Abstract
The farnesyl diphosphate synthase (FPPS) has previously been characterized in trypanosomes as an essential enzyme for their survival and as the target for bisphosphonates, drugs that are effective both in vitro and in vivo against these parasites. Enzymes from the isoprenoid pathway have been assigned to different compartments in eukaryotes, including trypanosomatids. We here report that FPPS localizes to the cytoplasm of both Trypanosoma cruzi and T. brucei, and is not present in other organelles such as the mitochondria and glycosomes.
Keywords: Bisphosphonates, farnesyl diphosphate synthase, mevalonate pathway, trypanosome
1. Introduction
Trypanosomiases are parasitic diseases affecting millions of people in the American and African continents. Recent work has shown that nitrogen-containing bisphosphonates, such as those used to treat bone resorption diseases, are competitive inhibitors of the farnesyl diphosphate synthase of both Trypanosoma cruzi (Montalvetti, 2001) and T. brucei (Montalvetti, 2003) and effective in vitro and in vivo against these parasites (Urbina, 1999; Szajnman, 2001 and 2003; Martin, 2001 and 2002; Garzoni, 2004; Bouzahzah, 2005). Farnesyl diphosphate synthase (FPPS) catalyzes the consecutive condensation of isopentenyl diphosphate (IPP, C5) with dimethylallyl diphosphate (DMAPP, C5), and with geranyl diphosphate (GPP, C10), all products of the mevalonate pathway in trypanosomatids, to form the 15-carbon isoprenoid compound, farnesyl diphosphate (FPP, C15). FPP is the substrate for enzymes catalyzing the first committed step for biosynthesis of sterols, ubiquinones, dolichols, heme a, and prenylated proteins. The three-dimensional structures of both T. cruzi (Gabelli, 2005) and T. brucei (Mao, 2004 and 2006) FPPS have been solved.
In trypanosomatids, the localization studies of some of the enzymes of the mevalonate pathway have yielded controversial results. 3-Hydroxy-3-methyl-glutaryl-CoA reductase (HMGR), the first enzyme of this pathway, was localized to the mitochondrion of T. cruzi and L. major (Pena-Diaz, 2004), although it was first reported to be glycosomal in T. cruzi (Concepcion, 1998), and was shown to be mitochondrial with some possible association to other organelles, such as the endoplasmic reticulum (ER) and glycosomes in T. brucei (Heise, 2000). We previously reported the presence of a long chain solanesyl diphosphate synthase (SPPS) in the glycosomes of T. cruzi, and detected the presence of another putative polyprenyl synthase in their cytoplasm (Ferella, 2006). Squalene synthase, which is involved in the first step of sterol biosynthesis, was reported to have multiple locations in T. cruzi and Leishmania mexicana (Urbina, 2002). The short chain farnesyl diphosphate synthase, described in all three trypanosomatids (Montalvetti, 2001 and 2003; Ortiz-Gomez, 2006), was reported to be a cytosolic enzyme in L. major (Ortiz-Gomez, 2006). An SKL-like sequence is present in the T. cruzi enzyme although it is not in the C-terminus of the protein as other motifs involved in glycosomal targeting (Montalvetti, 2001). In mammals and plants, there is a similar trend of different polyprenyl synthases located in different compartments. For example, FPPS has been found in the cytosol and associated with the endoplasmic reticulum (Hugueney, 1996), mitochondria (Cunillera, 1997), and plastids (Sanmiya, 1999) in plants, and the peroxisomes in animals (Biardi, 1996). These variable locations of the components of this pathway make it necessary to study this issue in detail in each organism.
We here report that FPPS is localized in the cytoplasm of T. cruzi epimastigotes and T. brucei procyclic and bloodstream trypomastigotes, as demonstrated by its release from digitonin-permeabilized cells together with cytosolic markers, and by immunofluorescence microscopy localization studies.
2. Materials and methods
2.1. Cell cultures
Epimastigotes from T. cruzi CL Brener were grown at 28°C in Liver infusion tryptose (LIT) media (Bone, 1956) supplemented with 5% FBS (Gibco) and streptomycin /penicillin (Gibco), pH 7.3. Procyclic trypomastigotes from T. brucei were grown at 28°C in SDM-79 medium pH 7, supplemented with 10% FBS (Gibco) and streptomycin /penicillin (Gibco). Bloodstream trypomastigotes from T. brucei (T. brucei brucei strain) were obtained and cultured as described before (Hesse, 1995).
2.2. Digitonin permeabilization assay
Approximately 2-4 × 109 parasites were collected and washed twice by centrifugation at 2500 rpm (∼1000 × g) for 10 min in buffer D (20 mM Tris-HCl, pH 7.2, 225 mM sucrose, 20 mM KCl, 10 mM KH2PO4, 1 mM EDTA, 5 mM MgCl2, 1 mM DTT), and finally resuspended in 3 ml of the same buffer containing a protease inhibitor cocktail (P8340, Sigma). An aliquot of 50 μl was mixed with an equal volume of 0.2 % Triton X-100 in 0.3 M NaCl, incubated at room temperature for 20 min, and used to determine the total protein concentration per ml of total cell suspension, using the BioRad Protein Assay (BioRad). The cell suspension was diluted to 1 mg of total protein/ml and distributed into 12 tubes for the digitonin assays. Increasing amounts of digitonin were added to each tube and then incubated at 28°C for 5 min. The samples were centrifuged for 2 min at 14,000-x g, and the supernatants were transferred to new tubes. The pellets were resuspended in buffer D, 0.3 mg digitonin was added, and incubated at 28°C for 30 min to release the total soluble protein content.
2.3. Enzymatic activities
Activities for cytoplasmic, mitochondrial and glycosomal markers were measured in the supernatants and pellets, together with FPPS activity, as described previously (Montalvetti, 2001), using the purified recombinant T. cruzi FPPS (TcFPPS) (Montalvetti, 2001) as a positive control. Measurements were performed in duplicates for each of three permeabilization experiments. Hexokinase was assayed as described before (Caceres, 2003) with minor modifications (higher MgCl2 concentration, and use of 0.1 M triethanolamine buffer). The reaction mixture contained 0.1 M triethanolamine pH 7.5, 3 mM MgCl2, 0.72 mM NADP+, 4 mM glucose, 1.5 mM ATP and 0.2 units of glucose-6-phosphate dehydrogenase in a total volume of 100 μl. The reaction was started by the addition of the mixture to the samples already distributed in a multi-well plate, and the absorbance was followed at 340 nm, and at 30°C, for several minutes. Phosphoenolpyruvate carboxykinase was measured in a carboxylation reaction as described (Urbina, 1987) using NaHCO3 instead of potassium carbonate. Glucose-6-phosphate dehydrogenase was assayed (Heise, 1999) in a reaction mixture containing 100 mM triethanolamine pH 7.5, 0.5 mM MgCl2, 0.5 mM NADP+, and 5 mM glucose-6-phosphate. The reaction was followed by absorbance at 340 nm at 30°C. For the citrate synthase activity (Adroher, 1988, with modifications) the reaction mixture contained 100 mM Tris-HCl, pH 8.1, 0.1 mM 5,5′-dithiobis(2-nitrobenzoic acid) (DTNB), 0.2 mM acetyl-CoA, and water to a final volume of 80 μl. The mixture was added to wells containing the samples and incubated for 3 min. To start the citrate synthase reaction 10 μl of 5 mM oxaloacetate were added, and the absorbance was measured at 412 nm, and at 25°C instead of 37°C.
2.4. Western blot analysis
Equal sample volumes (25 μl sample per lane) were loaded on a SDS/10% PAGE, run and transferred to polyvinylidene difluoride (PVDF) membranes. Membranes were blocked overnight at 4°C in PBS-0.1% Tween-20-5% skim milk and probed with rabbit anti-TcFPPS antibody (Montalvetti, 2001; 1:2,000), rabbit anti-TbgGAPDH (Rayyan, 1993; 1:3,000), and with secondary rabbit HRP-conjugated antibody (GE Healthcare; 1:20,000), as described before (Ferella, 2006). Immunoblots were visualized on radiographic film using the ECL enhanced chemiluminiscence detection kit according to the instructions of the manufacturer (GE Healthcare).
2.5. Immunofluorescence microscopy
Exponential cultures were pelleted and washed twice in PBS and fixed in 4% paraformaldehyde, 0.1 M cacodylate buffer and 0.1 % glutaraldehyde in PBS, pH 7.2. Fixed cells were adhered to polylysine coated cover slides, permeabilized with Triton X-100 in PBS, neutralized in 50 mM NH4Cl and blocked with 3% BSA in PBS. Incubation with primary and secondary antibodies in 3% BSA-PBS was done for one hour. The antibodies used were rabbit anti-TcFPPS (1:600) and goat anti-rabbit Alexa 488 (1:1000) or goat anti-rabbit Alexa 546 (1:800). For the co-localization studies, primary rabbit anti-BiP antibody was labelled using the Zenon rabbit IgG labelling kit Alexa 546 (Molecular Probes). Slides were incubated with the anti-TcFPPS and then incubated with the pre-labelled antibody. Glycosomes were detected using mouse monoclonal anti-TbPPDK (1:4). For mitochondrial staining Mitotracker (Molecular Probes) was used at a concentration of 0.02 μM and 10 min incubation of live cells. The cells were counterstained with DAPI (4′,6-diamidino-2-phenylindole) when mounted with ProLong Gold antifade reagent with DAPI (Molecular Probes). Fluorescent optical images were captured under non-saturating conditions and identical exposure times using an Olympus IX-71 inverted fluorescence microscope with a Photometrix CoolSnapHQ CCD (charge-coupled device) camera driven by DeltaVision software (Applied Precision).
3. Results
3.1 Permeabilization assays and western blot analysis
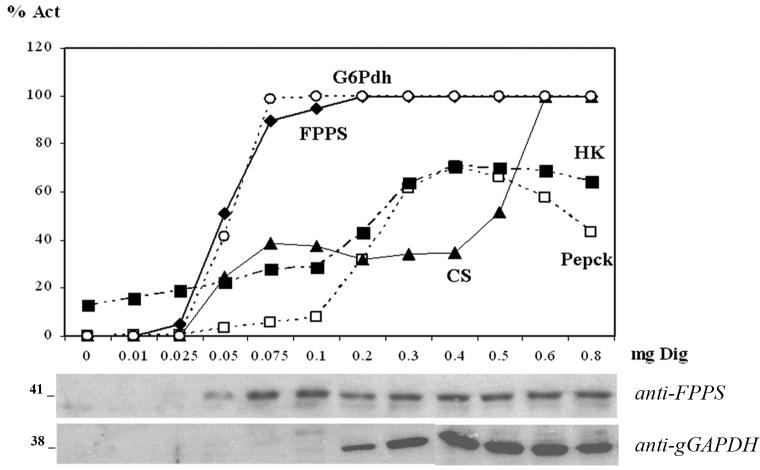
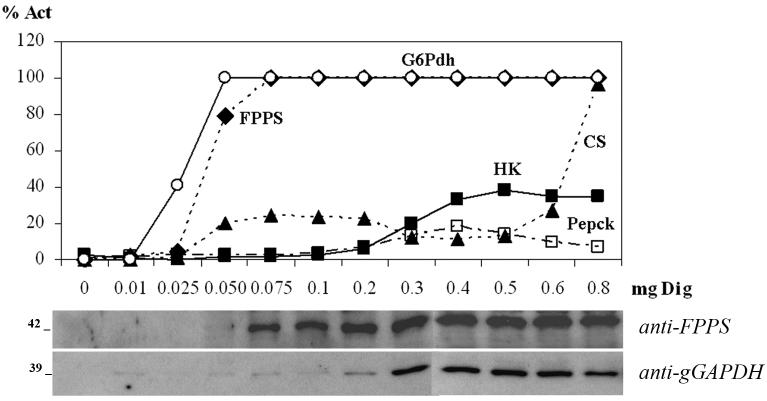
We found that the release of FPPS from both trypanosomes coincided with the release of the cytoplasmic marker, glucose-6-phosphate dehydrogenase (Figs. 1A and 1B). Incubation for 5 min with 0.05 mg of digitonin per mg of total cellular protein was sufficient to initiate the release of cytoplasmic components, while amounts of digitonin higher than 0.05 and 0.1 mg/ml were necessary to release the mitochondrial citrate synthase and the glycosomal content, respectively. The use of more than 0.5 mg/ml of digitonin partially inhibited the hexokinase (Hk) and phosphoenolpyruvate carboxykinase (Pepck) activities from both parasites.
Fig. 1.
Digitonin permeabilization assays. The graphs show the percentage of total recovered activity in the supernatants for each amount of digitonin used. A. T cruzi epimastigotes. B. T brucei procyclic trypomastigotes. Glucose-6-Phosphate dehydrogenase (G6PDH; ○); farnesyl diphosphate synthase (FPPS, ◆); citrate synthase (CS, ▲); hexokinase (Hk; □); phosphoenolpyruvate kinase (Pepck; ■). Immunoblot analyses of supernatants (25 μl sample per lane) are shown at the bottom of each figure. Mr is indicated at the left.
To confirm these results, we performed western blot analyses of the supernatants after digitonin treatment using antibodies against TcFPPS (Montalvetti, 2003) and T. brucei glycosomal glyceraldehyde-3-phosphate dehydrogenase (TbgGAPDH) (Rayyan, 1993) (Figs. 1A and 1B). The results were in agreement with the results of the enzymatic activities. TcFPPS and TbFPPS appear to be cytosolic and a glycosomal location can be excluded. We also performed western blot analysis of the digitonin fractions from T. cruzi using the anti-TcSPPS (Ferella, 2006), as the primary antibody (data not shown). The SPPS was only present in the pellets and was not released in the supernatants, suggesting a membrane association.
3.2. Immunofluorescence microscopy
To rule out a localization of TcFPPS and TbFPPS in the mitochondria, endoplasmic reticulum or glycosomes, we performed immunofluorescence microscopy studies of both, epimastigotes and procyclics trypomastigotes, using Mitotracker as a mitochondrial marker, and antibodies against BiP (Bangs, 1993) and PPDK (Bringaud, 1998), markers for the endoplasmic reticulum and glycosomes, respectively. No co-localization was detected with the glycosomal PPDK or in the mitochondrion, in neither epimastigotes (Fig. 2A) nor procyclic trypomastigotes (Fig. 2B). A weak labelling for FPPS was seen in the ER of both trypanosomes (Fig. 2A and B). The same pattern was observed for the bloodstream form in T. brucei (Supplementary Fig. 1).
Fig. 2.
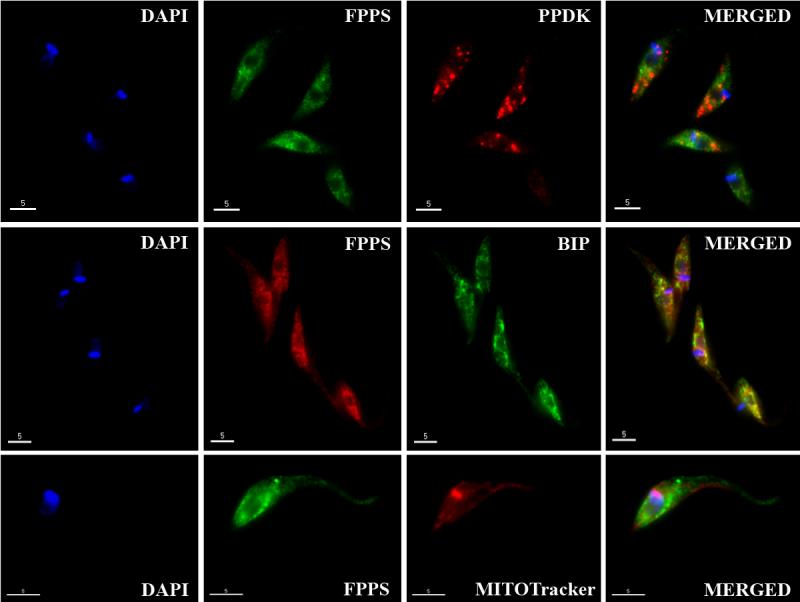
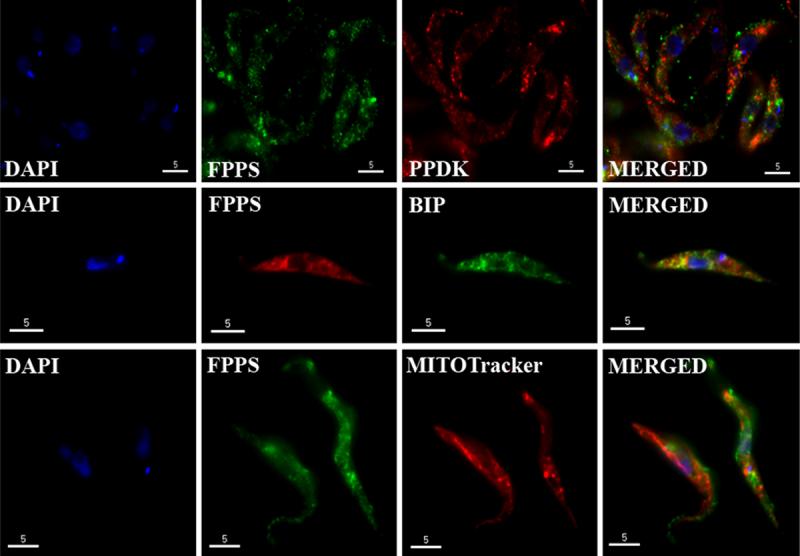
Immunofluorescence microscopy analysis. A. T. cruzi epimastigotes. B. T. brucei procyclic trypomastigotes. Colocalization of FPPS with: PPDK (glycosomal marker); BiP (endoplasmic reticulum marker) and Mitotracker (mitochondrion and kinetoplast stain). DAPI staining of the nuclei and kinetoplast is shown in blue. Bars = 5 μm.
4. Discussion
As pointed out in the introduction, the enzymes from the mevalonate pathway and particularly those of the isoprenoid biosynthetic route have been assigned to several different compartments in mammals and plants (20-23 Hugueney, 1996; Cunillera, 1997; Sanmiya, 1999; Biardi, 1996; Olivier, 2000; Hirooka, 2005; Leivar, 2005). Only a few enzymes of this pathway have been described in kinetoplastids (Montalvetti, 2001 and 2003; Pena-Diaz, 2004; Ferella, 2006; Urbina, 2002; Ortiz-Gomez, 2006), and have been located to the glycosome, mitochondria, and cytoplasm (Ferella, 2006; Pena-Diaz, 2004 and Ortiz-Gomez, 2006, respectively).
Although most of the genes are conserved among the three trypanosomatids, T. cruzi, T. brucei and L. major, and their metabolisms are similar, they still show some discrepancies.
Our results indicate that farnesyl diphosphate synthase, in both T. cruzi epimastigotes and T. brucei procyclic trypomastigotes, localizes mainly to the cytoplasm. This pattern can be assumed as well for the bloodstream forms of T. brucei as indicated by immunofluorescence microscopy analysis (Supplementary Fig. 1). This is in agreement with the cytosolic presence of FPPS in L. major (Ortiz-Gomez, 2006). And it can be extrapolated to the mammalian forms as seen by the immunofluorescence we performed over bloodstream T. brucei.
An internal SKL-like sequence, like those present in the C-terminus of other glycosomal proteins, was reported only in T. cruzi FPPS (Montalvetti et al., 2001) but not in T. brucei FPPS (Montalvetti et al., 2003). However this signal is not by itself a potential glycosomal targeting signal (Gatto, 2000 and 2003). The cytosolic localization described here, rules out a role for this putative signal in TcFPPS targeting, and confirms the conservation of the enzyme location throughout these three trypanosomes.
The localization of other enzymes of the isoprenoid pathway in other cell compartments, such as the TcSPPS present in the glycosome (Ferella, 2006), suggests that a mechanism must be available for the transport of the intermediates of this pathway between different cell compartments.
Supplementary Material
Acknowledgments
We are thankful to Dr J Bangs for the BiP antibody, to Dr F Opperdoes for the gGAPDH antibody and to Dr Frederic Bringaud for the PPDK antibody. We thank Dr. J.L. Concepcion for its help with the hexokinase activity and to Dr Martin Rottenberg for providing the bloodstream form of T. brucei. This work was supported in part by U.S. National Institutes of Health grant AI-68647 to R.D. MF was supported in part by an Ellison Medical Foundation Training Grant to the Center for Tropical and Emerging Global Diseases.
Index descriptors and abbreviations
- FPPS
farnesyl diphosphate synthase
- HMGR
3-hydroxy-3-methylglutaryl-CoA reductase
- SPPS
solanesyl diphosphate synthase.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Adroher FJ, Osuna A, Lupianez JA. Differential energetic metabolism during Trypanosoma cruzi differentiation. I. Citrate synthase, NADP-isocitrate dehydrogenase, and succinate dehydrogenase. Archives of Biochemistry and Biophysics. 1988;267:252–261. doi: 10.1016/0003-9861(88)90030-6. [DOI] [PubMed] [Google Scholar]
- Bangs JD, Uyetake L, Brickman MJ, Balber AE, Boothroyd JC. Molecular cloning and cellular localization of a BiP homologue in Trypanosoma brucei. Divergent ER retention signals in a lower eukaryote. Journal of Cell Science. 1993;105:1101–1113. doi: 10.1242/jcs.105.4.1101. [DOI] [PubMed] [Google Scholar]
- Biardi L, Krisans SK. Compartmentalization of cholesterol biosynthesis. Conversion of mevalonate to farnesyl diphosphate occurs in the peroxisomes. The Journal of Biological Chemistry. 1996;271:1784–1788. doi: 10.1074/jbc.271.3.1784. [DOI] [PubMed] [Google Scholar]
- Bone GJ, Steinert M. Induced change from culture form to blood-stream form in Trypanosoma mega. Nature. 1956;178:362. doi: 10.1038/178362a0. [DOI] [PubMed] [Google Scholar]
- Bouzahzah B, Jelicks LA, Morris SA, Weiss LM, Tanowitz HB. Risedronate in the treatment of Murine Chagas’ disease. Parasitology Research. 2005;96:184–187. doi: 10.1007/s00436-005-1331-9. [DOI] [PubMed] [Google Scholar]
- Bringaud F, Baltz D, Baltz T. Functional and molecular characterization of a glycosomal PPi-dependent enzyme in trypanosomatids: pyruvate, phosphate dikinase. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:7963–7968. doi: 10.1073/pnas.95.14.7963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caceres AJ, Portillo R, Acosta H, Rosales D, Quinones W, Avilan L, Salazar L, Dubourdieu M, Michels PA, Concepcion JL. Molecular and biochemical characterization of hexokinase from Trypanosoma cruzi. Molecular and Biochemical Parasitology. 2003;126:251–262. doi: 10.1016/s0166-6851(02)00294-3. [DOI] [PubMed] [Google Scholar]
- Concepcion JL, Gonzalez-Pacanowska D, Urbina JA. 3-Hydroxy-3-methyl-glutaryl-CoA reductase in Trypanosoma (Schizotrypanum) cruzi: subcellular localization and kinetic properties. Archives of Biochemistry and Biophysics. 1998;352:114–120. doi: 10.1006/abbi.1998.0577. [DOI] [PubMed] [Google Scholar]
- Cunillera N, Boronat A, Ferrer A. The Arabidopsis thaliana FPS1 gene generates a novel mRNA that encodes a mitochondrial farnesyl-diphosphate synthase isoform. The Journal of Biological Chemistry. 1997;272:15381–15388. doi: 10.1074/jbc.272.24.15381. [DOI] [PubMed] [Google Scholar]
- Ferella M, Montalvetti A, Rohloff P, Miranda K, Fang J, Reina S, Kawamukai M, Bua J, Nilsson D, Pravia C, Katzin A, Cassera MB, Aslund L, Andersson B, Docampo R, Bontempi EJ. A solanesyl-diphosphate synthase localizes in glycosomes of Trypanosoma cruzi. The Journal of Biological Chemistry. 2006;281:39339–39348. doi: 10.1074/jbc.M607451200. [DOI] [PubMed] [Google Scholar]
- Gabelli SB, McLellan JS, Montalvetti A, Oldfield E, Docampo R, Amzel LM. Structure and mechanism of the farnesyl diphosphate synthase from Trypanosoma cruzi: implications for drug design. Proteins. 2005;62:80–88. doi: 10.1002/prot.20754. [DOI] [PubMed] [Google Scholar]
- Garzoni LR, Caldera A, Meirelles Mde N, de Castro SL, Docampo R, Meints GA, Oldfield E, Urbina JA. Selective in vitro effects of the farnesyl pyrophosphate synthase inhibitor risedronate on Trypanosoma cruzi. International Journal of Antimicrobial Agents. 2004;23:273–285. doi: 10.1016/j.ijantimicag.2003.07.020. [DOI] [PubMed] [Google Scholar]
- Gatto GJ, Jr., Geisbrecht BV, Gould SJ, Berg JM. Peroxisomal targeting signal-1 recognition by the TPR domains of human PEX5. Nature Structural Biology. 2000;7:1091–1095. doi: 10.1038/81930. [DOI] [PubMed] [Google Scholar]
- Gatto GJ, Jr., Maynard EL, Guerrerio AL, Geisbrecht BV, Gould SJ, Berg JM. Correlating structure and affinity for PEX5:PTS1 complexes. Biochemistry. 2003;42:1660–1666. doi: 10.1021/bi027034z. [DOI] [PubMed] [Google Scholar]
- Heise N, Opperdoes FR. Purification, localisation and characterisation of glucose-6-phosphate dehydrogenase of Trypanosoma brucei. Molecular and Biochemical Parasitology. 1999;99:21–32. doi: 10.1016/s0166-6851(98)00176-5. [DOI] [PubMed] [Google Scholar]
- Heise N, Opperdoes FR. Localisation of a 3-hydroxy-3-methylglutaryl-coenzyme A reductase in the mitochondrial matrix of Trypanosoma brucei procyclics. Zeitschrift für Naturforschung. Section C: Biosciences. 2000;55:473–477. doi: 10.1515/znc-2000-5-626. [DOI] [PubMed] [Google Scholar]
- Hesse F, Selzer PM, Mühlstädt K, Duszenko M. A novel cultivation technique for long-term maintenance of bloodstream form trypanosomes in vitro. Molecular and Biochemical Parasitology. 1995;70:157–166. doi: 10.1016/0166-6851(95)00027-x. [DOI] [PubMed] [Google Scholar]
- Hirooka K, Izumi Y, An CI, Nakazawa Y, Fukusaki E, Kobayashi A. Functional analysis of two solanesyl diphosphate synthases from Arabidopsis thaliana. Bioscience, Biotechnology, and Biochemistry. 2005;69:592–601. doi: 10.1271/bbb.69.592. [DOI] [PubMed] [Google Scholar]
- Hugueney P, Bouvier F, Badillo A, Quennemet J, d’Harlingue A, Camara B. Developmental and stress regulation of gene expression for plastid and cytosolic isoprenoid pathways in pepper fruits. Plant Physiology. 1996;111:619–626. doi: 10.1104/pp.111.2.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leivar P, Gonzalez VM, Castel S, Trelease RN, Lopez-Iglesias C, Arro M, Boronat A, Campos N, Ferrer A, Fernandez-Busquets X. Subcellular localization of Arabidopsis 3-hydroxy-3-methylglutaryl-coenzyme A reductase. Plant Physiology. 2005;137:57–69. doi: 10.1104/pp.104.050245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao J, Gao YG, Odeh S, Robinson H, Montalvetti A, Docampo R, Oldfield E. Crystallization and preliminary X-ray diffraction study of the farnesyl diphosphate synthase from Trypanosoma brucei. Acta Crystallographica. Section D, Biological Crystallography. 2004;60:1863–1866. doi: 10.1107/S0907444904020633. [DOI] [PubMed] [Google Scholar]
- Mao J, Mukherjee S, Zhang Y, Cao R, Sanders JM, Song Y, Zhang Y, Meints GA, Gao YG, Mukkamala D, Hudock MP, Oldfield E. Solid-state NMR, crystallographic, and computational investigation of bisphosphonates and farnesyl diphosphate synthase-bisphosphonate complexes. Journal of the American Chemical Society. 2006;128:14485–14497. doi: 10.1021/ja061737c. [DOI] [PubMed] [Google Scholar]
- Martin MB, Grimley JS, Lewis JC, Heath HT, 3rd, Bailey BN, Kendrick H, Yardley V, Caldera A, Lira R, Urbina JA, Moreno SN, Docampo R, Croft SL, Oldfield E. Bisphosphonates inhibit the growth of Trypanosoma brucei, Trypanosoma cruzi, Leishmania donovani, Toxoplasma gondii, and Plasmodium falciparum: a potential route to chemotherapy. Journal of Medicinal Chemistry. 2001;44:909–916. doi: 10.1021/jm0002578. [DOI] [PubMed] [Google Scholar]
- Martin MB, Sanders JM, Kendrick H, de Luca-Fradley K, Lewis JC, Grimley JS, Van Brussel EM, Olsen JR, Meints GA, Burzynska A, Kafarski P, Croft SL, Oldfield E. Activity of bisphosphonates against Trypanosoma brucei rhodesiense. Journal of Medicinal Chemistry. 2002;45:2904–2914. doi: 10.1021/jm0102809. [DOI] [PubMed] [Google Scholar]
- Montalvetti A, Bailey BN, Martin MB, Severin GW, Oldfield E, Docampo R. Bisphosphonates are potent inhibitors of Trypanosoma cruzi farnesyl pyrophosphate synthase. The Journal of Biological Chemistry. 2001;276:33930–33937. doi: 10.1074/jbc.M103950200. [DOI] [PubMed] [Google Scholar]
- Montalvetti A, Fernandez A, Sanders JM, Ghosh S, Van Brussel E, Oldfield E, Docampo R. Farnesyl pyrophosphate synthase is an essential enzyme in Trypanosoma brucei. In vitro RNA interference and in vivo inhibition studies. The Journal of Biological Chemistry. 2003;278:17075–17083. doi: 10.1074/jbc.M210467200. [DOI] [PubMed] [Google Scholar]
- Olivier LM, Kovacs W, Masuda K, Keller GA, Krisans SK. Identification of peroxisomal targeting signals in cholesterol biosynthetic enzymes. AA-CoA thiolase, hmg-coa synthase, MPPD, and FPP synthase. Journal of Lipid Research. 2000;41:1921–1935. [PubMed] [Google Scholar]
- Ortiz-Gomez A, Jimenez C, Estevez AM, Carrero-Lerida J, Ruiz-Perez LM, Gonzalez-Pacanowska D. Farnesyl diphosphate synthase is a cytosolic enzyme in Leishmania major promastigotes and its overexpression confers resistance to risedronate. Eukaryotic Cell. 2006;5:1057–1064. doi: 10.1128/EC.00034-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pena-Diaz J, Montalvetti A, Flores CL, Constan A, Hurtado-Guerrero R, De Souza W, Gancedo C, Ruiz-Perez LM, Gonzalez-Pacanowska D. Mitochondrial localization of the mevalonate pathway enzyme 3-Hydroxy-3-methyl-glutaryl-CoA reductase in the Trypanosomatidae. Molecular Biology of the Cell. 2004;15:1356–1363. doi: 10.1091/mbc.E03-10-0720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayyan W, Kuntz DA, Opperdoes FR, Hoet PP. Expression in Bacillus subtilis of the glycosomal glyceraldehyde-phosphate dehydrogenase gene from Trypanosoma brucei. Biochemie. 1993;74:137–141. doi: 10.1016/0300-9084(92)90037-f. [DOI] [PubMed] [Google Scholar]
- Sanmiya K, Ueno O, Matsuoka M, Yamamoto N. Localization of farnesyl diphosphate synthase in chloroplasts. Plant & Cell Physiology. 1999;40:348–354. doi: 10.1093/oxfordjournals.pcp.a029549. [DOI] [PubMed] [Google Scholar]
- Szajnman SH, Bailey BN, Docampo R, Rodriguez JB. Bisphosphonates derived from fatty acids are potent growth inhibitors of Trypanosoma cruzi. Bioorganic & Medicinal Chemistry Letters. 2001;11:789–792. doi: 10.1016/s0960-894x(01)00057-9. [DOI] [PubMed] [Google Scholar]
- Szajnman SH, Montalvetti A, Wang Y, Docampo R, Rodriguez JB. Bisphosphonates derived from fatty acids are potent inhibitors of Trypanosoma cruzi farnesyl pyrophosphate synthase. Bioorganic & Medicinal Chemistry Letters. 2003;13:3231–3235. doi: 10.1016/s0960-894x(03)00663-2. [DOI] [PubMed] [Google Scholar]
- Urbina JA. The phosphoenolpyruvate carboxykinase of Trypanosoma (Schizotrypanum) cruzi epimastigotes: molecular, kinetic, and regulatory properties. Archives of Biochemistry and Biophysics. 1987;258:186–195. doi: 10.1016/0003-9861(87)90335-3. [DOI] [PubMed] [Google Scholar]
- Urbina JA, Concepcion JL, Rangel S, Visbal G, Lira R. Squalene synthase as a chemotherapeutic target in Trypanosoma cruzi and Leishmania mexicana. Molecular and Biochemical Parasitology. 2002;125:35–45. doi: 10.1016/s0166-6851(02)00206-2. [DOI] [PubMed] [Google Scholar]
- Urbina JA, Moreno B, Vierkotter S, Oldfield E, Payares G, Sanoja C, Bailey BN, Yan W, Scott DA, Moreno SN, Docampo R. Trypanosoma cruzi contains major pyrophosphate stores, and its growth in vitro and in vivo is blocked by pyrophosphate analogs. The Journal of Biological Chemistry. 1999;274:33609–33615. doi: 10.1074/jbc.274.47.33609. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.