Abstract
A significant decline in executive system function mediated by the prefrontal cortex (PFC) often occurs with normal aging. In vitro slice studies have shown that layer 2/3 pyramidal cells in the monkey PFC exhibit increased action potential (AP) firing rates which may, in part, contribute to this decline. Given that layer 5 cells also play a role in executive system function, it is important to determine if similar age-related changes occur in these cells. Whole-cell patch-clamp recordings in in vitro slices prepared from the PFC of young and aged behaviorally characterized rhesus monkeys were employed to answer this question. Basic membrane and repetitive AP firing properties were unaltered with age. Aged cells exhibited significantly decreased single AP amplitude, duration and fall time and increased slow afterhyperpolarization (sAHP) amplitude, but these changes were not associated with cognitive performance. This study demonstrates that layer 5 pyramidal cells, unlike layer 2/3 pyramidal cells, undergo only modest electrophysiological changes with aging, and that these changes are unlikely to contribute to age-related cognitive decline.
Keywords: in vitro slice, whole cell patch clamp, prefrontal cortex, normal aging, cognitive performance
The prefrontal cortex (PFC) mediates executive function domains such as working memory, cognitive flexibility, and set shifting (review: Fuster, 1997; Constantinidis and Procyk, 2004). Layer 2/3 cortico-cortical connections and a closed loop layer 5 cortico-striatal–thalamic– cortical circuit are both involved in the mediation of these functions, which are essential for normal daily living (for review: Alexander et al., 1986; Owen, 1997; Cavada et al., 2000). The process of normal, non-pathological aging is often accompanied by a significant decline in executive function in humans (review: Albert and Moss, 1999) and monkeys (review: Moss et al., 1999). Since changes in the action potential (AP) firing rates of pyramidal cells in the PFC are directly associated with transitions between different epochs of PFC-dependent cognitive tasks (for review: Goldman-Rakic, 1995; Fuster, 1997; Owen, 1997; Cavada et al., 2000), it is reasonable to postulate that perturbations in the electrophysiological properties of these neurons, such as AP firing rates, may contribute to cognitive deficits in senescence. Indeed, age-related alterations in firing rates have been reported. For example, in vivo single unit recordings of supragranular cells in the visual cortex (Schmolesky et al., 2000; Leventhal et al., 2003) and in vitro whole cell patch clamp recordings of layer 2/3 pyramidal cells in the PFC (Chang et al., 2005) demonstrate that neurons from aged monkeys exhibit significantly increased AP firing rates compared with those from young monkeys. In the aged monkey visual cortex in vivo, increased AP firing rates are associated with decreased stimulus selectivity (Schmolesky et al., 2000), and the increased AP firing rates of layer 2/3 pyramidal cells in in vitro PFC slices are associated with degree of cognitive impairment in aged monkeys (Chang et al., 2005). Wilson and coworkers (2005) have also demonstrated that the firing rates of CA3 pyramidal cells recorded in freely behaving rats are significantly increased with age, resulting in a failure to rapidly encode new spatial information. Taken together, these studies indicate that principal cells in a number of brain areas exhibit significantly altered AP firing properties with age, which may, at least in part, underlie age-associated cognitive decline.
Very little is known about the effects of age on the basic electrophysiological properties of layer 5 pyramidal cells. Given the importance of AP firing rates in the normal modulation of cognition, and the importance of the cortico-striatal–thalamic–cortical circuit in the modulation of executive functions, the current study was undertaken to determine whether there are significant alterations in the basic electrophysiological properties of neurons in layer 5 from the PFC of aged, cognitively characterized rhesus monkeys.
EXPERIMENTAL PROCEDURES
Experimental subjects
Seven young (6−12 years old) and eight aged (20−29 years old) rhesus monkeys (Macaca mulatta) were obtained from the Yerkes National Primate Research Center at Emory University (Atlanta, GA, USA) and then housed at the Boston University Laboratory Animal Science Center (LASC) in strict accordance with animal care guidelines as outlined in the NIH Guide for the Care and Use of Laboratory Animals and the U.S. Public Health Service Policy on Humane Care and Use of Laboratory Animals (Table 1). Both the Boston University LASC and the Yerkes Center are fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care and all procedures were approved by the Institutional Animal Care and Use Committees (IACUC) of both institutions. Every effort was made to minimize the number of animals used and their suffering.
Table 1.
Experimental subjects
| Monkey | Age (y) | Sex | DNMS basic | DNMS delay | DRST spatial |
|---|---|---|---|---|---|
| Young |
|
|
|
|
|
| AM188 | 6 | F | 64 | 0.83 | 2.76 |
| AM204 | 6 | M | 85 | 0.87 | 3.23 |
| AM198 | 7 | F | 104 | 0.78 | 3.71 |
| AM205 | 7.3 | M | 54 | 0.80 | 3.24 |
| AM199 | 10 | F | 168 | 0.79 | 3.01 |
| AM194 | 11.9 | F | 156 | 0.74 | 2.43 |
| AM195 | 12 | F | 56 | 0.83 | 3.07 |
| Mean | 8.6 | 98.1 | 0.81 | 3.06 | |
| SD | 2.7 | 47.1 | 0.04 | 0.40 | |
| Aged |
|
|
|
|
|
| AM190 | 20 | F | 149 | 0.81 | 2.33 |
| AM177 | 20.7 | F | 518 | 0.69 | 2.37 |
| AM162 | 22.3 | F | 163 | 0.79 | 2.03 |
| AM165 | 22.7 | M | 227 | 0.78 | 2.9 |
| AM179 | 23.8 | F | 505 | 0.69 | 1.84 |
| AM189 | 24.5 | M | 217 | 0.79 | 2.06 |
| AM181 | 27 | F | 792 | 0.71 | 2.45 |
| AM180 | 29 | F | 242 | 0.75 | 1.98 |
| Mean | 23.8 | 351.6* | 0.75+ | 2.25** | |
| SD | 3.1 | 229.1 | 0.05 | 0.34 |
DNMS basic, total number of errors; DNMS delay, % correct; DRST, average total span.
P<0.04.
P<0.01.
P<0.001.
Assessment of cognitive function
All animals completed a battery of cognitive tasks that assessed learning and working memory functions. Monkeys were tested on the following tasks: delayed non-match to sample (DNMS) basic (acquisition), DNMS with a delay period of 2 min, and the spatial modality of the delayed recognition span task (DRST). For a detailed description of implementation and assessment of performance on these tasks, see Herndon et al. (1997). Significant impairment on a task was defined as: >200 errors for DNMS basic, <78% correct for DNMS 2 min delay, and a span of <2.5 for DRST (Herndon et al., 1997).
Preparation of slices
Coronal brain slices were obtained from monkeys perfused as part of other on going studies. Monkeys were tranquilized with ketamine (10 mg/ml) and then deeply anesthetized with sodium pentobarbital (to effect 15 mg/kg, i.v.). While under deep anesthesia, monkeys were thoracotomized and craniotomies performed. Ten millimeter thick blocks of the PFC (area 46) containing both the upper and lower banks of the sulcus principalis were obtained by biopsy. Following the biopsy monkeys were killed by exsanguination with a 4% paraformaldehyde solution perfused through the ascending aorta. Coronal 400 μm thick slices were cut on a vibrating microtome, placed in 26 °C oxygenated (95% O2/5% CO2) Ringer's solution (concentrations in mM: 26 NaHCO3, 124 NaCl, 2 KCl, 3 KH2PO4, 10 glucose, 2.5 CaCl2, 1.3 MgCl2; pH=7.4, chemicals obtained from Sigma, St. Louis, MO, USA). Slices were then allowed to equilibrate for at least 1 h prior to use, and slices remained viable for up to 12 h. The time from the beginning of the perfusion of the monkey to obtaining slices of PFC was approximately 10−15 min. At the time of recording, a single slice was positioned under a nylon mesh in a submersion type slice-recording chamber (Harvard Apparatus, Holliston, MA, USA) and constantly superfused with 26 °C, oxygenated Ringer's solution at a rate of 2−2.5 ml per minute.
Whole cell patch clamp recordings
Layer 5 pyramidal cells in the lower bank of the sulcus principalis were visually identified with a Nikon E600 infrared-differential interference contrast (IR-DIC) microscope (Micro Video Instruments, Avon, MA, USA). Standard, tight-seal whole-cell patch clamp recordings (Luebke and Rosene, 2003; Luebke et al., 2004; Chang et al., 2005) were made with electrodes fabricated on a Flaming and Brown horizontal micropipette puller (Model P-87, Sutter Instruments, Novato, CA, USA) from nonheparinized microhematocrit capillary tubes (World Precision Instruments, Sarasota, FL, USA). Recording pipettes were filled with a potassium aspartate (KAsp) internal solution containing (in mM): 100 KAsp, 15 KCl, 3 MgCl2, 5 EGTA, 10 Na–Hepes, 0.3 NaGTP and 2 MgATP (pH=7.4, chemicals obtained from Fluka, NY, USA). The electrodes had a final resistance of 3−6 Mohm in Ringer's solution. Experiments were performed with List EPC-7 or EPC-9 patch clamp amplifiers and “Pulse” acquisition software from HEKA Electronik (Lambrecht, Germany). Recordings were low-pass filtered at 10 kHz and access resistance was monitored throughout each experiment.
Characterization of intrinsic membrane and AP firing properties
Cells were recorded in the current clamp mode throughout the course of all experiments. Resting membrane potential was determined by measuring the membrane voltage in the absence of current input. PFC layer 5 pyramidal cell types were classified based on membrane responses to a series of 2 s steps (ranging from +30 to +330 pA) applied from a potential of −70 mV. Passive membrane characteristics were assessed as described in detail elsewhere (Chang et al., 2005). Briefly, a series of 200 ms current steps (11 steps, ranging from −120 to +80 pA) was applied to the cell from a potential of −70 mV and input resistance determined by the slope of the best-fit line on the resulting voltage–current (V–I) graph. The membrane time constant (tau) was determined by fitting the membrane potential response to a small hyperpolarizing pulse to a single exponential function. Single AP characteristics, including amplitude, threshold, and kinetics (rise time, duration at half AP amplitude, and fall time) were analyzed. The first AP produced by the 200-ms current-clamp series was used for single AP measurements. The threshold for firing was measured by expanding the time scale of the digitized trace in the PulseFit oscilloscope window to 1 ms per gradation and, using a linear measurement software function, measuring the voltage at the point in the trace when upward deflection begins. Maximal AP amplitude was measured from threshold to the peak of the spike on the voltage axis. Duration at half-maximal amplitude was measured at half-amplitude from threshold to peak, while rise time and decay time were measured as the duration from threshold to peak amplitude, and peak to the beginning of the fast afterhyperpolarization (AHP), respectively. AHP amplitudes were measured from baseline (membrane potential during prepulse) to maximal amplitude. Medium afterhyperpolarization (mAHP) was measured following the first AP spike generated at rheobase from a 200 ms current step. Frequency–current (f–I) plots were generated by plotting the frequency of APs generated vs. amplitude of the depolarizing current step. In order to quantitatively compare differences in AP firing rate adaptation of cells from aged versus young monkeys during repetitive-firing, interspike intervals (ISI) over the course of a 280 pA, 2000 ms depolarizing step were also measured at 100 ms intervals. All electrophysiological data were initially analyzed using the “Pulse-Fit” analysis program (HEKA Elektronik), and further analyzed with “Igor Pro” software (Wave-Metrics, Inc., Lake Oswego, OR, USA). Only cells which had a resting membrane potential of <−55 mV, stable access resistance over the course of the recording, and the presence of an AP overshoot were included in analyses.
Consideration of possible differences in the effect of the experimental paradigm on young versus aged slices and cells
One concern that applies to in vitro aging studies such as this, is that aged tissue/cells may respond differently or be more negatively impacted by the slicing and/or recording procedure. Having recorded from both young and aged monkey slices for a number of years (St. John et al., 1997; O'Brien et al., 2003; Luebke and Rosene, 2003; Luebke et al., 2004; Chang et al., 2005), we believe that this is not the case. First, under IR-DIC visualization, slices from both age groups appear healthy and show no evidence of pyknotic nuclei, atrophy or other signs of cell death or degeneration. Second, it is equally straightforward to obtain viable cells in slices from both age groups; indeed the highest number of cells we have recorded from a single subject (31 in one recording session in a different ongoing study) was from slices prepared from a 30-year-old monkey. Third, the basic electrophysiological properties of most aged neurons meet criteria for “healthy” cells, exhibiting high amplitude APs with an overshoot, an AP threshold close to −44 mV, and a resting membrane potential near −70 mV. Finally, the proportion of cells that do not reach criteria for inclusion in slices prepared from aged than from young subjects is the same.
Statistical analyses
All behavioral data were analyzed for statistical significance using the two-tailed Student's t-test with significance defined at P<0.05. Differences in the electrophysiological properties in cells from aged versus young monkeys were analyzed using generalized linear models via generalized estimating equations with significance defined at P<0.05. These models were used to account for the fact that a variable number of recordings were obtained from each subject (a minimum of three layer 5 pyramidal cells were recorded from in slices from each monkey, with the average being 5.2±0.8 cells and the range being from three to seven cells). Relationships between electrophysiological properties that differed between age groups were correlated with performance scores on behavioral tests within each age group and examined using both linear regression analysis and with a quadratic function. Data are reported as mean±S.E.M. in the figures and ±S.D. in Table 1.
RESULTS
Aged monkeys are impaired on tasks of rule learning and working memory
All monkeys completed testing on the DNMS basic, DNMS 2 min delay, and DRST spatial tasks (Table 1). As a group, aged subjects were impaired on rule learning (DNMS basic; P<0.01), object working memory (DNMS 2 min delay, P<0.04) and spatial working memory (DRST; P<0.001) compared with young animals. Thus, as a group, aged monkeys exhibited impaired cognitive performance compared with the young group, although some aged animals were relatively unimpaired on specific tasks and one young animal (AM 194) was cognitively impaired (DNMS 2 min and DRST; Table 1).
Population of cells examined
Data were obtained from 44 layer 5 pyramidal cells in PFC slices from seven young monkeys and 44 layer 5 pyramidal cells in PFC slices from eight aged monkeys. Cells were categorized as regular spiking (RS) type 1, RS type 2 or fast adapting (FA). Briefly, RS type 1 cells did not exhibit significant AP threshold and amplitude changes duringa2s spike train, whereas in RS2 and FA cells AP threshold increased significantly and AP amplitude decreased significantly during the train. In FA cells, complete adaptation of AP firing was observed within 600 ms. Due to the relatively low numbers and variability in the properties of FA cells they were not included in the present study. The predominant cell types were RS1 and RS2 cells (comprising ∼80% of all cells) and the approximate percentages of RS1 and RS2 cells were equivalent between the aged and young groups (of young cells 62% were RS1 and 18% were RS2 and of aged cells 60% were RS1 and 20% were RS2). Hence, data from these two RS slowly-adapting cell types were pooled for age comparisons.
Intrinsic membrane properties are not altered with age
None of the intrinsic membrane properties of layer 5 pyramidal cells were altered with age. The mean resting membrane potential of aged cells was −68.2±1.0 mV versus −66.0±0.8 mV in young cells (Fig. 1A). The membrane time constant of aged cells was 22.4±1.5 mV compared with 22.4±2 mV in young cells (Fig. 1B). Input resistance of aged cells also did not differ from young cells (155±9.0 mΩ and 149±7.6 mΩ respectively; Fig. 1C).
Fig. 1.
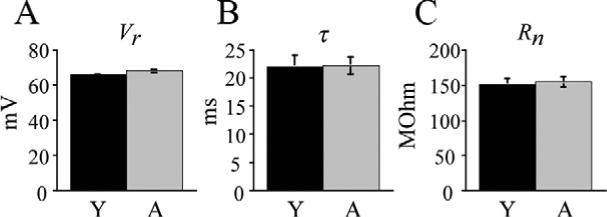
Intrinsic membrane properties of layer 5 pyramidal cells are unaltered with age. (A) Mean resting membrane potentials for young versus aged cells. (B) Mean membrane time constant for young versus aged cells. (C) Mean input resistance for young versus aged cells.
Single AP properties are altered with age
Single AP properties were evaluated from APs evoked by a depolarizing current stimulus at rheobase. Threshold for AP firing was not different in aged compared with young cells (−41.5±0.2 mV vs. −42.2±0.6 mV; Fig. 2B). AP amplitude was significantly lower in aged cells compared with young cells with mean values of 82.2±0.6 mV and 85.7±1.1 mV respectively (P<0.05; Fig. 2A, C). AP rise time did not differ in the two age groups with mean times of 0.73±0.03 ms (aged) and 0.78±0.04 ms (young) (Fig. 2D). The AP duration at half-maximal amplitude was significantly shorter in aged cells than young cells with mean times of 1.2±0.07 ms and 1.42±0.1 ms respectively (P<0.01, Fig. 2E). Finally, AP fall time was also significantly shorter in aged cells than in young cells with mean times of 1.8±0.1 ms and 2.3±0.2 ms respectively (P<0.005; Fig. 2F). Linear regression analysis demonstrated that AP parameters that changed with age co-varied with each other (duration vs. fall time, r=0.89, P<0.001; amplitude vs. duration, r=0.46, P<0.001; amplitude vs. fall time, r=0.42, P<0.001; not shown).
Fig. 2.
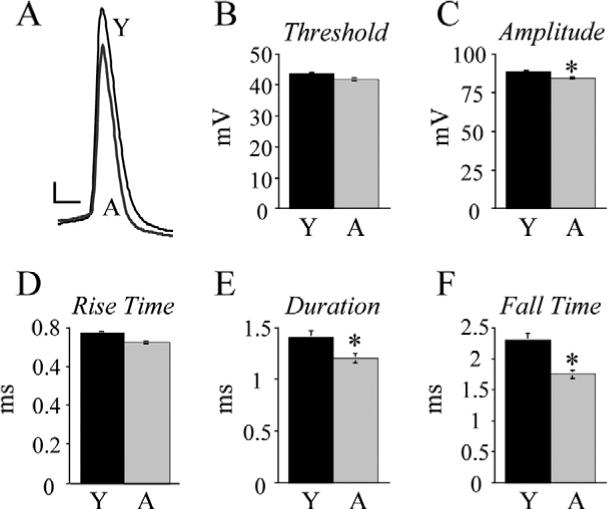
Aged layer 5 pyramidal cell single APs exhibit significantly decreased amplitude, duration and fall time. (A) Superimposed traces of single APs evoked by a just-threshold depolarizing current step from representative young (Y) and aged (A) cells. Scale bar=10 mV/1 ms. (B) Mean AP threshold in young vs. aged cells. Note: S.E.M. was very small at ±1.0 mV for aged cells and ±0.8 mV for young cells, and hence not visible on the graph. (C) Mean AP amplitude in young vs. aged cells, * P<0.05. (D) Mean AP rise time in young vs. aged cells. (E) Mean AP duration at half-maximal amplitude in young vs. aged cells, * P<0.01. (F) Mean AP fall times in young vs. aged cells, * P<0.005.
Slow afterhyperpolarization (sAHP) amplitude increases with age
Single APs were followed by both a fast afterhyperpolarization (fAHP) and a mAHP, although the mAHP often masked the fAHP resulting in an AHP that appeared to be monophasic or had only a small notch indicative of the fAHP (Fig. 3A). Thus fAHP amplitude measurements were not performed in the present study. mAHP amplitude in aged cells was not statistically different from young cells with mean values of 12.4±1.0 mV and 10.5±0.7 mV respectively (Fig. 3B, left). The amplitude of the sAHP following AP trains evoked by 2 s depolarizing current steps was significantly greater in aged than in young cells however, with mean values of 4.3±0.4 mV and 3.05±0.5 mV following a train of APs evoked by a 2 s 230 pA depolarizing step (P<0.01; Fig. 3B, right; C).
Fig. 3.
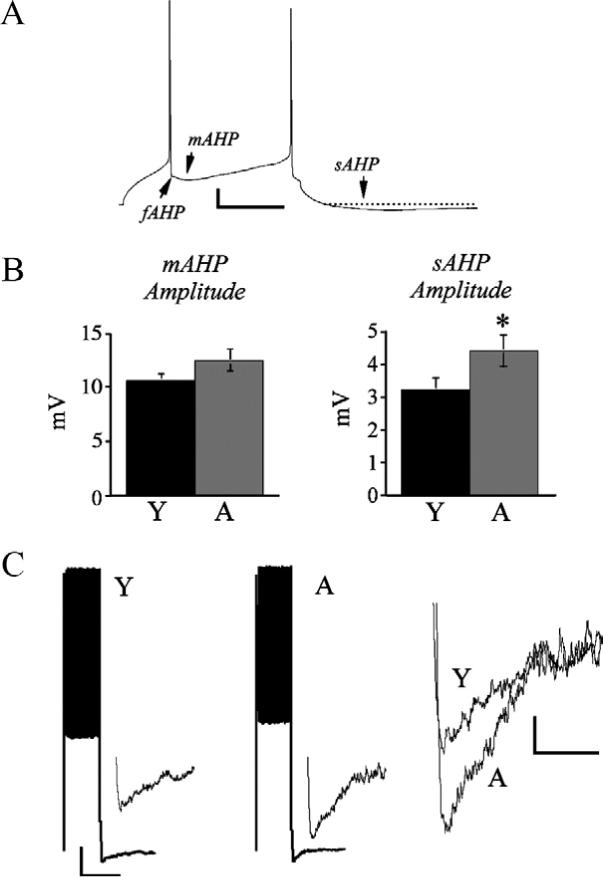
mAHP amplitude is unaltered and sAHP amplitude is increased in aged layer 5 pyramidal cells. (A) Representative current clamp trace from an aged layer 5 cell demonstrating fAHP, mAHP and sAHPs. Scale bar=10 mV/50 ms. (B) Bar graphs of mean mAHP amplitude (left panel) and sAHP amplitude following a train of spikes evoked by a 230 pA current step (right panel). * P<0.01. (C) Left: traces demonstrating a train of APs elicited by a 230 pA current step followed by a sAHP in representative young and aged cells. Scale bar=20 mV/2 s. Insets: sAHPs on an expanded time and amplitude scale. Right: superimposed trace of sAHPs from the young and aged cells. Scale bar=1 mV/500 ms.
AP firing rates are not altered with age
AP trains were evoked by application of increasing amplitude 2 s depolarizing current steps (30−330 pA). Unlike the case with aged layer 2/3 pyramidal cells (Chang et al., 2005), the firing rates of layer 5 pyramidal cells did not change with age (Fig. 4A, B). Consistent with this finding, the ISI, measured at 100 ms intervals, for these cells also did not change with age (Fig. 4C).
Fig. 4.
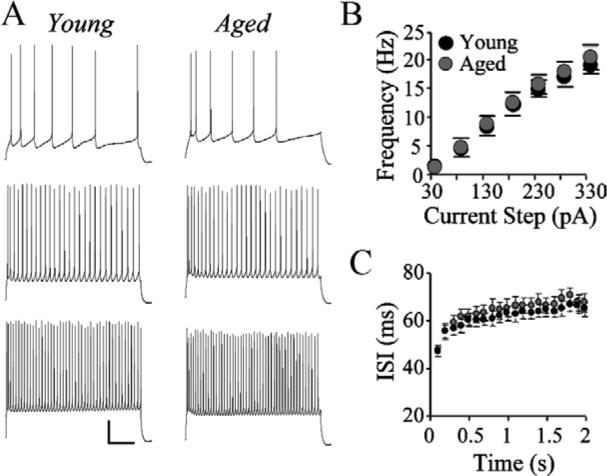
Layer 5 pyramidal cell AP firing rates are not altered with age. (A) Trains of APs elicited by a 2 s depolarizing current steps of 30 pA (top), 130 pA (middle), and 280 pA (top). Scale bar=20 mV/250 ms. (B) Mean frequency of AP firing at different depolarizing current steps for young versus aged cells. (C) ISIs in 100 ms increments across a 2 s, 280 pA current step for representative young and aged cells.
Correlation of electrophysiological changes with cognitive performance
The relationship between electrophysiological variables that changed with age (single AP amplitude, duration and fall time, and sAHP amplitude), was plotted versus the behavioral performance scores of aged monkeys. These data were then fit with linear or quadratic relationships. There was no significant relationship between these electrophysiological variables and cognitive performance within the aged group of subjects (data not shown).
DISCUSSION
The present study was undertaken to determine if layer 5 pyramidal cells in the rhesus monkey PFC undergo significant alterations in basic electrophysiological properties with age and whether any such changes are associated with cognitive decline. Layer 5 cells from aged monkeys did not differ from cells from young monkeys with regard to: resting membrane potential, membrane time constant, input resistance, single AP threshold or rise time, mAHP amplitude, or AP firing rates. However, cells from aged monkeys showed a significantly decreased AP amplitude, duration and fall time, and an increased sAHP amplitude.
Intrinsic membrane properties
There was no effect of age on resting membrane potential, membrane time constant, or input resistance of layer 5 cells. This result differs from that seen in layer 2/3 pyramidal cells from the PFC (Chang et al., 2005) and dentate granule cells from the hippocampus (Luebke and Rosene, 2003) of aged monkeys where age-related increases in input resistance have been observed.
Single AP properties are altered with age
In layer 5 cells, AP amplitude, duration, and fall time of single APs decreased with age. Similar changes in amplitude, duration, and fall time have been reported in monkey layer 2/3 cells (Chang et al., 2005). Age-related AP amplitude changes in these cells may result from altered expression of voltage-activated sodium channel subunits; indeed, reductions in expression of the beta-2 (SCN2B) and the beta-3 (SCN3B) voltage-activated sodium channel subunits have been reported in the PFC gray matter of aged humans (Lu et al., 2004; Erraji-Benchekroun et al., 2005). Shorter AP duration and fall time may also be due to altered expression of potassium channels such as IK,IA, and ID (Foehring and Surmeier, 1993; Korngreen and Sakmann, 2000). As with the voltage-activated sodium channel, there are age-related alterations in the expression of a number of voltage-activated potassium channel subunits in humans (Erraji-Benchekroun et al., 2005). Interestingly, there is an age-related increase in expression of the Kv9.1 subunit (KCNS1 gene), a voltage-gated delayed rectifier alpha subunit (Erraji-Benchekroun et al., 2005). Delayed rectifier currents are thought to determine the rate of the falling phase of APs, hence increased expression would likely result in decreased AP duration and fall time such as was observed in the present study. Detailed voltage clamp studies are needed to further examine the currents that may underlie age-related changes in pyramidal cell AP properties.
sAHP amplitude is significantly greater with age
The amplitude and duration of the mAHP were unchanged with age, which was consistent with the finding that there was no difference in AP firing rates or ISIs with age. However aged cells demonstrated a significant increase in sAHP amplitude. This finding is similar to those previously reported in layer 2/3 pyramidal cells in the aged monkey PFC (Chang et al., 2005), and in rabbit or rodent hippocampal pyramidal cells (review: Thibault et al., 1998). However, in layer 5 cells, the age-related increase in sAHP amplitudes was not accompanied by reduced AP firing rates. In in vitro studies in rodent CA1 pyramidal neurons this increased sAHP amplitude and duration correlates with decreased firing pyramidal cell firing rates and increased spike frequency adaptation (Landfield and Pitler, 1984; Disterhoft et al., 1996; Power et al., 2002). Of particular note is that increased sAHP amplitudes are associated with learning deficits (Landfield and Pitler, 1984; Thompson et al., 1996; Moyer et al., 2000; Weiss et al., 2000; Power et al., 2002) and poor Morris water maze performance (Tombaugh et al., 2005). In the neocortex, the effect of sAHPs on firing rate is less straightforward. In both human (Lorenzon and Foehring, 1992) and rat neo-cortical cells (Abel et al., 2004), increased numbers of APs for a given step are associated with increased sAHP amplitudes. Although an age-related increase in sAHP amplitude and increased firing rate were both observed in layer 2/3 pyramidal cells from monkeys, firing rate was not correlated with sAHP amplitudes (Chang et al., 2005). Furthermore, in the present study, sAHP amplitudes and firing rate were also not correlated. Thus while an age-related increase in sAHP amplitude appears to be a common finding among diverse cell types, brain areas and species, the functional consequences of this increase is not uniformly readily apparent.
AP firing rates of layer 5 pyramidal cells are unchanged with age
A number of studies have demonstrated that neocortical cells in aged animals exhibit increased AP firing rates that are associated with perturbations in visual or cognitive function (Schmolesky et al., 2000; Leventhal et al., 2003; Chang et al., 2005; Hua et al., 2006). The frequency and temporal firing patterns of APs in cortical and subcortical areas encode information required for the completion of cognitive tasks (review: Funahashi, 2001; Constantinidis and Procyk, 2004). Thus, alterations in AP firing patterns during aging could contribute to the factors which underlie age-related cognitive decline. The results of the current study demonstrate that, unlike layer 2/3 cells, layer 5 pyramidal cell firing rates do not change with age. Although firing rates for cells from aged animals did not differ from cells from young animals, aged animals as a group were impaired on all tasks, suggesting that layer 5 firing rates do not contribute to age-related cognitive deficits measured in tasks used in this study. Indeed, results from this study suggest that layer 5 cells are not, for the most part, functionally altered with age. Although aged cells exhibit changes in electrophysiological characteristics such as decreased AP amplitude, duration, and fall time, and increased sAHP amplitudes, these changes are not associated with alterations in AP firing rates or with cognitive decline.
Acknowledgments
The authors wish to thank Dr. Douglas Rosene for performing perfusions of the monkeys and providing brain tissue from which recordings were obtained. We are also grateful to Dr. Anne Rocher for careful reading of the manuscript. This work was supported by NIH/NIA grants PO1-AG00001 and R01-AG025062.
Abbreviations
- AHP
afterhyperpolarization
- AP
action potential
- DNMS
delayed non-match to sample
- DRST
delayed recognition span task
- FA
fast adapting
- fAHP
fast afterhyperpolarization
- IR-DIC
infrared-differential interference contrast
- ISI
interspike interval
- KAsp
potassium aspartate
- LASC
Laboratory Animal Science Center
- mAHP
medium afterhyperpolarization
- PFC
prefrontal cortex
- RS
regular spiking
- sAHP
slow afterhyperpolarization
REFERENCES
- Abel HJ, Lee JC, Callaway JC, Foehring RC. Relationships between intracellular calcium and afterhyperpolarizations in neo-cortical pyramidal neurons. J Neurophysiol. 2004;91(1):324–335. doi: 10.1152/jn.00583.2003. [DOI] [PubMed] [Google Scholar]
- Albert M, Moss MB. Cognition in normal aging. In: Peters A, Morrison J, editors. Cerebral cortex. Vol. 14. Plenum Press; New York: 1999. pp. 1–20. [Google Scholar]
- Alexander GE, DeLong MR, Strick PL. Parallel organization of functionally segregated circuits linking basal ganglia and cortex. Annu Rev Neurosci. 1986;9:357–381. doi: 10.1146/annurev.ne.09.030186.002041. [DOI] [PubMed] [Google Scholar]
- Cavada C, Company T, Tejedor J, Cruz-Rizzolo RJ, Reinoso-Suarez F. The anatomical connections of the Macaque monkey orbitofrontal cortex. A review. Cereb Cortex. 2000;10:220–242. doi: 10.1093/cercor/10.3.220. [DOI] [PubMed] [Google Scholar]
- Chang YM, Rosene DL, Killiany RJ, Mangiamele LA, Luebke JI. Increased action potential firing rates of layer 2/3 pyramidal cells in the prefrontal cortex are significantly related to cognitive performance in aged monkeys. Cereb Cortex. 2005;15:409–418. doi: 10.1093/cercor/bhh144. [DOI] [PubMed] [Google Scholar]
- Constantinidis C, Procyk E. The primate working memory networks. Cogn Affect Behav Neurosci. 2004;4(4):444–465. doi: 10.3758/cabn.4.4.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disterhoft JF, Thompson LT, Moyer JR, Jr, Mogul DJ. Calcium-dependent afterhyperpolarization and learning in young and aging hippocampus. Life Sci. 1996;59(5−6):413–420. doi: 10.1016/0024-3205(96)00320-7. [DOI] [PubMed] [Google Scholar]
- Erraji-Benchekroun L, Underwood MD, Arango V, Galfalvy H, Pavlidis P, Smyrniotopoulos P, Mann JJ, Sibille E. Molecular aging in human prefrontal cortex is selective and continuous throughout adult life. Biol Psychiatry. 2005;57(5):549–558. doi: 10.1016/j.biopsych.2004.10.034. [DOI] [PubMed] [Google Scholar]
- Foehring RC, Surmeier DJ. Voltage-gated potassium currents in acutely dissociated rat cortical neurons. J Neurophysiol. 1993;70(1):51–63. doi: 10.1152/jn.1993.70.1.51. [DOI] [PubMed] [Google Scholar]
- Funahashi S. Neuronal mechanisms of executive control by the prefrontal cortex. Neurosci Res. 2001;39(2):147–165. doi: 10.1016/s0168-0102(00)00224-8. [DOI] [PubMed] [Google Scholar]
- Fuster JM. The prefrontal cortex: Anatomy, physiology and neurophysiology of the frontal lobe. 3rd edition Lippencott-Raven Publishers; Philadelphia: 1997. [Google Scholar]
- Goldman-Rakic PS. Cellular basis of working memory. Neuron. 1995;14:477–485. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- Herndon JG, Moss MB, Rosene DL, Killiany RJ. Patterns of cognitive decline in aged rhesus monkeys. Behav Brain Res. 1997;87(1):25–34. doi: 10.1016/s0166-4328(96)02256-5. [DOI] [PubMed] [Google Scholar]
- Hua T, Li X, He L, Zhou Y, Wang Y, Leventhal AG. Functional degradation of visual cortical cells in old cats. Neurobiol Aging. 2006;27(1):155–162. doi: 10.1016/j.neurobiolaging.2004.11.012. [DOI] [PubMed] [Google Scholar]
- Korngreen A, Sakmann B. Voltage-gated K+ channels in layer 5 neocortical pyramidal neurones from young rats: subtypes and gradients. J Physiol. 2000;525(Pt 3):621–639. doi: 10.1111/j.1469-7793.2000.00621.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landfield PW, Pitler TA. Prolonged Ca2+-dependent afterhyperpolarizations in hippocampal neurons of aged rats. Science. 1984;226(4678):1089–1092. doi: 10.1126/science.6494926. [DOI] [PubMed] [Google Scholar]
- Leventhal AG, Wang Y, Pu M, Zhou Y, Ma Y. GABA and its agonists improved visual cortical function in senescent monkeys. Science. 2003;300(5620):812–815. doi: 10.1126/science.1082874. [DOI] [PubMed] [Google Scholar]
- Lorenzon NM, Foehring RC. Relationship between repetitive firing and afterhyperpolarizations in human neocortical neurons. J Neurophysiol. 1992;67(2):350–363. doi: 10.1152/jn.1992.67.2.350. [DOI] [PubMed] [Google Scholar]
- Lu T, Pan Y, Kao SY, Li C, Kohane I, Chan J, Yankner BA. Gene regulation and DNA damage in the ageing human brain. Nature. 2004;429(6994):883–891. doi: 10.1038/nature02661. [DOI] [PubMed] [Google Scholar]
- Luebke JI, Chang YM, Moore TL, Rosene DL. Normal aging results in decreased synaptic excitation and increased synaptic inhibition of layer 2/3 pyramidal cells in the monkey prefrontal cortex. Neuroscience. 2004;125(1):277–288. doi: 10.1016/j.neuroscience.2004.01.035. [DOI] [PubMed] [Google Scholar]
- Luebke JI, Rosene DL. Aging alters dendritic morphology, input resistance, and inhibitory signaling in dentate granule cells of the rhesus monkey. J Comp Neurol. 2003;460(4):573–584. doi: 10.1002/cne.10668. [DOI] [PubMed] [Google Scholar]
- Moss MB, Killiany RJ, Herndon JG. Age-related cognitive decline in the rhesus monkey. In: Peters A, Morrison J, editors. Cerebral cortex. Kluwer Academic/Plenum Publishers; New York: 1999. pp. 21–47. [Google Scholar]
- Moyer JR, Jr, Power JM, Thompson LT, Disterhoft JF. Increased excitability of aged rabbit CA1 neurons after trace eyeblink conditioning. J Neurosci. 2000;20(14):5476–5482. doi: 10.1523/JNEUROSCI.20-14-05476.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Brien SE, Rosene DL, Luebke JI. GABAA receptor-mediated neurotransmission in the dentate gyrus of the rhesus monkey; a comparison with the rat. Synapse. 2003;49(4):287–289. doi: 10.1002/syn.10237. [DOI] [PubMed] [Google Scholar]
- Owen AM. Cognitive planning in humans: Neuropsychological, neuroanatomical, and neuropharmacological perspectives. Prog Neurobiol. 1997;53:431–450. doi: 10.1016/s0301-0082(97)00042-7. [DOI] [PubMed] [Google Scholar]
- Power JM, Wu WW, Sametsky E, Oh MM, Disterhoft JF. Age-related enhancement of the slow outward calcium-activated potassium current in hippocampal CA1 pyramidal neurons in vitro. J Neurosci. 2002;22(16):7234–7243. doi: 10.1523/JNEUROSCI.22-16-07234.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmolesky MT, Wang Y, Pu M, Leventhal AG. Degradation of stimulus selectivity of visual cortical cells in senescent rhesus monkeys. Nat Neurosci. 2000;3(4):384–390. doi: 10.1038/73957. [DOI] [PubMed] [Google Scholar]
- St. John JL, Rosene DL, Luebke JI. Morphology and electrophysiology of dentate granule cells in the rhesus monkey: a comparison with the rat. J Comp Neurol. 1997;387:136–147. doi: 10.1002/(sici)1096-9861(19971013)387:1<136::aid-cne11>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Thibault O, Porter NM, Chen KC, Blalock EM, Kaminker PG, Clodfelter GV, Brewer LD, Landfield PW. Calcium dysregulation in neuronal aging and Alzheimer's disease: history and new directions. Cell Calcium. 1998;24(5−6):):417–433. doi: 10.1016/s0143-4160(98)90064-1. [DOI] [PubMed] [Google Scholar]
- Thompson LT, Moyer JR, Jr, Disterhoft JF. Transient changes in excitability of rabbit CA3 neurons with a time course appropriate to support memory consolidation. J Neurophysiol. 1996;76(3):1836–1849. doi: 10.1152/jn.1996.76.3.1836. [DOI] [PubMed] [Google Scholar]
- Tombaugh GC, Rowe WB, Rose GM. The slow afterhyperpolarization in hippocampal CA1 neurons covaries with spatial learning ability in aged Fisher 344 rats. J Neurosci. 2005;25(10):2609–2616. doi: 10.1523/JNEUROSCI.5023-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss C, Preston AR, Oh MM, Schwarz RD, Welty D, Disterhoft JF. The M1 muscarinic agonist CI-1017 facilitates trace eyeblink conditioning in aging rabbits and increases the excitability of CA1 pyramidal neurons. J Neurosci. 2000;20(2):783–790. doi: 10.1523/JNEUROSCI.20-02-00783.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson IA, Ikonen S, Gallagher M, Eichenbaum H, Tanila H. Age-associated alterations of hippocampal place cells are subregion specific. J Neurosci. 2005;25(29):6877–6886. doi: 10.1523/JNEUROSCI.1744-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]


