S. R., Uno H., Faras A. J. Molecular cloning and characterization of the DNA of two papillomaviruses from monkeys. Virology. 1988 Sep;166(1):30–40. doi: 10.1016/0042-6822(88)90143-2. [DOI] [PubMed] [Google Scholar]
- Lambert P. F., Spalholz B. A., Howley P. M. A transcriptional repressor encoded by BPV-1 shares a common carboxy-terminal domain with the E2 transactivator. Cell. 1987 Jul 3;50(1):69–78. doi: 10.1016/0092-8674(87)90663-5. [DOI] [PubMed] [Google Scholar]
- Matlashewski G., Schneider J., Banks L., Jones N., Murray A., Crawford L. Human papillomavirus type 16 DNA cooperates with activated ras in transforming primary cells. EMBO J. 1987 Jun;6(6):1741–1746. doi: 10.1002/j.1460-2075.1987.tb02426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mincheva A., Gissmann L., zur Hausen H. Chromosomal integration sites of human papillomavirus DNA in three cervical cancer cell lines mapped by in situ hybridization. Med Microbiol Immunol. 1987;176(5):245–256. doi: 10.1007/BF00190531. [DOI] [PubMed] [Google Scholar]
- Ostrow R. S., Bender M., Niimura M., Seki T., Kawashima M., Pass F., Faras A. J. Human papillomavirus DNA in cutaneous primary and metastasized squamous cell carcinomas from patients with epidermodysplasia verruciformis. Proc Natl Acad Sci U S A. 1982 Mar;79(5):1634–1638. doi: 10.1073/pnas.79.5.1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostrow R. S., LaBresh K. V., Faras A. J. Characterization of the complete RhPV 1 genomic sequence and an integration locus from a metastatic tumor. Virology. 1991 Mar;181(1):424–429. doi: 10.1016/0042-6822(91)90519-h. [DOI] [PubMed] [Google Scholar]
- Ostrow R. S., McGlennen R. C., Shaver M. K., Kloster B. E., Houser D., Faras A. J. A rhesus monkey model for sexual transmission of a papillomavirus isolated from a squamous cell carcinoma. Proc Natl Acad Sci U S A. 1990 Oct;87(20):8170–8174. doi: 10.1073/pnas.87.20.8170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pater M. M., Pater A. Human papillomavirus types 16 and 18 sequences in carcinoma cell lines of the cervix. Virology. 1985 Sep;145(2):313–318. doi: 10.1016/0042-6822(85)90164-3. [DOI] [PubMed] [Google Scholar]
- Phelps W. C., Yee C. L., Münger K., Howley P. M. The human papillomavirus type 16 E7 gene encodes transactivation and transformation functions similar to those of adenovirus E1A. Cell. 1988 May 20;53(4):539–547. doi: 10.1016/0092-8674(88)90570-3. [DOI] [PubMed] [Google Scholar]
- Pirisi L., Yasumoto S., Feller M., Doniger J., DiPaolo J. A. Transformation of human fibroblasts and keratinocytes with human papillomavirus type 16 DNA. J Virol. 1987 Apr;61(4):1061–1066. doi: 10.1128/jvi.61.4.1061-1066.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirisi L., Yasumoto S., Feller M., Doniger J., DiPaolo J. A. Transformation of human fibroblasts and keratinocytes with human papillomavirus type 16 DNA. J Virol. 1987 Apr;61(4):1061–1066. doi: 10.1128/jvi.61.4.1061-1066.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider-Gädicke A., Schwarz E. Different human cervical carcinoma cell lines show similar transcription patterns of human papillomavirus type 18 early genes. EMBO J. 1986 Sep;5(9):2285–2292. doi: 10.1002/j.1460-2075.1986.tb04496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz E., Freese U. K., Gissmann L., Mayer W., Roggenbuck B., Stremlau A., zur Hausen H. Structure and transcription of human papillomavirus sequences in cervical carcinoma cells. Nature. 1985 Mar 7;314(6006):111–114. doi: 10.1038/314111a0. [DOI] [PubMed] [Google Scholar]
- Shih C., Weinberg R. A. Isolation of a transforming sequence from a human bladder carcinoma cell line. Cell. 1982 May;29(1):161–169. doi: 10.1016/0092-8674(82)90100-3. [DOI] [PubMed] [Google Scholar]
- Storey A., Osborn K., Crawford L. Co-transformation by human papillomavirus types 6 and 11. J Gen Virol. 1990 Jan;71(Pt 1):165–171. doi: 10.1099/0022-1317-71-1-165. [DOI] [PubMed] [Google Scholar]
- Thierry F., Yaniv M. The BPV1-E2 trans-acting protein can be either an activator or a repressor of the HPV18 regulatory region. EMBO J. 1987 Nov;6(11):3391–3397. doi: 10.1002/j.1460-2075.1987.tb02662.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson G. D., Soranson J. A., Lewis A. A. Cell kinetics of mouse kidney using bromodeoxyuridine incorporation and flow cytometry: preparation and staining. Cell Tissue Kinet. 1987 Mar;20(2):125–133. doi: 10.1111/j.1365-2184.1987.tb01091.x. [DOI] [PubMed] [Google Scholar]
- zur Hausen H. Papillomaviruses in anogenital cancer as a model to understand the role of viruses in human cancers. Cancer Res. 1989 Sep 1;49(17):4677–4681. [PubMed] [Google Scholar]