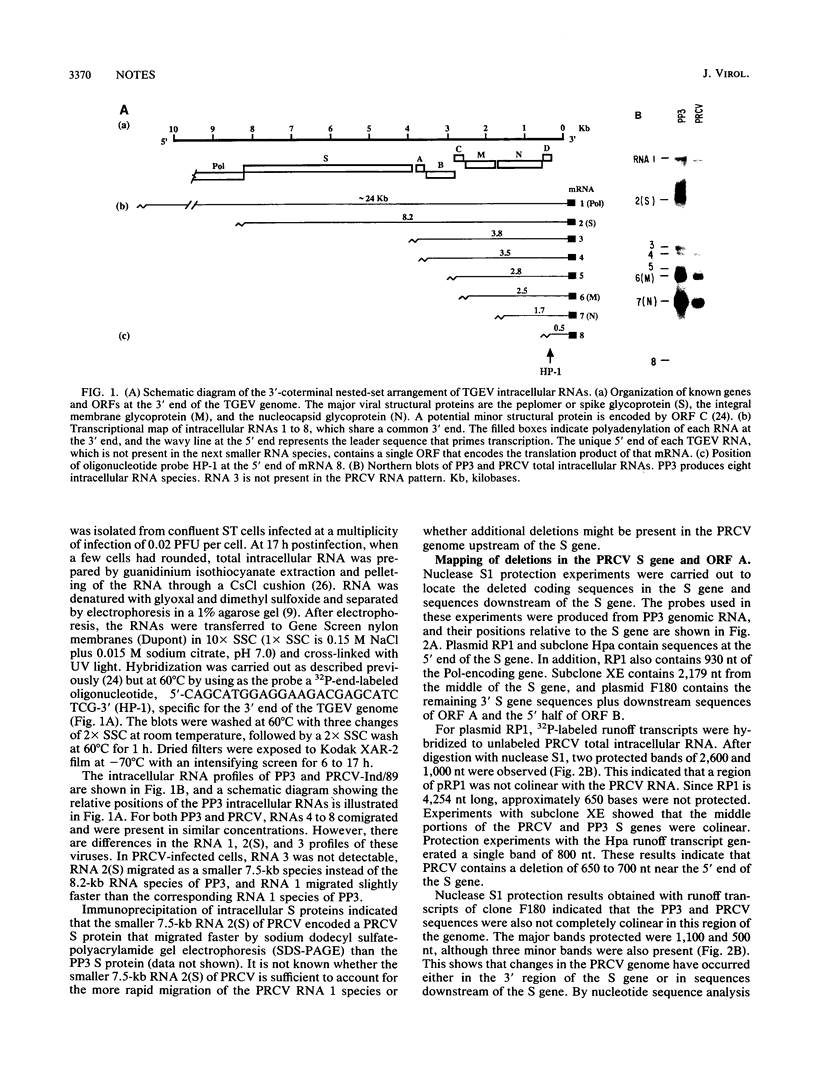
Abstract
The genome and transcriptional pattern of a newly identified respiratory variant of transmissible gastroenteritis virus were analyzed and compared with those of classical enterotropic transmissible gastroenteritis virus. The transcriptional patterns of the two viruses indicated that differences occurred in RNAs 1 and 2(S) and that RNA 3 was absent in the porcine respiratory coronavirus (PRCV) variant. The smaller RNA 2(S) of PRCV was due to a 681-nucleotide (nt) deletion after base 62 of the PRCV peplomer or spike (S) gene. The PRCV S gene still retained information for the 16-amino-acid signal peptide and the first 6 amino acid residues at the N terminus of the mature S protein, but the adjacent 227 residues were deleted. Two additional deletions (3 and 5 nt) were detected in the PRCV genome downstream of the S gene. The 3-nt deletion occurred in a noncoding region; however, the 5-nt deletion shortened the potential open reading frame A polypeptide from 72 to 53 amino acid residues. Significantly, a C-to-T substitution was detected in the last base position of the transcription recognition sequence upstream of open reading frame A, which rendered RNA 3 nondetectable in PRCV-infected cell cultures.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Britton P., Lopez Otin C., Martin Alonso J., Parra F. Sequence of the coding regions from the 3.0 kb and 3.9 kb mRNA. Subgenomic species from a virulent isolate of transmissible gastroenteritis virus. Arch Virol. 1989;105(3-4):165–178. doi: 10.1007/BF01311354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox E., Pensaert M. B., Callebaut P., van Deun K. Intestinal replication of a porcine respiratory coronavirus closely related antigenically to the enteric transmissible gastroenteritis virus. Vet Microbiol. 1990 Jun;23(1-4):237–243. doi: 10.1016/0378-1135(90)90154-N. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher T. M., Parker S. E., Buchmeier M. J. Neutralization-resistant variants of a neurotropic coronavirus are generated by deletions within the amino-terminal half of the spike glycoprotein. J Virol. 1990 Feb;64(2):731–741. doi: 10.1128/jvi.64.2.731-741.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garwes D. J., Stewart F., Britton P. The polypeptide of Mr 14,000 of porcine transmissible gastroenteritis virus: gene assignment and intracellular location. J Gen Virol. 1989 Sep;70(Pt 9):2495–2499. doi: 10.1099/0022-1317-70-9-2495. [DOI] [PubMed] [Google Scholar]
- Hattori M., Sakaki Y. Dideoxy sequencing method using denatured plasmid templates. Anal Biochem. 1986 Feb 1;152(2):232–238. doi: 10.1016/0003-2697(86)90403-3. [DOI] [PubMed] [Google Scholar]
- Hofmann M. A., Sethna P. B., Brian D. A. Bovine coronavirus mRNA replication continues throughout persistent infection in cell culture. J Virol. 1990 Sep;64(9):4108–4114. doi: 10.1128/jvi.64.9.4108-4114.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemeny L. J., Wiltsey V. L., Riley J. L. Upper respiratory infection of lactating sows with transmissible gastroenteritis virus following contact exposure to infected piglets. Cornell Vet. 1975 Jul;65(3):352–362. [PubMed] [Google Scholar]
- Lai M. M. Coronavirus: organization, replication and expression of genome. Annu Rev Microbiol. 1990;44:303–333. doi: 10.1146/annurev.mi.44.100190.001511. [DOI] [PubMed] [Google Scholar]
- McClurkin A. W., Norman J. O. Studies on transmissible gastroenteritis of swine. II. Selected characteristics of a cytopathogenic virus common to five isolates from transmissible gastroenteritis. Can J Comp Med Vet Sci. 1966 Jul;30(7):190–198. [PMC free article] [PubMed] [Google Scholar]
- Moon H. W. Mechanisms in the pathogenesis of diarrhea: a review. J Am Vet Med Assoc. 1978 Feb 15;172(4):443–448. [PubMed] [Google Scholar]
- Page K. W., Britton P., Boursnell M. E. Sequence analysis of the leader RNA of two porcine coronaviruses: transmissible gastroenteritis virus and porcine respiratory coronavirus. Virus Genes. 1990 Dec;4(4):289–301. doi: 10.1007/BF00570024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker S. E., Gallagher T. M., Buchmeier M. J. Sequence analysis reveals extensive polymorphism and evidence of deletions within the E2 glycoprotein gene of several strains of murine hepatitis virus. Virology. 1989 Dec;173(2):664–673. doi: 10.1016/0042-6822(89)90579-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pensaert M., Callebaut P., Vergote J. Isolation of a porcine respiratory, non-enteric coronavirus related to transmissible gastroenteritis. Vet Q. 1986 Jul;8(3):257–261. doi: 10.1080/01652176.1986.9694050. [DOI] [PubMed] [Google Scholar]
- Rasschaert D., Duarte M., Laude H. Porcine respiratory coronavirus differs from transmissible gastroenteritis virus by a few genomic deletions. J Gen Virol. 1990 Nov;71(Pt 11):2599–2607. doi: 10.1099/0022-1317-71-11-2599. [DOI] [PubMed] [Google Scholar]
- Rasschaert D., Gelfi J., Laude H. Enteric coronavirus TGEV: partial sequence of the genomic RNA, its organization and expression. Biochimie. 1987 Jun-Jul;69(6-7):591–600. doi: 10.1016/0300-9084(87)90178-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasschaert D., Laude H. The predicted primary structure of the peplomer protein E2 of the porcine coronavirus transmissible gastroenteritis virus. J Gen Virol. 1987 Jul;68(Pt 7):1883–1890. doi: 10.1099/0022-1317-68-7-1883. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethna P. B., Hofmann M. A., Brian D. A. Minus-strand copies of replicating coronavirus mRNAs contain antileaders. J Virol. 1991 Jan;65(1):320–325. doi: 10.1128/jvi.65.1.320-325.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sethna P. B., Hung S. L., Brian D. A. Coronavirus subgenomic minus-strand RNAs and the potential for mRNA replicons. Proc Natl Acad Sci U S A. 1989 Jul;86(14):5626–5630. doi: 10.1073/pnas.86.14.5626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siddell S. G., Anderson R., Cavanagh D., Fujiwara K., Klenk H. D., Macnaughton M. R., Pensaert M., Stohlman S. A., Sturman L., van der Zeijst B. A. Coronaviridae. Intervirology. 1983;20(4):181–189. doi: 10.1159/000149390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesley R. D., Cheung A. K., Michael D. D., Woods R. D. Nucleotide sequence of coronavirus TGEV genomic RNA: evidence for 3 mRNA species between the peplomer and matrix protein genes. Virus Res. 1989 Jun;13(2):87–100. doi: 10.1016/0168-1702(89)90008-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesley R. D. Nucleotide sequence of the E2-peplomer protein gene and partial nucleotide sequence of the upstream polymerase gene of transmissible gas gastroenteritis virus (Miller strain). Adv Exp Med Biol. 1990;276:301–306. doi: 10.1007/978-1-4684-5823-7_41. [DOI] [PubMed] [Google Scholar]
- Wesley R. D., Woods R. D., Cheung A. K. Genetic basis for the pathogenesis of transmissible gastroenteritis virus. J Virol. 1990 Oct;64(10):4761–4766. doi: 10.1128/jvi.64.10.4761-4766.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesley R. D., Woods R. D., Hill H. T., Biwer J. D. Evidence for a porcine respiratory coronavirus, antigenically similar to transmissible gastroenteritis virus, in the United States. J Vet Diagn Invest. 1990 Oct;2(4):312–317. doi: 10.1177/104063879000200411. [DOI] [PubMed] [Google Scholar]
- Wesley R. D., Woods R. D. Identification of a 17000 molecular weight antigenic polypeptide in transmissible gastroenteritis virus-infected cells. J Gen Virol. 1986 Jul;67(Pt 7):1419–1425. doi: 10.1099/0022-1317-67-7-1419. [DOI] [PubMed] [Google Scholar]