Abstract
Background
The authors define a DNA biobank as a repository of genetic information correlated with patient medical records. DNA biobanks may assist in the research and identification of genetic factors influencing disease and drug interactions, but may raise ethical issues. How healthcare providers perceive DNA biobanks is unknown.
Objectives
To determine how useful healthcare professionals believe DNA biobanks will be and whether these attitudes differ between private and socialized healthcare systems.
Design
The authors surveyed 200 healthcare professionals, including research and non-research focused doctors, nurses and other staff from medical centers and independent practice in both the United States and Scotland. The survey included fifteen items evaluated for general receptiveness toward biobanks, presumed usefulness of biobanks and perceived attitudes in recruiting patients for a biobank.
Measurements
A total of 81 (45%) of 179 eligible participants responded: 41 from the U.S. and 40 from Scotland. Of these respondents, most (70%) were from academic centers.
Results
Results indicate that there is a broadly favorable attitude in both locations toward the creation of a DNA biobank (83%) and its perceived benefit (75%). This enthusiasm is tempered in Scotland when respondents evaluated their comfort in consenting patients for entry into a biobank; 16 of 40 respondents (40%) were uncomfortable doing so, representing a significant difference from those in the U.S. (p=0.001).
Conclusions
Despite systematic differences in healthcare practice between the U.S. and Scotland, health care professionals in both nations believe DNA biobanks will be useful in curing disease. This finding appears to support further development of such a research tool.
Introduction
Before completion of the Human Genome Project in 2003, academic medical centers and government health agencies already planned to create large biobanks of patient DNA. 1,2 A main motivating factor in creating such biobanks is their ability to correlate small differences in genetic variability with phenotypic changes. In 1998 Britain's Wellcome Trust was charged with creating a nationwide DNA collection called UK Biobank, and in 2002 the Center for Genetic Medicine at Northwestern University began recruiting patients for NUgene. Data from the NUgene project has facilitated an on-going, but unpublished, study investigating abdominal aortic aneurysm. 3 Other database efforts focused on making the results of genotype/phenotype studies available for broader use. For example, the National Center for Biomedical Informatics' database of Genotype and Phenotype (dbGaP) is intended as a widely-accessible archive to facilitate research that may yield commercial products or further public health. 4
Recent designs for biobank recruitment rely on the use of “leftover” blood from routine lab tests. 5 Inherent in the large-scale collection of such genetic information are ethical, legal and technical considerations. 6 These include using a consent process that will facilitate testing future hypotheses without re-contacting or re-consenting donors every time a new protocol is conducted. Another concern relates to the ownership of genetic information and the question of who should deserve benefits from discoveries made through biobank research. 7 Other issues relate to the possible identification of sample donors and ensuring privacy of DNA through the use of de-identifying technology. 8
The expanding interest in DNA biobanks stems from their potential to assist researchers in conducting large, population-based studies investigating the genetic, lifestyle and environmental factors associated with common disease and drug response. 9 Effective identification of causation, or even correlation, between genetic sequences and clinical manifestation requires the recognition of candidate genes. Recent technologic breakthroughs making this process easier, more affordable and more accessible have led to increasing numbers of targets for both monogenic and polygenic disease. 10
The concepts of genotype-phenotype correlation and genome-wide association are extensions of the premise that specific inherited genetic sequences can cause or, in concert with lifestyle factors, predispose individuals toward disease. Classical evaluation of SNP sequences and more recent phylogenetic studies aim to help identify putative genes involved in disease. 11 Other tools invoke genome-wide association to interrogate human genetic sequences vis-à-vis a sophisticated map of genetic variation. This model is therefore aided by the collection of large volumes of DNA samples along with the well-defined phenotypic and clinical characteristics. 12 In this way, these concepts promise to help identify candidate and later specific genetic sequences that may be responsible for phenotypic and pathologic manifestations.
Public opinion on issues surrounding medical biobanks such as confidentiality, informed consent and ownership of information has also been evaluated. 13 Much of the concern surrounding confidentiality relates to maintaining the anonymity of donors and making sure insurers and employers do not have access to any findings. 14 Public opinion has favored strictly authorizing fresh consent before any new research is conducted, even on existing patient genetic information and medical records. 15 Another sentiment held by the public is that ownership of DNA biobanks should exclude commercial interests. 15 The attitudes of medical professionals have not been characterized. 16
To determine how healthcare professionals viewed these concerns and the DNA biobank concept in general, the authors conducted a survey of research and non-research focused physicians, nurses and ancillary staff from separate medical centers in both the United States and Scotland in order to understand their views on the usefulness of such biobanks. The authors hypothesized that there would be a generally positive attitude toward creating a DNA biobank among both groups of professionals in both countries. Due to the nationalized system of health care delivery in Scotland, in which patients are entitled to care and an unbiased collective notion of coverage exists, the authors believed perceptions of DNA biobanks would differ with respect to the privatized healthcare environment that remains in the United States.
Methods
Subjects and Settings
The study population consisted of 200 healthcare professionals including doctors, nurses and technicians from the United States and Scotland. The U.S. cohort included 100 participants randomly selected from among those practicing in the Nashville, TN area: 50 randomly selected from a list of area private practices, and 50 from the Vanderbilt University Medical Center directory. This group consisted of 70% doctors, 20% nurses and 10% ancillary technical staff. The Scottish cohort also had 100 subjects randomly selected from practitioners located in the Dundee area. Software from www.graphpad.com was used to generate a list of random numbers to help select participants in both locations. The subject sample was distributed in this way to obtain a cross-section of opinions form those most likely to be utilizing or recruiting patients into a DNA biobank. In Scotland, there are no dedicated private practitioners because of the government-operated National Health Service. Participants were selected from either general practices in the Dundee area or from the Tayside Hospital associated with the University of Dundee Medical School; this group approximates the “academic” and “private” dichotomy found in the United States sample. Due to practical concerns about contacting non-physician health professionals outside the hospital, only doctors from general practices were contacted. The overall cohort included 35 physicians working in general practice and 50 physicians, 10 nurses, and 5 ancillary technical staff from the hospital.
Survey Design
In 2000 Vanderbilt University conducted two focus groups with members of the general public to investigate potential concerns associated with creating a DNA biobank. 17 The main areas identified related to issues of privacy, benefit to the donor and access to knowledge and results from research. These areas were therefore considered categories of concern and were used to create novel questions included in the survey of health professionals. These survey items were ranked by participants on a 5-point Likert-like scale that ranged from “Strongly Agree” to “Strongly Disagree.” Additional questions probed receptiveness toward creating a biobank and rating the concept of collecting samples for the biobank, e.g., using “spare blood.” Other items on the survey asked whether such biobanks would be useful in the help to cure disease and whether the respondent would use information from the biobank in their practice. Further questions dealt with consenting patients to participate in the biobank project.
A packet including a cover letter and a 1-page survey was mailed to all 200 participants. During pilot testing the questionnaire was found to take approximately 5 minutes to complete. Participants were asked to enter demographic data and complete the 1-page survey that contained 15 questions in the U.S. and 16 in Scotland, covering three main areas, namely receptiveness toward a “spare blood” DNA biobank, concerns related to privacy and autonomy and perceived attitude of patients (▶). The survey was distributed and completed in 2006. The additional question in the Scottish survey was included to query whether participants would be “willing to spend 5 minutes consenting a patient.” This item was added to clarify logistical concerns that may prevent health care providers from discussing DNA biobank participation with a patient, but was not included in the ultimate statistical analysis. One follow-up mailing was sent to all non-respondents in both locations.
Table 1.
Table 1 Survey Results by Question
| Item | US Response | Scottish Response | Significance | |
|---|---|---|---|---|
| 1. | I am receptive to the idea of creating a database of DNA. (n=79) | 4.35 | 3.70 | p<0.001 |
| 2. | Using “spare” or extra blood is a good way to obtain DNA. (n=80) | 4.24 | 3.90 | p=0.115 |
| 3. | I would be likely to use information from such a DNA database in my practice. (n=79) | 3.41 | 2.85 | p=0.034 |
| 4. | I would feel comfortable consenting a patient for a “spare blood” DNA sample. (n=80) | 3.65 | 2.93 | p<0.001 |
| 5. | A DNA database would be beneficial in providing information needed in the work to cure disease. (n=80) | 4.46 | 3.80 | p<0.001 |
| 6. | Some populations would be more likely to benefit from this project than others. (n=79) | 3.58 | 3.80 | p=0.921 |
| 7. | The database would not be secure. (n=79) | 2.32 | 2.85 | p=0.116 |
| 8. | Information from a DNA database might be sold even if the creators say the data is private. (n=78) | 2.75 | 2.95 | p=0.327 |
| 9. | Participants' genetic information might be used for unintended purposes, e.g., notification of their employer. (n=79) | 2.46 | 2.69 | p=0.426 |
| 10. | Participants should be alerted if a potential cure were developed for their disease. (n=80) | 4.15 | 3.75 | p=0.082 |
| 11. | It is important that patient participants receive a portion of any profits that may result from advances made using the DNA database. (n=80) | 2.37 | 2.23 | p=0.642 |
| 12. | Patients should be able to know the results of their DNA test. (n=80) | 4.51 | 3.25 | p=0.194 |
| 13. | Patients would feel coerced to donate a DNA sample. (n=80) | 2.24 | 2.70 | p=0.027 |
| 14. | Patients would be able to understand the reasons for donating a DNA sample. (n=80) | 3.49 | 3.18 | p=0.194 |
| 15. | Patients will be likely to donate a DNA sample. (n=80) | 3.46 | 3.25 | p=0.191 |
| 16. | I would be willing to spend 5 minutes consenting a patient for a “spare blood” DNA sample. (n = 40) | N/A | 2.26 | N/A ∗ |
∗ No statistical comparison between locations was completed for item #16.
Numerical values are weighted average response where 5.0 represents “Strongly Agree” and 0 represents “Strongly Disagree.” Significance indicates difference in responses between study locations.
Data Analysis
Participant responses were returned via mail and sorted by location. Descriptive statistics were generated. Differences in categorical variables were determined using Exact testing of contingency tables. To determine whether survey responses differed on the basis of respondent location, gender or age category, the authors performed T testing, applying a Bonferroni adjustment to the alpha value (i.e., the likelihood of committing a type I error when testing the hypothesis that there is a difference in the survey response based on whether the respondent was from the United States or from Scotland) to account for multiple testing, leading to a P value of less than 0.003 indicating significance. The authors performed multivariate logistic regression to account for the effect of location, gender and categories of age on survey responses. To determine whether responses were impacted by end-aversion (i.e., that respondents in one location were more or less likely to score survey items towards a neutral response), the authors performed T testing to compare the likelihood that scaled responses were more likely to be 1 or 5 versus 2 or 4 on the 5-point scale, by location. All statistical analyses were performed using Stata SE software, version 8.0.
Results
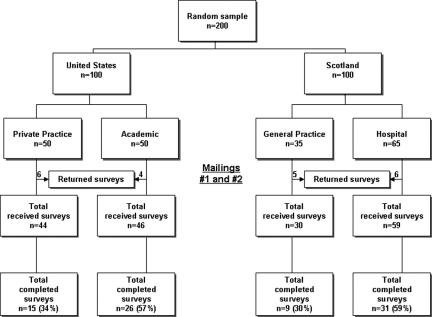
From an initial pool of 200 potential participants, 21 were excluded from the study because they had retired (9 subjects), their listed address was incorrect (5 subjects), they believed the survey would take too long to complete (3 subjects), were not interested in the subject matter of the study (2 subjects) or did not meet inclusion criteria to participate in the study because they were already participating in DNA biobank-related research (2 subjects) (▶). The response rate for subjects from the United States was 46% (41/90) and from those in Scotland it was 45% (40/89) (P=0.533). In the United States 10 surveys were returned incomplete; reasons cited for non-response included 5 potential subjects had either left work or retired, 4 addresses were incorrect and 1 did not meet entrance requirements because of concurrent participation in another DNA biobank project. In Scotland, 11 surveys were returned incomplete for reasons including 4 had either left work or retired, 3 believed the survey would take too long to complete, 2 reported lack of interest in the subject, 1 address was incorrect and 1 person was excluded. Of the remaining 179 potential subjects, the overall response rate among both locations was 45% (81/179). The initial mailing in the United States yielded 22 completed surveys with 8 coming from private practice and 14 from professionals working in an academic environment. After the follow-up mailing 19 more surveys were returned complete. In Scotland, the first mailing yielded 27 completed surveys and another 13 were returned after the follow-up.
Figure 1.
Study recruitment flowchart.
Survey results are presented in ▶. Respondents generally agreed with the statements “I am receptive to the idea of creating a database of DNA,” “Using ‘spare’ or extra blood is a good way to obtain DNA,” “A DNA database would be beneficial in providing information needed in the work to cure disease,” “Some populations would be more likely to benefit from this project than others,” “Participants should be alerted if a potential cure were developed for their disease,” “Patients would be able to understand the reasons for donating a DNA sample,” “Patients will be likely to donate a DNA sample.”
Respondents generally disagreed with the statements “The database would not be secure,” “Information from a DNA database might be sold even if the creators say the data is private,” “Participants genetic information might be used for unintended purposes, e.g., notification of their employer,” “It is important that patients participants received a portion of any profits that may result from advances made using the DNA database,” “Patients would feel coerced to donate a DNA sample.”
Despite the overall positive attitude toward some of the questions, three revealed a significant difference between locations. Item 1, “I am receptive to the idea of creating a database of DNA,” (P=0.001), item 4, “I would feel comfortable consenting a patient for a ‘spare’ blood DNA sample,” (P=0.001) and item 5, “A DNA database would be beneficial in providing information needed in the work to cure ease,” (P=0.001) had significantly different responses by location. Overall, the rate of respondents avoiding extreme values did not differ between the locations, with 20% avoiding the extremes in Scotland and 17% in the U.S. (P=0.223).
Discussion
The authors found that a majority of health professionals in both the U.S. and Scotland favored the creation and use of a DNA biobank in the work to cure disease. This response lends support for the development of such a research tool. Despite this apparent enthusiasm, it is important to know whether health care professionals believe they would use the biobank and whether they believe patients would agree to donate a DNA sample. These questions deal with both ethical and practical concerns and will factor significantly in the ultimate success of recruiting and sustaining such a program. These issues relate to feelings about biobanks in general, and specifically to concerns about security of information and perceived utility for patients. Previous research dealt with these issues in the general public and we aimed to qualify them among healthcare professionals.
While an overall majority of respondents were supportive of the creation of a DNA biobank, there was a difference in attitude between locations. Although different, the responses are distinguished not by a diverging overall outlook, but rather in a more subtle way by degree of favorability (“Strongly agree” versus “Agree”). End-aversion differences were not significant in general, but they were seen in this particular question. Earlier studies show that among the most significant concerns patients and researches have about biobanks are those that relate to privacy. 13,18 Our study indicates health professionals do not share this concern. This result may be predicted in Scotland, where the NHS guarantees care and fears of consequences from a third party (e.g., insurers) would be minimal. A less expected result was for American responses to be similar to the Scottish. This dichotomy between those hoping to use the information and those donating it may ultimately inhibit efforts for researchers to maximize recruitment and the utility of any biobank. A considerable effort to alleviate fears the public may have will be necessary to avoid this conflict. It should also be noted that while the views expressed by the public and in the survey results represent current relevance, the fields in question are evolving and fast moving. It is possible therefore that these attitudes will change in the future and this may justify subsequent quantification.
Any biobank design will necessarily balance the implications of ensuring total privacy with the cost of re-identifying patients and of treating an individual versus the community. One trade-off in designing a system that ensures privacy, by automatically removing a patient's medical record number when linking a history with the corresponding DNA information, is losing the ability to re-contact that patient in the future. Likewise any patient with an at-risk genotype, e.g., BRCA-1 and BRCA-2, could not be notified directly. 8 Such privacy also precludes reimbursing specific donors for any discoveries made from their DNA. While some incentive is often associated with increasing participation in medical research, a purely altruistic DNA biobank enrollment makes this a more difficult proposition. This also may prove to be a hurdle in recruitment.
Another option may be to create various consent processes aimed at addressing the concerns of potential donors and health care professionals alike. In this schema, those participants not interested in continuous re-consenting could sign a blanket form, while others who wish to be contacted about results or discoveries would be. Ultimately, this model might become a separate arm of a future DNA biobank or even morph into a genetic counseling endeavor. Such possibilities will need to be addressed prior to the design of future biobanks.
Beyond concerns to protect individual donors, other practical issues remain. One way to reduce the consenting burden includes using “spare” blood. In this scenario, blood that has already been drawn as part of a hospital visit is designated for analysis. The strong support in both study locations for this indicates it may be a feasible way to enhance donor enrollment while minimizing intrusion into the existing workflow.
When asked whether they would feel comfortable consenting a patient, respondents were no longer uniformly positive. In this case, respondents in America were more likely to be willing to consent patients than their Scottish peers. While initial assessment might suggest that Scottish providers are contradicting their enthusiasm for creating a DNA biobank, closer examination indicates this is unlikely the case. Rather, the real differences in healthcare system structure between the United States and Scotland may be playing a role.
The majority of physicians in Britain work for the National Health Service exclusively, while most of their American counterparts are privately employed. 19 The per capita money spent on healthcare and accessibility to services varies as well. Americans spend more on healthcare than the British; while all citizens in the U.K. are guaranteed access to healthcare services, only a fraction of U.S. patients share this status. 20 Scottish doctors, meanwhile, to an even greater degree than their American peers, face time constraints when seeing patients. 20 While many U.S. practices are expected to see patients in 12–15 minutes, Scottish doctors are expected to perform the same visit in 7–10 minutes. The additional burden of consenting, or even explaining a biobank project, may be an overwhelming challenge to integrate into the existing workflow.
A further possibility rests on the additional question asked of Scottish participants, i.e., willingness to spend “five minutes” consenting patients. Participants responded negatively toward this question in what may have been a further clarification of their clinical time constraints. This phrase may have the potential to bias respondents' answers based on a preconceived notion of the time necessary for consenting. Specifically, it may be implicated in creating a more negative view toward consent relative to Americans. However, this item was purposely placed last on the Scottish survey so that respondents would interact with all other questions in a manner as nearly equivalent to their American counterparts as possible. Although it is conceivable participants would have read the entire questionnaire before marking their answers, the authors believe this scenario would be unusual. However, a formal correlation analysis might be able to further clarify this relationship, though the authors do not believe this study has enough statistical power to do this. Evaluating this relationship in greater detail may represent an important consideration for future work.
It is likely that in both the U.S. and Scottish models conventional approaches to obtain consent will prove infeasible. Traditional consent procedures require researchers to contact participants each time a new investigation is undertaken with the same existing information. Trying to employ such a process in the context of a DNA biobank would prove cumbersome and intrusive to both researcher and participant, while also stifling a project's mandate to facilitate a broad range of research topics using the same data. It may also introduce a selection bias into the participant population and decrease the scientific validity of the study. 21 A more effective approach is a one-time, blanket consent completed with other paperwork patients fill out during a physician visit. This consent would also include an opt-out, rather than opt-in, clause allowing unfettered use of DNA samples for all future research. 7,22
Conclusion
Despite systematic differences in healthcare practice between America and Scotland, the fact that health care professionals in both nations believe a DNA biobank will be useful in curing disease appears to support the investment needed to develop such a research tool. Future challenges will focus on incorporating biobank recruitment and discussion into current work flow. Researchers will also need to be aware that patients' fears of losing privacy may affect their willingness to donate a DNA sample.
Footnotes
The project was supported in part by a Grant from the United States National Library of Medicine (Rosenbloom, 5K22 LM008576-02) and by the Vanderbilt University School of Medicine.
References
- 1.Mitka M. Banking (on) genes: DNA sought as key to disease causes and cures JAMA 2002;288(23):2951-2952. [DOI] [PubMed] [Google Scholar]
- 2.Funded volunteer gene bank Chemistry and Industry6 May:4 2002.
- 3.Crown E. Aneurysm Study Uses Gene Bank Samples. Northwestern News 28 September, 2004.
- 4.National Center for Biomedical Informaticshttp://www.ncbi.nlm.nih.gov/entrez/query/Gap/gap_tmpl/about.html 2002. Accessed Jan 12, 2008.
- 5.Pinto C. VU to put patient DNA in vast research pool: Blood samples included unless people opt outThe Tennessean. 2006; 2006. 20 June.
- 6.Caulfield T, Upshur RE, Daar A. DNA databanks and consent: a suggested policy option involving an authorization model BMC Med Ethics 2003;4:E1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wendler D. One-time general consent for research on biological samples BMJ 2006;332(7540):544-547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Malin B, Sweeney L. Determining the identifiability of DNA database entries Proc AMIA Symp 2000:537-541. [PMC free article] [PubMed]
- 9.Collins FS, Green ED, Guttmacher AE, Guyer MS. A vision for the future of genomics research Nature 2003;422(6934):835-847. [DOI] [PubMed] [Google Scholar]
- 10.Chanock SJ, Manolio T, Boehnke M, Boerwinkle E, Hunter DJ, et al. NCI-NHGRI Working Group on Replication in Association Studies Replicating genotype–phenotype associations: What constitutes replication of a genotype–phenotype association, and how best can it be achieved? Nature 2007;447(7):655-660. [DOI] [PubMed] [Google Scholar]
- 11.Habib F, Johnson AD, Bundschuh R, Janies D. Large scale genotype–phenotype correlation analysis based on phylogenetic trees Bioinform 2007;23(7):785-788. [DOI] [PubMed] [Google Scholar]
- 12.Christensen KaJCM. What genome-wide association studies can do for medicine New Engl J Med 2007;356(11):1094-1097. [DOI] [PubMed] [Google Scholar]
- 13.Einsiedel E. Whose Genes, Whose Safe, How Safe?. Publics' and Professionals' Views of Biobanks. Ottawa: Canadian Biotechnology Advisory Committee; 2003. March.
- 14. Canadian perceptions of health information confidentiality. Canadian Medical Association/Angus Reid Survey; 1999.
- 15. Report to the Human Genetics Commission on public attitudes to the uses of human genetic informationLondon, UK: Human Genetics Commission; 2000.
- 16.Hapgood RDS, Alastair Kent. Consultation with primary care health professionals on issues relating to the recruitment of patients to a DNA collection studyLondon: Wellcome Trust; 2001. 31 January.
- 17.Lorenzi NM. Transforming health care through information2nd ed.. New York, London: Springer; 2004.
- 18.Clark AaF I. Attaining adequate consent for the use of electronic patient records: An opt-out strategy to reconcile individuals' rights and public benefit J Royal Inst Pub Health 2005;119(11):1003-1010. [DOI] [PubMed] [Google Scholar]
- 19.Banks J, Marmot M, Oldfield Z, Smith JP. Disease and Disadvantage in the United States and in England JAMA 2006;295(17):2037-2045. [DOI] [PubMed] [Google Scholar]
- 20.Simon C. Oxford handbook of general practiceOxford: Oxford University Press; 2002.
- 21.Hansson MG, Dillner J, Bartram CR, Carlson JA, Helgesson G. Should donors be allowed to give broad consent to future biobank research? Lancet Oncol 2006;7(3):266-269. [DOI] [PubMed] [Google Scholar]
- 22.Maschke KJ. Alternative consent approaches for biobank research. In The Lancet Oncology; 2006. Letter responding to article by M. Hansson. [DOI] [PubMed]