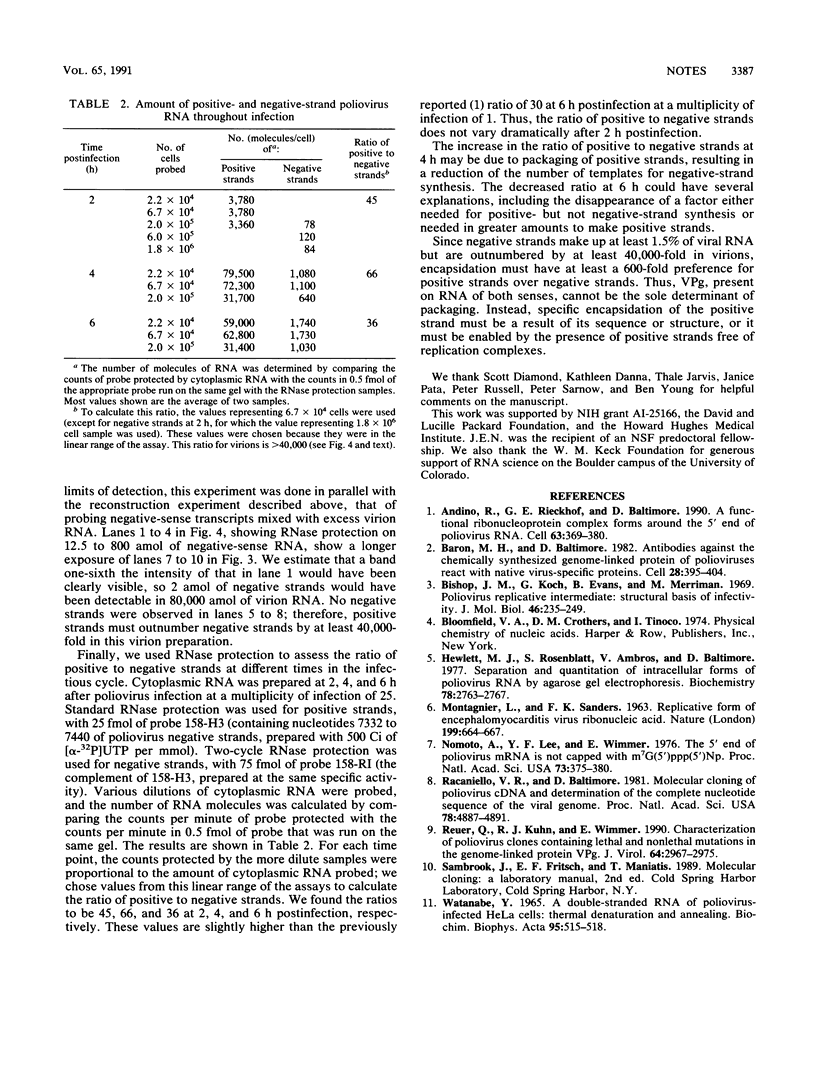
Abstract
We have developed a ribonuclease protection method suitable for sensitive detection of an RNA species in the presence of a large excess of its complementary strand, as for the detection of negative strands of positive-strand RNA viruses. By using this method to probe for poliovirus negative strands in virions, we found that positive strands are present in at least 40,000-fold excess over negative strands. Thus, we have confirmed that poliovirus encapsidation is highly specific for positive strands and have demonstrated that the genome-linked protein VPg, which is covalently attached to the 5' ends of both positive and negative strands, cannot be the sole determinant of RNA packaging. We tested the ratios of viral positive strands to negative strands in cells at different times during infection; this value ranged from approximately 40/1 to 70/1, being highest at 4 h and lower at 2 and 6 h postinfection.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andino R., Rieckhof G. E., Baltimore D. A functional ribonucleoprotein complex forms around the 5' end of poliovirus RNA. Cell. 1990 Oct 19;63(2):369–380. doi: 10.1016/0092-8674(90)90170-j. [DOI] [PubMed] [Google Scholar]
- Baron M. H., Baltimore D. Antibodies against the chemically synthesized genome-linked protein of poliovirus react with native virus-specific proteins. Cell. 1982 Feb;28(2):395–404. doi: 10.1016/0092-8674(82)90357-9. [DOI] [PubMed] [Google Scholar]
- Bishop J. M., Koch G., Evans B., Merriman M. Poliovirus replicative intermediate: structural basis of infectivity. J Mol Biol. 1969 Dec 14;46(2):235–249. doi: 10.1016/0022-2836(69)90419-7. [DOI] [PubMed] [Google Scholar]
- Hewlett M. J., Rozenblatt S., Ambros V., Baltimore D. Separation and quantitation of intracellular forms of poliovirus RNA by agarose gel electrophoresis. Biochemistry. 1977 Jun 14;16(12):2763–2767. doi: 10.1021/bi00631a027. [DOI] [PubMed] [Google Scholar]
- MONTAGNIER L., SANDERS F. K. REPLICATIVE FORM OF ENCEPHALOMYOCARDITIS VIRUS RIBONUCLEIC ACID. Nature. 1963 Aug 17;199:664–667. doi: 10.1038/199664a0. [DOI] [PubMed] [Google Scholar]
- Nomoto A., Lee Y. F., Wimmer E. The 5' end of poliovirus mRNA is not capped with m7G(5')ppp(5')Np. Proc Natl Acad Sci U S A. 1976 Feb;73(2):375–380. doi: 10.1073/pnas.73.2.375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racaniello V. R., Baltimore D. Molecular cloning of poliovirus cDNA and determination of the complete nucleotide sequence of the viral genome. Proc Natl Acad Sci U S A. 1981 Aug;78(8):4887–4891. doi: 10.1073/pnas.78.8.4887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuer Q., Kuhn R. J., Wimmer E. Characterization of poliovirus clones containing lethal and nonlethal mutations in the genome-linked protein VPg. J Virol. 1990 Jun;64(6):2967–2975. doi: 10.1128/jvi.64.6.2967-2975.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATANABE Y. A DOUBLE-STRANDED RNA OF POLIOVIRUS-INFECTED HELA CELLS: THERMAL DENATURATION AND ANNEALING. Biochim Biophys Acta. 1965 Mar 15;95:515–518. doi: 10.1016/0005-2787(65)90200-5. [DOI] [PubMed] [Google Scholar]