Abstract
The administration of neuroleptics in animal models has been extensively reported and plays an important role in the study of schizophrenia. Our study was designed to address the following questions: (1) Is it possible to achieve steady-state receptor occupancy levels administering neuroleptics in drinking water? (2) Is there an appropriate dose to obtain clinically comparable receptor occupancies? (3) Is there a correlation between plasma drug levels and receptor occupancy? Thus, we tested three neuroleptic drugs administered in drinking water for 7 days. Plasma drug levels were measured, and in vivo receptor occupancy assays were performed in order to determine peak and trough dopamine D2 receptor occupancies in striatal brain samples. Overall, our study indicates that in rodents the administration of appropriate doses of haloperidol and olanzapine in drinking water achieves receptor occupancies comparable to the clinical occupancy levels, but this appears not to be the case for clozapine.
Keywords: Haloperidol, Clozapine, Olanzapine, Raclopride, Schizophrenia, Catalepsy, Drug efficacy, D2 receptor, Dopamine
Introduction
The use of animal models in schizophrenia research and specifically in studies involving antipsychotic drug (APD) treatments is very frequent, and such studies are crucial for understanding the mechanisms of action and side effects of these medications (see review by Lipska and Weinberger 2000). However, modeling APD treatment in animals is complicated by several factors including differences in drug metabolism across species, evaluation of the efficacy of the drugs, the route of drug administration and the achievement of clinically relevant doses. For instance, metabolism of antipsychotic drugs (APDs) in rodents is four to six times faster than in humans, and may also vary with different routes of drug administration (Cheng and Paalzow 1992; Baldessarini et al. 1993; Aravagiri et al. 1997; Bezchlibnyk-Butler and Jeffries 1999). Most of the symptoms (i.e., delusions and hallucinations) in patients treated by APD cannot be modeled in animals, which makes evaluation of drug efficacy very difficult (Lipska and Weinberger 2000). Measurements of D2 receptor occupancies in the brain have been used as a surrogate of drug efficacy in animal models (Kapur et al. 2003), even though the majority of APDs do not interact exclusively with the D2 receptor (Kapur and Seeman 2001), and other receptors most likely play a role in APD performance (Kapur and Seeman 2001; Kapur et al. 2003).
Several methods of drug administration have been extensively used in animal models, each of them with their particular strengths and weaknesses. For example, the administration of drugs by various injection techniques are very accurate in determining the amount of drug delivered, but can produce a high rate of stress to the animals in multi-dosing experiments (Stahle and Ungerstedt 1986; Schmitt et al. 1999; Schleimer et al. 2005) and large differences in drug availability between injections (see Kapur et al. 2003). Another widely used approach is the use of osmotic mini-pumps since this methodology reduces the stress associated with repeated injections and handling, which could alter the subject's behavioral profile (Mitchell and Redfern 2005). However, this later method could be difficult to use in large-scale experiments, as suggested by the study by Kapur et al. (2003), and may be inappropriate for the delivery of some APDs like clozapine or quetiapine. Finally, the administration of drugs in drinking water has been also extensively used (see, i.e., Tamminga et al. 1990; Kaneda et al. 1992; Roberts et al. 1995, 2002; Gao et al. 1997, 2005; Kelley et al. 1997; Roberts 2001; Sakai et al. 2001a, b; Roberts and Lapidus 2003; Kelley and Roberts 2004), whose major strengths include the avoidance of environmental stressors and the similarity of administration to the clinical situation. Although, in order to obtain an accurate measurement of the dose of the drug administered, close monitoring of water intake and other parameters such as plasma drug levels are necessary.
In order to obtain data as comparable as possible to the clinical situation, the use of the appropriate drug doses and method of administration should be priorities in such studies. Despite the critical importance of these issues, numerous studies have neglected them, leading to potentially confounding and misleading conclusions (see Kapur et al. 2000, 2003). An elegant study by Kapur et al. (2003) addresses this issue for some of the most commonly used APD administered through subcutaneous injections and mini-pumps, but no information about oral treatments was given. Thus, the present study was designed to determine the accuracy of administering APDs in drinking water, the reliability of achieving clinically relevant D2 receptor occupancies, and the validity of measuring plasma drug levels as an indicator of receptor occupancy. This work has been presented in preliminary form (Perez-Costas et al. 2005, 2006).
Materials and methods
Animals
Adult male Sprague–Dawley rats [n = 90, 10 animals per treatment group, 5 per treatment subgroup] (Charles River, Wilmington, MA, USA), weighing 175–200 g at the beginning of the experiment were individually housed in transparent polycarbonate cages, and maintained in a 12 h light–dark cycle (lights turned on at 7:00 AM and turned off at 7:00 PM) with access to food and water ad libitum. All procedures were carried out in accordance with an experimental protocol approved by the Institutional Animal Care and Use Committee of the University of Maryland School of Medicine (Baltimore). Water consumption was monitored daily in order to calculate average water consumption for the preparation of drug solutions to be delivered in drinking water.
Drug dose selection and preparation
Drug doses were selected following several criteria: for haloperidol we chose doses that have been previously used in published work by our group (Kelley et al. 1997; Roberts et al. 2002; Roberts and Lapidus 2003; Kelley and Roberts 2004), and that have also been frequently reported in treatments in rodents (see, i.e., Gao et al. 1997; Schleimer et al. 2005; Ceresoli-Borroni et al. 2006; Pillai and Mahadik 2006; Rosengarten et al. 2006; Terry et al. 2006). Clozapine doses were chosen based on frequently used doses and previous studies that reported significant plasma drug levels using this antipsychotic in rodents (Gao et al. 1997; Schmitt et al. 1999, 2004; Weigmann et al. 1999; Ossowska et al. 2002; Schleimer et al. 2005; Ceresoli-Borroni et al. 2006). Finally, in the case of olanzapine we chose three doses (2, 4 and 5 mg/kg/day) based on the doses previously used in rodent treatment by our group and others (Roberts 2001; Pouzet et al. 2003; Fell et al. 2005; Pillai and Mahadik 2006), and unpublished data from our group that indicate that doses between 4 and 5 mg/kg/day produce significant plasma drug levels of olanzapine.
Haloperidol was purchased from Sigma–Aldrich (St. Louis, MO, USA), clozapine was kindly provided by Novartis (Basel, Switzerland) and olanzapine was kindly donated by Eli Lilly and Co, (Indianapolis, IN, USA). All drugs were dissolved in a minimum volume of glacial acetic acid, the solution was further diluted with drinking water, and the pH was adjusted using 10 N NaOH to the normal pH of the drinking water (pH ranged between 6 and 6.25).
Drug treatments
Animals (n = 90, each treatment group n = 10) were housed in our animal facility and allowed an adaptation period of 1 week. After that, they were randomly assigned to different treatment groups (see Table 1 for treatment groups and doses). All drugs were delivered in drinking water taking into account the average daily water consumption of each animal during the prior 7 days of adaptation.
Table 1.
Targeted dose and average dose achieved for each treatment group based on water consumption (X ± SD: average dose achieved ± standard deviation)
| Targeted dose (mg/kg/day) | Dose achieved X ± SD (mg/kg/day) |
|---|---|
| Haloperidol: 1.5 | 1.81 ± 0.15 |
| Haloperidol: 2.0 | 2.15 ± 0.26 |
| Clozapine: 20 | 22.56 ± 3.40 |
| Clozapine: 40 | 43.42 ± 6.31 |
| Olanzapine: 2.0 | 2.35 ± 0.30 |
| Olanzapine: 4.0 | 4.47 ± 0.43 |
| Olanzapine: 5.0 | 5.90 ± 0.65 |
Water consumption monitoring
Animals were treated for 1 week, water consumption was monitored daily, and the dose of the drug was adjusted daily on the basis of water consumption (see Table 1 for targeted dose and actual dose delivered). Control animals received water from the same source than the water used for the dilution of the drugs. For water consumption monitoring animals were provided everyday with an initial volume of 100 ml, measuring everyday at the same hour the remaining volume in the bottle using a graduated cylinder. Freshly prepared solutions were provided everyday.
Catalepsy test
A bar catalepsy test was performed on all animals at 3 different time points during the study: before the beginning of the treatment (baseline), the third day of treatment, and the last day of treatment. This test has been successfully used to measure catalepsy in rodents (see, i.e., Prinssen et al. 1999; Kleven et al. 2005). Briefly, the forelimbs of the animals were placed on a horizontally placed, cylindrical bar (diameter 1.25 cm) at a height of 10 cm and the time that the forelimbs remained on the bar was determined for a period of 30 s. For each testing period the test was repeated 3 and 6 min after the first test, in order to calculate the mean value of the time that the forelimbs remained on the bar. All animals were tested during the light cycle and returned to their home cage between tests.
D2 receptor binding potential and occupancy
After 1 week of treatment, animals were randomly selected from each group and euthanized for determination of either peak or trough occupancies. Peak was considered as the occupancy levels that animals had during the most active period in a 24 h time span. Thus, for the measurement of peak occupancies, animals were euthanized 6 h after the dark cycle started. Trough was considered the occupancy levels that animals will have during a low-active period in a 24 h time span. Thus, for the measurement of trough occupancies, animals were euthanized 6 h after the light cycle started.
In all cases, 30 min before euthanasia each animal was injected in the tail vein with 7.5 μCi of [3H] raclopride (Amersham Biosciences, Pittsburgh, PA, USA) diluted in 0.4 ml of 0.9% NaCl. After that, animals were decapitated, the brains were immediately removed from the skull, and the cerebellum and striatum were dissected out and collected separately. Striatal and cerebellar (control tissue lacking D2 receptors) samples from each animal were weighed, diluted 1:10 in distilled water and homogenized. Total protein concentration was calculated for each of these samples using the Lowry method (Lowry et al. 1951). The same amount of protein sample (based on total protein concentration) for each specimen was used. Thus, 2 ml of solvent NCS-II (Amersham Biosciences) were added to each sample and incubated on a shaker for 2 h. After that, 10 ml of BCS-NA scintillation fluid (Amersham Biosciences) were added to each sample. [3H] raclopride radioactivity was measured using a Packard 2200CA liquid scintillation analyzer. Striatal and cerebellar counts were obtained and expressed as disintegrations per minute per milligram of protein (DPM/mg). The D2 receptor binding potential (D2BP) was obtained for each animal using the following formula: [(striatum DPM/mg − cerebellum DPM/mg)/cerebellum DPM/mg]. The receptor occupancy for each rat was determined with reference to the D2BP in the control group (animals that did not receive any drug in their drinking water) by applying the previously published formula [% OCCUPANCY = 100 × [(D2BP control − D2BP indiv)/D2BP control] (Farde et al. 1988; Kapur et al. 1999, 2003).
Plasma antipsychotic levels
At the time of euthanasia trunk blood samples from each animal were collected in blood sample tubes containing EDTA. Blood samples were centrifuged for 10 min at 2,000g at 4°C, and the resulting plasma was stored at −20°C. Plasma drug levels were measured at the Analytical Psychopharmacology Laboratory of the Nathan Kline Institute (Orangesburg, NY, USA). In brief, plasma levels of haloperidol were determined using a modified gas-liquid chromatography method that quantifies haloperidol and reduced haloperidol as a major metabolite (Bianchetti and Morselli, 1978). Clozapine and its inactive metabolite norclozapine were measured using high-performance gas chromatography with electrochemical detection (Simpson and Cooper 1978). Finally, plasma levels of olanzapine were detected using high performance liquid chromatography (Catlow et al. 1995).
Statistics
For each treatment group and subgroup, mean values and standard deviation were obtained for receptor occupancy as well as for plasma drug levels measurements.
Unpaired t tests were performed in order to compare the receptor occupancies achieved in subgroups of animals treated with the same doses of antipsychotic drugs, but euthanized at peak versus trough. The same kind of test was used to compare the occupancy values achieved between two groups of animals treated with two different doses of the same drug (haloperidol or clozapine). One-way ANOVA analysis was used to compare receptor occupancies achieved when more than two different doses were tested (olanzapine). In addition, a correlation analysis between receptor occupancy achieved and plasma drug levels obtained was also performed.
Results
For all drugs and doses tested the actual dose achieved (based on the data obtained monitoring daily water consumption) was slightly higher than the targeted dose (Table 1).
Catalepsy test
In our study none of the animals in any treatment group developed cataleptic symptoms. In fact, in all cases, animals showed a total lack of delay in removing their paws from the wooden bar in the catalepsy bar test. Since all rats removed their paws immediately data are not shown.
D2 receptor occupancy
The D2 receptor occupancies are presented in Table 2 and Fig. 1a-c for the comparison between different doses of the same drug, as well as for the comparison of occupancies achieved at peak and trough with the same doses.
Table 2.
Average D2 receptor occupancies and plasma levels obtained for each drug treatment group, and for the peak and trough subgroups
| Treatment group (mg/kg/day) |
Average D2 receptor occupancya |
Average D2 receptor occupancya (peak + trough) |
Average plasma drug levels (ng/ml) |
Average plasma levels (ng/ml) (peak + trough) |
|---|---|---|---|---|
| Haloperidol: | ||||
| 1.5 Peak | 85.78 ± 3.69 | 5.02 ± 1.96 | ||
| 1.5 Trough | 68.43 ± 5.99 | 77.11 ± 10.54 | 1.84 ± 0.53 | 3.43 ± 2.26 |
| 2.0 Peak | 86.43 ± 4.66 | 3.36 ± 1.58 | ||
| 2.0 Trough | 70.94 ± 7.43 | 78.68 ± 10.46 | 1.68 ± 0.43 | 2.52 ± 1.51 |
| Clozapine: | ||||
| 20 Peak | 16.07 ± 11.79 | 8.6 ± 1.02 | ||
| 20 Trough | −13.32 ± 10.85 | 1.37 ± 19.84 | 1.8 ± 3.6 | 5.25 ± 4.40 |
| 40 Peak | 26.93 ± 18.42 | 29.8 ± 17.27 | ||
| 40 Trough | −6.33 ± 18.23 | 10.30 ± 26.09 | 6.0 ± 3.10 | 17.9 ± 18.12 |
| Olanzapine: | ||||
| 2 Peak | 32.50 ± 9.26 | 1.54 ± 0.67 | ||
| 2 Trough | 12.78 ± 11.78 | 22.64 ± 15.47 | 0.16 ± 0.32 | 0.87 ± 0.95 |
| 4 Peak | 42.20 ± 19.69 | 7.58 ± 4.95 | ||
| 4 Trough | 36.98 ± 14.59 | 39.88 ± 18.88 | 1.24 ± 1.38 | 4.81 ± 5.22 |
| 5 Peak | 44.14 ± 13.03 | 8.62 ± 2.87 | ||
| 5 Trough | 33.44 ± 7.86 | 39.39 ± 12.99 | 0.8 ± 0.26 | 5.13 ± 4.72 |
D2 receptor occupancy values are shown as the percentage of control animals. In human patients clinically relevant receptor occupancy values are 65–80% for haloperidol and olanzapine, and 45–65% for clozapine (Kapur et al. 2003)
Fig. 1.
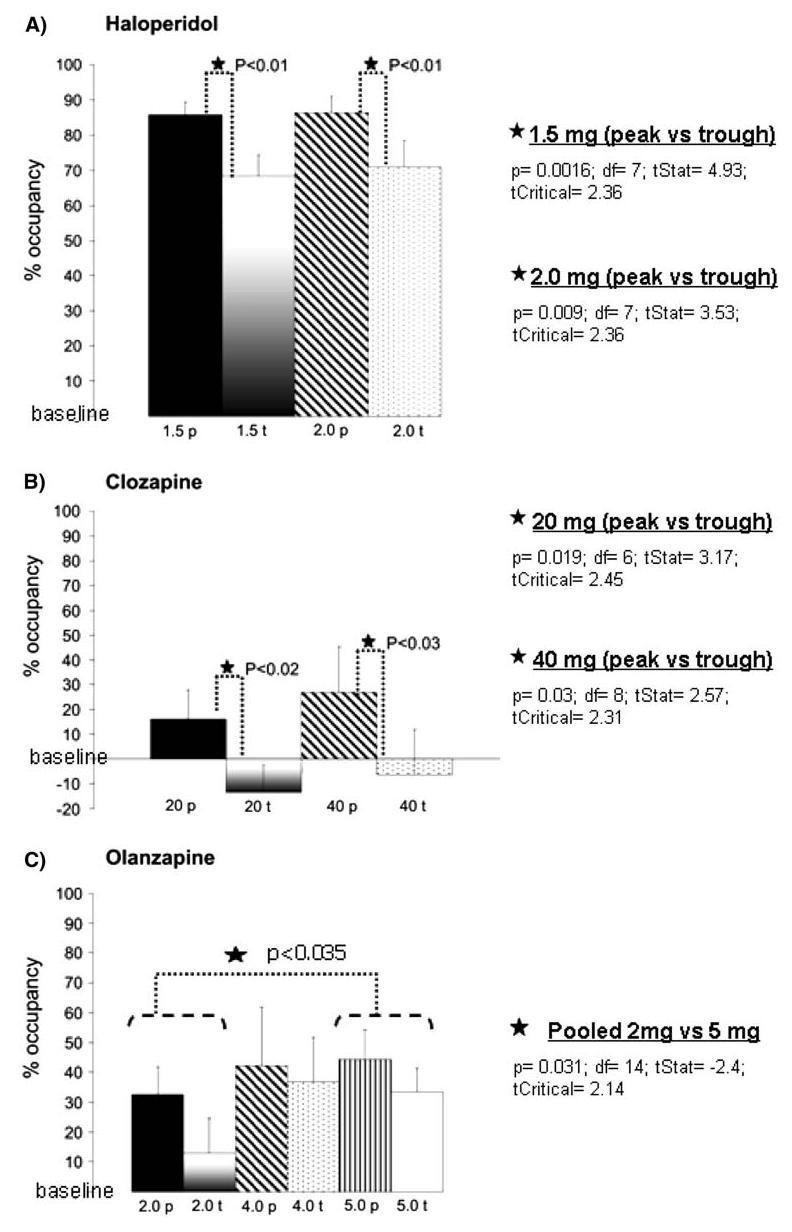
Graphs showing D2 receptor occupancies achieved at peak and trough time points. a Haloperidol: note the high occupancy levels present even at trough with the two doses tested. Significant differences in receptor occupancy were found between the peak and trough groups of each dose used (*P < 0.01). b Clozapine: Statistically significant differences in receptor occupancy were found between the peak and trough groups of each dose used (*P < 0.03). Note that in the trough groups of both treatment doses, D2 receptor occupancy values drop below the levels of the average occupancy of the controls. c Olanzapine: No statistically significant differences in receptor occupancy were found between the peak and trough groups at any of the doses used. The highest dose used (5 mg) produced less variability and considerable levels of D2 receptor occupancy even at trough. Note also that there is a significant difference (P < 0.035) when comparing the 2 and 5 mg dose group. In all graphs the “baseline” represents the average receptor occupancy value of the controls. p Peak; t trough. Numbers indicate doses (mg/kg/day) used in each animal group
Peak versus trough receptor occupancies
Haloperidol
For both doses used, t test analysis of receptor occupancies showed statistically significant differences between the peak and trough subgroups of animals treated with the same dose (Fig. 1a). Despite these statistically significant differences, the mean values of D2 receptor occupancy were in all cases in the range or above the range reported in clinical situations (Table 2).
Clozapine
Unpaired t test revealed that there were significant differences between the peak and trough subgroups at both doses used (Fig. 1b). Neither of the doses used achieved clinically comparable D2 receptor occupancies. Moreover, the mean occupancy values showed high variability, as reflected by the presence of high standard deviation values (Table 2).
Olanzapine
There were no significant differences in receptor occupancy levels between the peak and trough subgroups for any of the three doses used (Fig. 1c). None of the doses used achieved occupancies in the clinically comparable range, but the higher doses yielded mean occupancy values that were closer to the clinical level (Table 2).
Comparison of receptor occupancies achieved using different drug doses
For the analysis of these data, the peak and trough subgroups of each treatment dose were pooled together to include in the analysis the variability that would be present in receptor occupancies along a 24 h time period.
Haloperidol
There were no significant differences in receptor occupancy (P > 0.7) between the two doses (Fig. 1a, Table 2). Furthermore, the mean receptor occupancies of both doses were in the clinically comparable range (77.11% for the 1.5 mg/kg/day group, and 78.68% for the 2.0 mg/kg/day group). The variability within the same treatment group was small as indicated by the presence of small values for the standard deviation (Table 2).
Clozapine
There were no significant differences between the two treatment groups (P > 0.4). Neither dose achieved receptor occupancy levels that could be considered in or close to the clinically comparable range (Fig. 1b, Table 2). In addition, the variability within each dose and subgroup was very high as demonstrated by the presence of very high standard deviation values (Fig. 1b, Table 2).
Olanzapine
One-way ANOVA comparison of the receptor occupancies obtained for the three tested doses showed a trend towards a statistically significant difference (P < 0.065). Then, unpaired t tests showed a significant difference (P < 0.035) only between the 2 and 5 mg/kg/ day groups (Fig. 1c).
Plasma drug levels and their correspondence with receptor occupancies
Haloperidol
Plasma drug levels showed higher mean values in the peak than in the trough groups (Table 2; Fig. 2a). There were no significant differences in plasma levels between the two doses used. These results coincide with the ones obtained for the receptor occupancy measurements. In addition, an analysis of the correlation between plasma levels and receptor occupancy achieved showed a very high correlation between these two parameters (Pearson's correlation > 79.5% for both treatment doses).
Fig. 2.
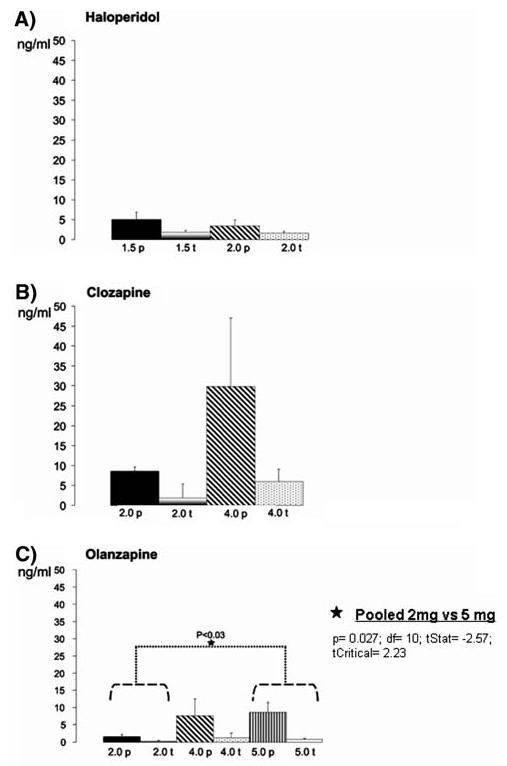
Graphs showing the plasma drug levels achieved at peak and trough time points in the different treatment groups. a Haloperidol: Plasma levels were equivalent between the two doses used. No statistically significant differences were found between the 2 doses tested (peak + trough subgroups of each dose pooled together for the analysis). b Clozapine: note the high variability present (especially in the 40 mg peak group). No statistically significant differences were found between the two doses tested (peak + trough subgroups of each dose pooled together for the analysis). c Olanzapine: note that plasma levels are very low at trough for all three doses. One-way ANOVA did not reveal statistically significant differences among the three doses tested (peak + trough subgroups of each dose pooled together for the analysis) but a t test analysis between the different groups revealed significant differences between the 2 and 5 mg/kg/day treatment groups (*P < 0.03)
Clozapine
Plasma levels of clozapine were higher in animals in the peak subgroups than in the trough subgroups (Table 2; Fig. 2b). Drug plasma levels tended to be different when comparing animals treated with 20 versus 40 mg/kg/day (P < 0.06). Correlation analysis comparing plasma levels and receptor occupancy data for each treatment group revealed poor correlations (Pearson's correlation was 35.45% for the 20 mg/kg/day group and 20.78% for the 40 mg/kg/day group).
Olanzapine
Peak subgroups presented higher plasma drug levels than the trough subgroups of animals treated with the same dose of olanzapine, and mean plasma levels were higher in the groups of animals treated with the higher doses (Table 2; Fig. 2c). One-way ANOVA comparison revealed non significant differences in plasma levels among the three doses. On the contrary, unpaired t test analysis comparing the different treatment groups (2 vs. 4 mg/kg/day, 2 vs. 5 mg/kg/day, 4 vs. 5 mg/kg/day) revealed significant differences in plasma levels between the 2 versus 5 mg treatment groups (P < 0.03), and a trend to significant differences in the 2 versus 4 mg groups (P < 0.055). No significant difference was found between the plasma levels obtained for the 4 and 5 mg treatment groups (P > 0.89). These results are in accordance with the ones obtained for the receptor occupancy measurements (see above). The analysis of the correlation between plasma levels and receptor occupancy achieved showed a very high correlation between these two parameters (Pearson's correlation > 70% for all the treatment doses).
Discussion
In this work we have analyzed the D2 receptor occupancy levels in adult male rats in which three different antipsychotic drugs (haloperidol, clozapine and olanzapine) were administered orally in drinking water. In our study none of the animals in any treatment group developed cataleptic symptoms, a result that indicates that a progressive intake of the drug reduce the possibility to develop undesirable cataleptic side effects. The absence of catalepsy in the haloperidol treated animals is consistent with the fact that none of the animals reached the threshold of D2 receptor occupancy (93% and above) reported as cataleptic in previous dose studies in rats (Wadenberg et al. 2001).
The analysis of our data demonstrates that the administration of haloperidol or olanzapine in drinking water using proper doses is a reliable method to obtain steady-state receptor occupancy levels, and to maintain a clinically relevant D2 receptor occupancy even in the trough groups. Our results also indicate that the use of plasma drug levels as a surrogate of D2 receptor occupancy could be a valid monitoring tool for treatments with haloperidol and olanzapine, even when plasma drug levels are generally low and the variability (standard deviation value) of plasma drug levels within the same group of animals is higher than that of receptor occupancy measurements.
Previous studies in which plasma drug levels have been used as a drug efficacy monitoring parameter have shown high individual variability within groups of animals treated with the same dose of haloperidol or olanzapine in drinking water (see, i.e., plasma levels reported by Sakai et al. 2001a, b; Gao et al. 2005) supporting that this variability is not due to methodological issues. In addition, this variability has been shown not only in the case of animals treated through drinking water but also in the case of animals treated with mini-pumps, subcutaneous or intraperitoneal injections (see, i.e., Aravagiri et al. 1999; Kapur et al. 2003; Perrone et al. 2004), supporting that this variability could be due to the fast clearance of these drugs and drug-derived metabolites from the bloodstream. In fact, it has been demonstrated that the half-life of these drugs is much shorter in rodents than in humans. The half life of haloperidol is 1.5 h in rodents compared to 12–36 h in humans (Cheng and Paalzow 1992; Bezchlibnyk-Buttler and Jeffries 1999), the half life of olanzapine is 2.5 h in rodents versus 20–54 h in humans (Aravagiri et al. 1997; Bezchlibnyk-Buttler and Jeffries 1999), and the half life of clozapine is 1.5 h in rodents compared to 5–16 h in humans (Baldessarini et al. 1993; Bezchlibnyk-Buttler and Jeffries 1999). All these data indicate that for an accurate and reliable measurement of plasma drug levels in animals treated with drugs dissolved in drinking water, a careful design of the experiment is a key player, avoiding factors that can increase the variability such as the existence of delays between the last drug intake and blood collection for analysis. Even in the case of carefully performed experiments, the variability in plasma drug levels can be considerably high for certain drugs (present results). Since plasma levels tend to follow the same pattern as receptor occupancy levels (present results), this suggests that plasma drug levels can be used as an indicator of drug receptor occupancy achievement for olanzapine and haloperidol administered in drinking water.
One of the most striking findings of our study were the results obtained using different doses of clozapine, where a “withdraw-like” effect (meaning that the receptor occupancy levels decrease to values similar to animals not treated with any antipsychotic: baseline D2 receptor occupancy level) was observed for receptor occupancy levels at trough with either dose. If we compare our clozapine treatment data with other administration methods previously used for this drug, a withdraw-like effect for clozapine has also been reported by Kapur et al. (2003) in animals treated with subcutaneous injections of this drug. These authors reported occupancy values that in some cases were below the receptor occupancy of the controls (baseline). In fact, using the same receptor occupancy assay techniques employed in the present study they reported mean D2 occupancy values of 0.2 ± 22 at trough for animals treated with 15 mg/kg/day of clozapine. This suggests that trough withdraw-like effect is not a matter of the method of drug delivery or a measurement error.
Plasma drug level measurements for clozapine revealed an even higher variability than receptor occupancy values for this drug. Statistical analysis comparing plasma drug levels and receptor occupancy data for clozapine revealed a poor correlation between plasma and receptor occupancies (present results). On the contrary, olanzapine and haloperidol treated animals presented a high correlation between plasma drug levels and receptor occupancies achieved (present results).
In summary, our study demonstrates that the administration of appropriate doses of haloperidol and olanzapine in drinking water produces D2 receptor occupancies comparable to clinical occupancy levels. In addition, plasma drug levels for these drugs can be used as a treatment-efficacy monitoring tool once the appropriate dose has been determined using more accurate methodologies like D2 receptor occupancy.
Acknowledgments
We wish to thank Elli Lilly and Co. (Indianapolis, IN, USA) for the donation of the olanzapine used in this study, and Novartis (Basel, Switzerland) for the donation of the clozapine. This work was supported by NIH grant MH66123.
Contributor Information
Emma Perez-Costas, Department of Psychiatry and Behavioral Neurobiology, University of Alabama at Birmingham, SPARKS Center (SC865), 1720 7th Avenue South, Birmingham, AL 35294, USA.
Paolo Guidetti, Maryland Psychiatric Research Center, Department of Psychiatry, University of Maryland School of Medicine, Baltimore, MD 21228, USA.
Miguel Melendez-Ferro, Department of Psychiatry and Behavioral Neurobiology, University of Alabama at Birmingham, SPARKS Center (SC865), 1720 7th Avenue South, Birmingham, AL 35294, USA.
Joyce J. Kelley, Maryland Psychiatric Research Center, Department of Psychiatry, University of Maryland School of Medicine, Baltimore, MD 21228, USA
Rosalinda C. Roberts, Department of Psychiatry and Behavioral Neurobiology, University of Alabama at Birmingham, SPARKS Center (SC865), 1720 7th Avenue South, Birmingham, AL 35294, USA
References
- Aravagiri M, Ames D, Wishing WC, Marder SR. Plasma level monitoring of olanzapine in patients with schizophrenia: determination by high-performance liquid chromatography with electrochemical detection. Ther Drug Monit. 1997;19:307–313. doi: 10.1097/00007691-199706000-00011. [DOI] [PubMed] [Google Scholar]
- Aravagiri M, Teper Y, Marder SR. Pharmacokinetics and tissue distribution of olanzapine in rats. Biopharm Drug Dispos. 1999;20:369–377. doi: 10.1002/1099-081x(199911)20:8<369::aid-bdd200>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Baldessarini RJ, Centorrino F, Flood JG, Volpicelli SA, Huston-Lyons D, Cohen BM. Tissue concentrations of clozapine and its metabolites in the rat. Neuropsychopharmacol. 1993;9:117–124. doi: 10.1038/npp.1993.50. [DOI] [PubMed] [Google Scholar]
- Bezchlibnyk-Butler KZ, Jeffries JJ, editors. Clinical handbook of psychotropic drugs. Hogrefe and Huber Publishers; Toronto: 1999. [Google Scholar]
- Bianchetti G, Morselli PL. Rapid and sensitive method for determination of haloperidol in human samples using nitrogen–phosphorus selective detection. J Chromatogr. 1978;153:203–209. doi: 10.1016/s0021-9673(00)89873-8. [DOI] [PubMed] [Google Scholar]
- Catlow JT, Barton RD, Clemens M, Gillespie TA, Goodwin M, Swanson SP. Analysis of olanzapine in human plasma utilizing reversed-phase high performance liquid chromatography with electrochemical detection. J Chromatogr. 1995;668:85–90. doi: 10.1016/0378-4347(95)00061-m. [DOI] [PubMed] [Google Scholar]
- Ceresoli-Borroni G, Rassoulpour A, Wu HQ, Guidetti P, Schwarcz R. Chronic neuroleptic treatment reduces endogenous kynurenic acid levels in rat brain. J Neural Transm. 2006;113:1355–1365. doi: 10.1007/s00702-005-0432-z. [DOI] [PubMed] [Google Scholar]
- Cheng YF, Paalzow LK. Linear pharmacokinetics of halperidol in the rat. Biopharm Drug Dispos. 1992;13:69–76. doi: 10.1002/bdd.2510130106. [DOI] [PubMed] [Google Scholar]
- Farde L, Wiesel FA, Halldin C, Sedwall G. Central D2-dopamine receptor occupancy in schizophrenic patients treated with antipsychotic drugs. Arch Gen Psychiatr. 1988;45:71–76. doi: 10.1001/archpsyc.1988.01800250087012. [DOI] [PubMed] [Google Scholar]
- Fell MJ, Neill JC, Rao C, Marshall KM. Effects of sub-chronic antipsychotic drug treatment on body weight and reproductive function in juvenile female rats. Psychopharmacology (Berl) 2005;182:499–507. doi: 10.1007/s00213-005-0131-3. [DOI] [PubMed] [Google Scholar]
- Gao XM, Hashimoto T, Cooper TB, Tamminga CA. The dose-response characteristics of rat oral dyskinesias with chronic haloperidol administration. J Neural Transm. 1997;104:97–104. doi: 10.1007/BF01271298. [DOI] [PubMed] [Google Scholar]
- Gao XM, Cooper T, Suckow RF, Tamminga CA. Multidose risperidone treatment evaluated in a rodent model of tardive dyskinesia. Neuropsychopharmacol. 2005;23:1–5. doi: 10.1038/sj.npp.1300975. [DOI] [PubMed] [Google Scholar]
- Kaneda H, Shirakawa O, Dale J, Goodman L, Bachus SE, Tamminga CA. Co-administration of probabide inhibits haloperidol-induced oral dyskinesias. Eur J Pharmacol. 1992;212:43–49. doi: 10.1016/0014-2999(92)90070-k. [DOI] [PubMed] [Google Scholar]
- Kapur S, Seeman P. Does fast dissociation from the dopamine D2 receptor explain the action of atypical antipsychotics?: a new hypothesis. Am J Psychiat. 2001;159:360–369. doi: 10.1176/appi.ajp.158.3.360. [DOI] [PubMed] [Google Scholar]
- Kapur S, Zipursky R, Remington G. Clinical and theoretical implications of 5-HT2 and D2 receptor occupancy of clozapine, risperidone and olanzapine in schizophrenia. Am J Psychiat. 1999;156:286–293. doi: 10.1176/ajp.156.2.286. [DOI] [PubMed] [Google Scholar]
- Kapur S, Wadenberg ML, Remington G. Are animal studies of antipsychotics appropriately dosed. Lessons from the bedside to the bench. Can J Psychiat. 2000;45:241–246. doi: 10.1177/070674370004500302. [DOI] [PubMed] [Google Scholar]
- Kapur S, Vanderspek SC, Brownlee BA, Nobrega J. Antipsychotic dosing in preclinical models is often unrepresentative of the clinical condition: a suggested solution based on in vivo occupancy. J Pharmacol Exp Ther. 2003;305:625–631. doi: 10.1124/jpet.102.046987. [DOI] [PubMed] [Google Scholar]
- Kelley JJ, Roberts RC. Effects of haloperidol on cholinergic striatal interneurons: relationship to oral dyskinesias. J Neural Transm. 2004;111:1075–1091. doi: 10.1007/s00702-004-0131-1. [DOI] [PubMed] [Google Scholar]
- Kelley JJ, Gao XM, Tamminga CA, Roberts RC. The effect of chronic haloperidol treatment on dendritic spines in the rat striatum. Exp Neurol. 1997;146:471–478. doi: 10.1006/exnr.1997.6552. [DOI] [PubMed] [Google Scholar]
- Kleven MS, Assie MB, Cosi C, Barret-Grevoz C, Newman-Tancredi A. Anticataleptic properties of α2 adrenergic antagonists in the crossed leg position and bar tests: differential mediation by 5-HT1A receptor activation. Psychopharmacology. 2005;177:373–380. doi: 10.1007/s00213-004-1970-z. [DOI] [PubMed] [Google Scholar]
- Lipska BK, Weinberger DR. To model a psychiatric disorder in animals: schizophrenia as a reality test. Neuropsychopharmacol. 2000;23:223–239. doi: 10.1016/S0893-133X(00)00137-8. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Mitchell PJ, Redfern PH. Animal models of depressive illness: the importance of chronic drug treatment. Curr Pharm Design. 2005;11:171–203. doi: 10.2174/1381612053382250. [DOI] [PubMed] [Google Scholar]
- Ossowska K, Pietraszek M, Wardas J, Dziedzicka-Wasylewska M, Nowicka D, Wolfarth S. Chronic treatments with haloperidol and clozapine alter the level of NMDA-R1 mRNA in the rat brain: an in situ hybridization study. Pol J Pharmacol. 2002;54:1–9. [PubMed] [Google Scholar]
- Perez-Costas E, Guidetti P, Kelley JJ, Melendez-Ferro M, Roberts RC. Neuroleptics and animal models: feasibility of oral treatment monitored by plasma levels and receptor occupancy assays. Society for Neuroscience; Washington: 2005. (Program No.914.18). 2005 Abstract viewer/Itinerary planner. On line. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Costas E, Guidetti P, Kelley JJ, Melendez-Ferro M, Roberts RC. Feasibility of antipsychotic oral treatments in animal models monitored by plasma levels and receptor occupancy assays. FENS Abstr. 2006;3:A059. doi: 10.1007/s00702-007-0004-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrone JA, Chabla JM, Hallas BH, Horowitz JM, Torres G. Weight loss dynamics during combined fluoxetine and olanza-pine treatment. BMC Pharmacol. 2004;4:27. doi: 10.1186/1471-2210-4-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai A, Mahadik SP. Differential effects of haloperidol and olanzapine on levels of vascular endothelial growth factor and angiogenesis in rat hippocampus. Schizophr Res. 2006;87:48–59. doi: 10.1016/j.schres.2006.06.017. [DOI] [PubMed] [Google Scholar]
- Pouzet B, mow T, Kreilgaard M, Velschow S. Chronic treatment with antipsychotics in rats as a model for antipsychotic-induced gain weight in human. Pharmacol Biochem Be. 2003;75:133–140. doi: 10.1016/s0091-3057(03)00042-x. [DOI] [PubMed] [Google Scholar]
- Prinssen EPM, Kleven MS, Koek W. Interactions between Neuroleptics and 5-HT1A ligands in preclinical behavioral models for antipsychotic and extrapyramidal effects. Psychopharmacology. 1999;144:20–29. doi: 10.1007/s002130050972. [DOI] [PubMed] [Google Scholar]
- Roberts RC. Effect of chronic olanzapine treatment on striatal synaptic organization. Synapse. 2001;39:8–15. doi: 10.1002/1098-2396(20010101)39:1<8::AID-SYN2>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Roberts RC, Lapidus B. Ultrastructural correlates of haloperidol-induced oral dyskinesias in rats: a study of unlabeled and enkephalin-labeled striatal terminals. J Neural Transm. 2003;110:961–975. doi: 10.1007/s00702-003-0013-y. [DOI] [PubMed] [Google Scholar]
- Roberts RC, Gaither LA, Gao XM, Kashyap SM, Tamminga CA. Ultrastructural correlates of haloperidol-induced oral dyskinesias in rat striatum. Synapse. 1995;20:234–243. doi: 10.1002/syn.890200307. [DOI] [PubMed] [Google Scholar]
- Roberts RC, Force M, King L. Dopaminergic synapses in the matrix of the ventromedial striatum after chronic haloperidol treatment. Synapse. 2002;45:78–85. doi: 10.1002/syn.10081. [DOI] [PubMed] [Google Scholar]
- Rosengarten H, Bartoszyk GD, Quartermain D, Lin Y. The effect of chronic administration of sarizotan, 5-HT1A agonist/D3/D4 ligand, on haloperidol-induced repetitive jaw movements in rat model of tardive dyskinesia. Prog Neuro-Psychoph. 2006;30:273–279. doi: 10.1016/j.pnpbp.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Sakai K, Gao XM, Hashimoto T, Tamminga CA. Traditional and new antipsychotic drugs differentially alter neurotransmission markers in basal ganglia-thalamocortical neural pathways. Synapse. 2001a;39:152–160. doi: 10.1002/1098-2396(200102)39:2<152::AID-SYN6>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- Sakai K, Gao XM, Tamminga CA. Scopolamine fails to diminish chronic haloperidol-induced purposeless chewing in rats. Psychopharmacology. 2001b;153:191–195. doi: 10.1007/s002130000570. [DOI] [PubMed] [Google Scholar]
- Schleimer SB, Johnston GAR, Henderson JM. Novel oral drug administration in an animal model of neuroleptic therapy. J Neurosci Method. 2005;146:159–164. doi: 10.1016/j.jneumeth.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Schmitt A, Weber S, Jatzko A, Braus DF, Henn FA. Hippocampal volume and cell proliferation after acute and chronic Clozapine or haloperidol treatment. J Neural Transm. 2004;111:91–100. doi: 10.1007/s00702-003-0070-2. [DOI] [PubMed] [Google Scholar]
- Schmitt U, Dahmen N, Fischer V, Weigmann H, Rao M, Reuss SCH. Chronic oral haloperidol and clozapine in rats: a behavioral evaluation. Neuropsychobiology. 1999;39:86–91. doi: 10.1159/000026566. [DOI] [PubMed] [Google Scholar]
- Simpson GM, Cooper TB. Clozapine plasma levels and convulsions. Am J Psychiat. 1978;135:99–100. doi: 10.1176/ajp.135.1.99. [DOI] [PubMed] [Google Scholar]
- Stahle L, Ungerstedt U. Effects of neuroleptic drugs on the inhibition of exploratory behavior induced by a low dose of apomorphine: implications for the identity of dopamine receptors. Pharmacol Biochem Behav. 1986;25:473–480. doi: 10.1016/0091-3057(86)90026-2. [DOI] [PubMed] [Google Scholar]
- Tamminga CA, Dale JM, Goodman L, Kaneda H, Kaneda N. Neuroleptic-induced vacuous chewing movements as an animal model of tardive dyskinesia: a study in three rat strains. Psychopharmacology. 1990;102:474–478. doi: 10.1007/BF02247127. [DOI] [PubMed] [Google Scholar]
- Terry AV, Jr, Gearhart DA, Mahadik SP, Warsi S, Waller JL. Chronic treatment with first or second generation antipsychotics in rodents: effects on high affinity nicotinic and muscarinic acetylcholinic receptors in the brain. Neuroscience. 2006;140:1277–1287. doi: 10.1016/j.neuroscience.2006.03.011. [DOI] [PubMed] [Google Scholar]
- Wadenberg MLG, Soliman A, VanderSpek SC, Kapur S. Dopamine D2 receptor occupancy is a common mechanism underlying animal models of antipsychotics and their clinical effects. Neuropsychopharmacology. 2001;25:633–641. doi: 10.1016/S0893-133X(01)00261-5. [DOI] [PubMed] [Google Scholar]
- Weigmann H, Hartter s, Fischer V, Dahmen N, Hiemke C. Distribution of clozapine and desmethylclozapine between blood and brain in rats. Eur Neuropsychopharm. 1999;9:253–256. doi: 10.1016/s0924-977x(98)00036-4. [DOI] [PubMed] [Google Scholar]


