Abstract
Variation in emotional processes may contribute to aggressive and defiant behavior. This study assessed these problem behaviors in a large sample of normal healthy children and adolescents in relation to the volume of two cortical regions with prominent roles in emotion processing, the anterior cingulate cortex (ACC) and ventromedial prefrontal cortex (vmPFC). 117 participants (61 boys, 56 girls), age 7–17, were recruited from the community. Aggressive and defiant behavior was measured using the Pediatric Behavior Scale, a parent- and teacher-reported questionnaire and volumetric measures were generated using structural MRI. Regression analyses indicated a significant sex X ACC volume interaction in predicting aggressive and defiant behavior, without significant results for the vmPFC. Followup analyses showed that aggressive and defiant behavior is associated with decreased right ACC volume in boys and a non-significant reduction in left ACC volume in girls. These results are consistent with the notion that the right ACC acts as a neuroanatomical correlate of aggression and defiance in boys. We discuss this finding in light of its implications for understanding the neural correlates of antisocial behavior.
Keywords: antisocial, children, FreeSurfer, sex differences, structural MRI
Humans are an exceptionally social species and, as such, much of our cognitive and behavioral repertoire is adapted to accommodate a social existence. Over the course of development new competencies emerge that facilitate prosocial behavior, such as the ability to modulate aggression and hostile behavior in accord with the contextual appropriateness of such behavior. The emergence of prosocial behavior likely relies on a combination of learning experiences and the maturation of specific neural systems in the brain, though the details of this process are not well understood. Also, variations in social behavior may reflect differences in the structural and functional organization of these neural systems. Better understanding of the neurobiological contribution to social behavior could offer important clues regarding the etiology of pathological antisocial behavior and violence.
Prominent theories suggest that neural systems that underlie emotional processes may play a critical role in the development of prosocial behavior, and deficits in emotion processing may contribute to antisocial behavior (Damasio, 2000; Davidson et al., 2000). Support for these theories is derived from converging evidence of three main types: 1) emotion deficits are often found in association with pathological antisocial behavior (Scarpa and Raine, 1997; Blair et al., 1999; Blair et al., 2001a; Blair et al., 2001b; Cimbora and McIntosh, 2003; Frick and Morris, 2004; Blair et al., 2005), 2) circumscribed damage to emotion processing regions of the brain sometimes leads to antisocial behavior (Damasio et al., 1990; Tonkonogy, 1991; Tranel, 1994; Grafman et al., 1996; Blair and Cipolotti, 2000), and 3) functional imaging studies have detected abnormal activity in emotion processing regions of the brain in association with pathological antisocial behavior (Veit et al., 2002; Sterzer et al., 2005). Two emotion processing regions of the cerebral cortex that have been implicated in social behavior are the anterior cingulate cortex (ACC) (Devinsky et al., 1995) and the ventromedial prefrontal cortex (vmPFC) (Damasio, 2000) (also see discussion).
Also of interest to research on emotion and social behavior is the issue of sex differences. The prevalence of pathological antisocial behavior in males is much higher relative to females (American Psychiatric Association, 1994), perhaps due to differences in the brain. There are early reports of hemispheric asymmetry in emotion processing structures that differ by sex. For instance, more severe emotional and behavioral impairments follow unilateral damage to the right vmPFC in males and left vmPFC in females (Tranel et al., 2002; Tranel et al., 2005). Preliminary reports of early-onset vmPFC damage also follow this pattern (Anderson et al., 2006).
The current study was designed to investigate whether aggressive and defiant behavior corresponds to volumetric differences in the ACC and vmPFC. Specifically, we hypothesized that in a large sample of normal healthy children and adolescents without any psychiatric diagnosis, subjects with higher aggression-defiance would have lower ACC and vmPFC volume, i.e. inversely correlated. Another aim of the study was to assess whether sex differences exist in the aforementioned relationship. Specifically, we hypothesized a stronger relationship of aggression-defiance and ACC / vmPFC volumes in the right hemisphere in boys and in the left hemisphere in girls.
MATERIALS AND METHODS
Participants
117 healthy children and adolescents (61 boys, 56 girls), age 7–17, were recruited from the community using local advertisements. These subjects served as a comparison group for another study on brain structure and function in children with cleft lip and palate (Nopoulos et al., 2007). A phone screening interview was performed to exclude subjects with any medical or neurological disease that required significant medical intervention. Additional exclusion criteria included any reported current or past diagnosis of a psychiatric disorder or learning disorder. The protocol was approved by the University of Iowa Human Subjects Institutional Review Board and written informed consent was obtained for all subjects prior to participation.
Demographics
Demographic data included age, parental socioeconomic status (SES), IQ, birth history, and handedness. SES was determined using a modified Hollingshead scale of 1 to 5, with a lower number corresponding to higher social class (Hollingshead, 1975). IQ was estimated using the full scale Wechsler Intelligence Scale for Children, 4th ed. (Wechsler, 2003). Prenatal, obstetrical, and developmental history was obtained using a 40 item Birth and Developmental History (DeLisi et al., 1988). Handedness was determined using the Physical and Neurologic Evaluation of Subtle Signs (Denckla, 1985).
Behavioral Measure
The Pediatric Behavior Scale, short version (PBS) is a screening tool for emotional and behavioral problems derived from the Child Behavior Checklist (CBCL) (Achenbach and Edelbrock, 1983) and Pediatric Behavior Scale (Lindgren and Koeppl, 1987). For each subject a parent and a teacher were asked to rate problems on a 4 point Likert scale (0 – 3), with a lower score indicating fewer problems. For the current study the conduct scale provided the behaviors of interest, which assessed symptoms of aggression and defiance. Individual questions included: 1) mean or cruel to others, 2) threatens, bullies, or picks on other children, 3) starts fights, 4) hits, bites, or throws things at people, 5) disobedient; won’t mind or follow rules, 6) argues or quarrels, 7) irritable; gets angry or annoyed easily, 8) loses temper; has temper tantrums, and 9) shouts or screams a lot. Due to a high co-occurrence of conduct problems with symptoms of impulsivity and hyperactivity a second PBS scale, the impulse control scale, was included. This scale assessed the following behaviors: 1) impulsive; acts without stopping to think, 2) can’t stand waiting; wants things right away, 3) interrupts, talks out of turn, or blurts things out, 4) fails to finish things he or she starts, 5) hyperactive; always “on the go,” 6) squirms or fidgets, 7) restless, can’t sit still. This scale was used as a covariate in statistical analyses to ensure that individual differences in impulsiveness and hyperactivity did not confound structure-function findings with regards to aggression-defiance.
The parent and teacher response rate for the PBS was 100% and 90% respectively. As a means of data reduction the PBS scores from the parent and teacher were summed. The parent’s rating was doubled for subjects without teacher-reported ratings (n=17). Other methods of data reduction were attempted (average, higher of two ratings) and produced the same results. The sum rating was selected because both parent and teacher ratings were preserved and the behavioral variance was maximized in the sample. The summed conduct ratings correlated significantly with individual parent- and teacher-reported ratings using Pearson correlation [r = .82, p = .000; r = .79, p = .000; respectively]. The reliability of the PBS conduct scale was established using a longer version of the PBS, which estimated the internal consistency coefficient of the conduct scale at .92 (Lindgren and Koeppl, 1987). The PBS scales were derived from factor analysis with varimax rotation from a sample of 600 children ages 6–12. The scales were obtained from a 4-factor solution with all eigenvalues greater than one. All questions in this analysis correspond to similar items on the CBCL. Also, all of the items on this scale do not cross-load to any significant degree (less than .30) with other PBS scales.
MRI Acquisition
MRI scans were obtained using a 1.5 Tesla General Electric SIGNA System (GE Medical Systems, Milwaukee, WI). Three-dimensional (3D) T1 weighted images were acquired in the coronal plane using a spoiled grass sequence with the following parameters: 1.5 mm coronal slices, 40° flip angle, 24 msec repetition time (TR), 5 ms echo time (TE), 2 number of excitations (NEX), 26 cm field of view (FOV) and a 256X192 matrix. The proton density (PD) and T2 weighted images were acquired with the following parameters: 3.0 mm coronal slices, 36 msec TE (for PD) or 96 msec TE (for T2), 3000 msec TR, 1 NEX, 26 cm FOV, 256X192 matrix and an echo train length=1.
Image Processing
MRI data were processed using BRAINS2 (Brain Research: Analysis of Images, Networks, and Systems), our locally developed software, described elsewhere (Magnotta et al., 2002). T1 weighted images were spatially normalized and resampled to 1.015625 mm³ voxels and the anterior-posterior axis of the brain was realigned parallel to the anterior commissure–posterior commissure line. The interhemispheric fissure was aligned by selecting points along the fissure in the coronal and axial views. T2 and PD weighted images were aligned to the spatially normalized T1 weighted image (Woods et al., 1992) to allow the use of a multimodal discriminant classifier. The resulting classified image was used for the application of an artificial neural network that creates an automated brain mask (Harris et al., 1999). This mask was visually inspected and manually edited by trained, reliable technicians. The resulting intracranial volume (ICV) mask includes all brain tissue and both internal and surface cerebrospinal fluid.
The T1 acquisition was processed using FreeSurfer (http://www.martinos.org/freesurfer), an automated parcellation software program (Desikan et al., 2006). The output of interest was volumetric measures of the total cerebral cortex gray matter volume and volume of the predefined regions of interest (ROIs), the ACC and vmPFC. The anatomical accuracy of FreeSurfer parcellation in the ROIs was visually inspected (by A.D.B. and P.N.) and those with unacceptable parcellation were excluded from all analyses (2 right ACC and 5 left ACC). Common reasons for exclusion included problems with the corpus callosum bleeding into the rostral ACC or only the outer portion of a double cingulate was labeled.
ACC and vmPFC Definition
Figure 1 displays the anatomical regions of interest (ROIs) for the current study, which were defined using standard FreeSurfer parcellation. The ACC includes a rostral and caudal division, (rACC and cACC, respectively). The vmPFC is composed of two regions defined by FreeSurfer, the medial orbitofrontal cortex (mOFC) and lateral orbitofrontal cortex (lOFC). The mOFC is composed of the ventral surface of the medial prefrontal cortex and the straight gyrus. The lOFC contains the rest of the OFC, excluding the lateral-most sector. The anatomical boundaries of these regions and the intraclass correlation coefficient describing the correlation of automated and manual parcellation methods for each region are reported elsewhere (Desikan et al., 2006).
Figure 1.
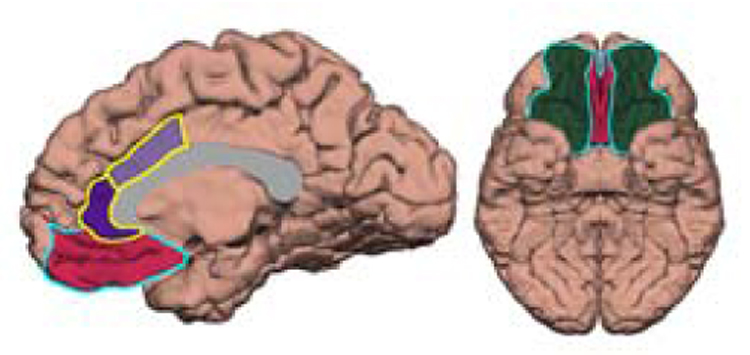
This figure shows a medial (left) and ventral (right) view of the cerebral cortex demonstrating the regions of interest for the current study. The anterior cingulate cortex is outlined in yellow and is composed of a rostral (dark purple) and caudal (light purple) divisions. The ventromedial prefrontal cortex is outlined in light blue and contains the medial orbitofrontal cortex (mOFC) in red and lateral orbitofrontal cortex (lOFC) in dark green, according to the FreeSurfer terminology (also see description in methods section). The corpus callosum (in gray) was generated manually and included to illustrate a relevant landmark.
Statistical Analysis
All analyses were performed using SPSS 13.0 for Windows (SPSS Inc. Chicago, IL). Independent samples t tests analyzed sex differences in quantitative descriptive data, including demographic, behavioral, and volumetric measures. The relationship of conduct ratings to multivariate predictors was assessed with hierarchical multiple regression. Significant structural findings in the regression model were followed up with Spearman partial correlation coefficients to investigate the nature of the relationship of the conduct ratings and volumetric measures. We performed two additional analyses aimed at assessing the specificity of our correlation test and ruling out alternative explanations. First, we chose a specific region of the cortex that we hypothesized would have no relationship to our behavioral measure, the occipital lobe, and correlated its gray matter volume with conduct ratings. Next, we performed the same statistical analysis as described for conduct ratings with the physical health PBS measure, predicting a non-significant correlation.
ROI volumes that significantly correlated with conduct ratings were compared in subjects with high versus low conduct scores using general linear models analysis of variance (ANCOVA). High and low conduct ratings were defined by separating subjects into tertiles according to their scores. The purpose of this analysis was to determine whether volumetric differences driving significant correlations reached statistical significance in comparing subjects at each end of the behavioral distribution. Also, demographic variables were compared among these “high” and “low” groups using independent t tests.
RESULTS
Descriptive
The boys and girls in the sample had similar outcomes on demographic measures (Table 1). IQ scores were somewhat above the population mean of 100 for both genders (consistent with the demographics of Iowa City and the surrounding area). The behavioral data revealed fairly low mean levels of conduct problems and impulsiveness \ hyperactivity in the sample, with a positive skew in the distribution. Boys had higher levels of behavioral problems relative to girls. Boys had larger total cortical gray matter volume than girls, though volume of the ROIs did not differ between sexes when expressed as a percentage of the cortical volume. Review of the Birth and Developmental History questionnaire revealed 1 subject with significant alcohol exposure prenatally.
Table 1.
Descriptive Data: Demographic, Behavioral, and Structural
| Boys | Girls | |||
|---|---|---|---|---|
| Variable | Measure | |||
| (n=61) | (n=56) | Sig. (p) | ||
| Age | Range | 7.75 – 17.92 | 7.08 – 17.58 | |
| Mean (s.d.) | 12.08 (2.71) | 12.49 (2.87) | .42 | |
| IQ | Mean (s.d.) | 112 (16) | 108 (13) | .11 |
| SES | Mean (s.d.) | 2.29 (.57) | 2.28 (.52) | .94 |
| Handedness | 53 RH, 7 LH, 1 A | 50 RH, 6 LH | ||
| Impulsivity Rating | Range | 0 – 27 | 0 – 23 | |
| Mean (s.d.) | 6.32 (5.39) | 3.94 (4.76) | .01 | |
| Conduct Rating | Range | 0 – 24 | 0 – 16 | |
| Mean (s.d.) | 5.85 (5.28) | 3.69 (3.36) | .01 | |
| Total Cortex Volume (cc) | Mean (s.d.) | 504 (35) | 468 (29) | .000 |
| R ACC* | Mean (s.d.) | .78 (.11) | .81 (.14) | .23 |
| L ACC* | Mean (s.d.) | .78 (.13) | .81 (.14) | .23 |
| R vmPFC* | Mean (s.d.) | 2.95 (.21) | 2.96 (.29) | .74 |
| L vmPFC* | Mean (s.d.) | 3.03 (.24) | 3.05 (.25) | .68 |
Sig. = Significance, independent samples t-test.
Regional structural measures are expressed as a percentage of total cortex volume.
ACC = anterior cingulate cortex, A = ambidextrious, L = left, LH = left handed, R = right, RH = right handed SES = parent socioeconomic status, vmPFC = ventromedial prefrontal cortex, Vol = volume
Multiple Regression
Results of the hierarchical multiple regression analysis are summarized in Table 2. Step 1 included demographic data (sex, IQ, SES, age), total gray matter volume of the cerebral cortex, and ratings of impulse control. Age and IQ were found to have a negligible effect on the model and were subsequently dropped. Step 2 included volumetric measures of the right and left ACC and vmPFC. Step 3 of the model included interaction terms for ROI volumes X sex. Non-significant interaction terms were dropped from the model. The volumetric data were centered by mean subtraction to avoid problems with multicollinearity. Behavioral data were rank-ordered to correct a positive skew. Change in R² was statistically significant for steps 1 and 3, with an effect size estimated at .29 and .10 using Cohen’s f², which is considered moderate and small respectively. Individual variables within step 1 that reached significance include SES and ratings of impulse control. In step 3 there was a significant interaction effect for sex X right ACC volume and sex X left ACC volume. As a result of the interaction effects subsequent analyses were performed separately for boys and girls.
Table 2.
Predictors of Conduct Behavioral Ratings
| Variable | β | Sig |
|---|---|---|
| Step 1a | ||
| SES | .20 | .01 |
| Sex | .02 | .78 |
| Total Cortex Volume | .14 | .15 |
| Impulse Control | .42 | <.0005 |
| Step 2b | ||
| R ACC | −.12 | .16 |
| L ACC | .02 | .76 |
| R vmPFC | .04 | .72 |
| L vmPFC | −.09 | .47 |
| Step 3c | ||
| R ACC * Sex | .72 | .01 |
| L ACC * Sex | −.53 | .04 |
R² Change = .23, F Change = 8.15, Sig. = .000
R² Change = .02, F Change = .66, Sig. = .61
R² Change = .07, F Change = 5.40, Sig. = .006
Correlation Findings
Spearman partial correlation analyses of conduct ratings and ACC volume are illustrated in Table 3. Covariates included SES, total cortex volume, and impulse control. There was a statistically significant inverse (negative) correlation of conduct ratings and volume of the right ACC in boys [r = −.42, p = .002], whereas in girls there was a nonsignificant negative correlation with left ACC [r = −.22, p = .12].
Table 3.
Correlation† of conduct ratings and anterior cingulate cortex volume
| Conduct Behavioral Measure |
||
|---|---|---|
| ROI | Boys (n = 61) | Girls (n = 56) |
| L ACC | .15 (.28) | −.22 (.12) |
| R ACC | −.42 (.002) | .15 (.27) |
Spearman partial correlations control for impulse control rating, total cortex volume, and parent socioeconomic status (SES).
ACC = anterior cingulate cortex, L = left, ROI = region of interest, R = right
Demonstration of Specificity
The Spearman partial correlations of occipital lobe volume and conduct ratings were not significant in the right or left hemisphere for either sex. There were no significant correlations of the PBS physical health ratings with ACC volume. These results are displayed in Table 4.
Table 4.
Correlations† demonstrating specificity
| Conduct | Physical Health | |||
|---|---|---|---|---|
| ROI | Boys | Girls | Boys | Girls |
| L Occipital Volume | .02 (.83) | −.06 (.67) | - | - |
| R Occipital Volume | −.03 (.80) | .06 (.63) | - | - |
| L ACC | - | - | −.08 (.57) | .24 (.09) |
| R ACC | - | - | .07 (.61) | −.03 (.78) |
Controlling for impulse control, socioeconomic status, and total cortex volume. N= 61 boys, 56 girls
Follow-up Analyses of High and Low Conduct Problem Groups
Table 5 reports demographic, behavioral, and volumetric data for boys separated into upper and lower tertiles according to their conduct rating (extreme group comparison). Quantitative demographic variables were compared among the two groups using independent t tests, which revealed nonsignificant differences for all demographic variables. Significant differences in ratings of conduct and impulse control ratings were noted (as expected based on the classification approach). The Table also shows results from an ANCOVA (controlling for SES, total cortical volume, and impulse control rating) used to test whether significant differences in the right ACC exist in comparing boys with High versus Low conduct score. Boys with higher scores had significantly less volume of the right ACC [F = 14.21, p = .001]. The effect size was large, estimated at .289 using partial Eta squared.
Table 5.
Extreme group comparison (Boys)
| Low | High | |||
|---|---|---|---|---|
| Measure | Sig | |||
| (n=20) | (n =20) | |||
| Age | Range | 9.00 – 17.92 | 7.92 – 16.92 | |
| Mean (s.d.) | 12.65 (2.77) | 11.80 (2.63) | .29 | |
| IQ | Mean (s.d.) | 114 (14) | 112 (17) | .50 |
| SES | Mean (s.d.) | 2.16 (.48) | 2.38 (.73) | .28 |
| Handedness | 16 RH, 4 LH | 18 RH, 2 LH | ||
| Impulsivity Rating | Range | 0 – 9 | 0 – 27 | |
| Mean (s.d.) | 3.85 (2.08) | 9.25 (6.06) | .01 | |
| Conduct Rating | Range | 0 – 3 | 5 – 24 | |
| Mean (s.d.) | 1.65 (1.18) | 11.40 (5.64) | .000 | |
| Total Cortex Volume (cc) | Mean volume (s.d) | 502.76 (3.54) | 515.17 (8.66) | .29 |
| R ACC Volume (cc) | Adjusted Mean volume (s.e.) | 4.37 (.12) | 3.64 (.12) | F = 14.21 (.001) |
ACC = anterior cingulate cortex, cc = cubic centimeters, L = left, LH, left handed, R = right, RH = right handed
The analyses were repeated after excluding the subject with prenatal alcohol exposure. The results were identical, and thus the subject’s data were included.
DISCUSSION
Our findings provide preliminary support for the right ACC as a neuroanatomical correlate of aggressive and defiant behavior in boys. Overall, the significant results were as follows: 1) there was a significant interaction of sex and ACC volume bilaterally in a regression model predicting aggressive and defiant behavior. 2) Spearman’s partial correlation revealed a significant negative correlation of aggression-defiance ratings and volume of the right ACC in boys (r = −.42). 3) In comparing subjects with high versus low levels of aggression-defiance, boys with high levels had significantly less volume of the right ACC.
In girls, the left ACC correlated negatively with aggression-defiance, but the result was not statistically significant. We did not find a significant relationship between aggression-defiance and vmPFC volumes in either boys or girls.
The finding of a neuroanatomical correlate of aggression-defiance in the ACC presents the possibility that anatomical variation in this cortical region may reflect functional variations manifest as behavioral differences. This idea is supported by convergent methods demonstrating a role of the ACC in social behavior and aggression.
The ACC, social behavior, and aggression
Several methodologies have implicated the ACC as a critical neural substrate of social behavior (Devinsky et al., 1995). Boys with conduct disorder have decreased activity in the right caudal ACC when viewing stimuli with negative emotional salience relative to a comparison group (Sterzer et al., 2005; Stadler et al., 2007). Studies of impulsive aggression have shown abnormally high levels of ACC activity (Eisenberger et al., 2006) and decreased serotonin transporter levels in the ACC (Frankle et al., 2005). Also, a known genetic risk factor for impulsive aggression is associated with decreased overall cingulate cortex volume (Meyer-Lindenberg et al., 2006).
Consensus regarding the functional contribution of the ACC to social behavior is lacking. In the field of developmental psychology there has been extensive work describing the temperamental correlates of antisocial behavior. Temperament may provide a phenotype that is more proximal to the structure and function of specific neural systems than behavioral conduct problems. With this in mind, we turn to a discussion of the possible role of the ACC in the two temperamental traits most commonly implicated in conduct problems and antisocial behavior: 1) negative emotionality and 2) low effortful regulation of behavior (Casey and Schlosser, 1994; Rothbart et al., 1994; Davidson et al., 2000; Melnick and Hinshaw, 2000; Blair et al., 2001a; Blair et al., 2001b; Izard et al., 2002; Eisenberg et al., 2005; Garnefski et al., 2005; Hill et al., 2006).
ACC and Negative Emotionality
Increased levels of negative emotions, such as anger, are commonly associated with temper tantrums, aggressive outbursts, and more generally, antisocial behavior. This may stem from hyperresponsive triggering of negative emotions, an inability to modulate negative emotions, or an inability to implement socially appropriate behavior under the influence of negative emotion. The ACC is believed to have a prominent role in modulating arousal, which is a central feature of negative emotions. ACC activity correlates with overall cortical arousal (Paus, 2000) and peripheral arousal (e.g. cardiovascular tone, skin conductance) (Critchley, 2005), which is blunted following ACC damage (Tranel and Damasio, 1994). The connectivity of the ACC and amygdala may provide a means by which emotional arousal is modulated (Ghashghaei et al., 2007). A recent study has shown that activity of the caudal ACC closely precedes inhibition of the amygdala, and decreased volume of the ACC corresponds to increased amygdala activity (Pezawas et al., 2005).
In contrast to overactive negative emotions, a severely restricted emotional repertoire may also be a vulnerability factor for antisocial behavior. Externalizing / antisocial behavior has been associated with fearlessness and guiltlessness (Rothbart et al., 1994; Keltner et al., 1995; Kochanska et al., 2002; Frick and Morris, 2004) as well as physiologic underarousal (Blair et al., 1997; Blair, 1999; Raine et al., 2000; Lorber, 2004; Ortiz and Raine, 2004; Herpertz et al., 2005). Such individuals may be insensitive to punishment and other social cues that guide prosocial behavior, and more prone to take risks or engage in sensation-seeking behavior. Hypo-emotionality may also be reflected in ACC morphometry, as decreased surface area of the right ACC was reported in association with fearlessness in a healthy sample of children (Pujol et al., 2002).
Low Effortful Control
Effortful control is typically conceptualized as the voluntary capacity to regulate behavior; a mechanism that may interact with the influence of more basic drives and emotions in goal directed behavior (Posner and Rothbart, 1998). Impairments in effortful control are associated with disruptive and antisocial behavior (Rothbart et al., 1994), particularly when coupled with increased negative emotionality (Eisenberg et al., 1996). Moreover, effortful control has a prominent role in the development of moral conduct (Kochanska and Aksan, 2006).
The ACC is believed to have a critical role in the development and implementation of effortful control (Rothbart et al., 2000). The ACC may facilitate bringing cognitive neural resources to bear on situations of conflict, supported by a positive correlative relationship of task performance requiring behavioral control and reciprocal activity of the caudal ACC-prefrontal cortex (Kerns et al., 2004). The ACC may also act as an interface between neural systems of emotion and cognition (Bush et al., 2000), in line with ACC activity being linked to the conscious awareness of emotion (Lane et al., 1998). The volume of the ACC may also reflect its role in effortful control, as children with lower ACC volume demonstrate decreased attention when effortful control is needed (Casey et al., 1997).
The picture that emerges from this evidence is that the ACC may be involved in several processes that have known associations with antisocial behavior and aggression. In the current study we did not address emotion or effortful regulation explicitly. Future work must tease apart the neurobiology of each of these more proximal temperaments associated with antisocial behavior. This will be challenging, though. Despite the seemingly opposite ends of the emotion spectrum, the neural systems that mediate excessive negative emotionality and hypo-emotionality may lie in close proximity or overlap. Damage to the vmPFC, for instance, often leads to a paucity of emotion and lack of empathy with the exception of outbursts of negative emotion (Anderson et al., 2006; Koenigs and Tranel, 2007). Similarly, traits that are highly comorbid with antisocial behavior (e.g. impulsiveness) may rely on adjacent or overlapping neural systems and this will complicate the study of traits such as aggression in isolation. Moreover, the environment has an influence on these behaviors (evidenced here by SES being a significant predictor of aggressive and defiant behavior) and the degree to which environmental factors alter brain development is poorly understood.
Hemispheric Asymmetry and Sex Differences
A second aim of the current study was to address whether sex differences exist in the relationship of aggression-defiance and structural measures, such that volume of ROIs would negatively correlate with conduct problems on the right side in boys and the left side in girls. The regression analysis did reveal a significant interaction of sex and ACC volume. Follow-up correlation tests fit the hypothesized pattern, with a negative correlation for the right ACC in boys and for the left ACC in girls. However, this structure-function relationship was not statistically significant for girls. The lack of significance may be attributable to much lower variance in conduct ratings in girls.
Neurobiology of Normal Behavior
It is important to emphasize that the participants in this study were normal healthy children who were not demonstrating pathological conduct problems. There are some advantages to studying normal variance in behavior. It is possible to recruit large samples without co-morbid substance abuse or medication use that may confound structure-function relationships in pathological groups. A limitation, though, is much lower behavioral variance relative to a between-group comparison of subjects with pathological conduct problems versus a normal comparison group. Moreover, we cannot directly address whether the findings generalize to more severe antisocial problems. There is evidence that symptoms of antisocial behavior exist along a continuum with non-pathological symptoms, as a dimensional rather than categorical construct (Edens et al., 2006). It is reasonable, then, to postulate that a similar continuum may exist in the neural correlates to these symptoms, though future work will be needed to address this issue empirically.
Other limitations of the analysis include the absence of a clinical interview to assess substance use or the possibility of unreported psychopathology. Because this was a normal sample of children and adolescents it is unlikely that the conclusions of the study were significantly influenced by these factors. However, because our conclusions focus on the normal, healthy status of the subjects it must be noted that psychopathology was not formally assessed. Nonetheless, we feel that even with such limitations, the data provide a convincing preliminary demonstration of a brain-behavior relationship.
The current study contributes to the ongoing effort to elucidate the biological underpinnings of antisocial behavior, which may complement alternate approaches such as functional neuroimaging and identifying the genes involved. Advances in this endeavor may ultimately inform the diagnostic approach and efforts at intervention for antisocial disorders. Ultimately preventative efforts may target specific genetic, neural, and environmental risk factors.
Acknowledgments
Supported by NIDCR 1 RO1 DE01 14399 01 A1, NIDA R01 DA022549, and NINDS P01 NS19632
Footnotes
No authors report any conflicts of interest, financial or otherwise.
REFERENCES
- Achenbach TM, Edelbrock C. Manual for the child behavior checklist and revised child behavior profile. Burlington: University of Vermont; 1983. [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th Edition. Washington, DC: American Psychiatric Association; 1994. [Google Scholar]
- Anderson SW, Barrash J, Bechara A, Tranel D. Impairments of emotion and real-world complex behavior following childhood- or adult-onset damage to ventromedial prefrontal cortex. Journal of the International Neuropsychological Society. 2006;12:224–235. doi: 10.1017/S1355617706060346. [DOI] [PubMed] [Google Scholar]
- Blair RJ. Responsiveness to distress cues in the child with psychopathic tendencies. Personality and Individual Differences. 1999;27:135–145. [Google Scholar]
- Blair RJ, Cipolotti L. Impaired social response reversal. A case of ‘acquired sociopathy’. Brain. 2000;123:1122–1141. doi: 10.1093/brain/123.6.1122. [DOI] [PubMed] [Google Scholar]
- Blair RJ, Colledge E, Mitchell DG. Somatic markers and response reversal: is there orbitofrontal cortex dysfunction in boys with psychopathic tendencies? Journal of Abnormal Child Psychology. 2001a;29:499–511. doi: 10.1023/a:1012277125119. [DOI] [PubMed] [Google Scholar]
- Blair RJ, Jones L, Clark F, Smith M. The psychopathic individual: a lack of responsiveness to distress cues? Psychophysiology. 1997;34:192–198. doi: 10.1111/j.1469-8986.1997.tb02131.x. [DOI] [PubMed] [Google Scholar]
- Blair RJ, Colledge E, Murray L, Mitchell DG. A selective impairment in the processing of sad and fearful expressions in children with psychopathic tendencies. Journal of Abnormal Child Psychology. 2001b;29:491–498. doi: 10.1023/a:1012225108281. [DOI] [PubMed] [Google Scholar]
- Blair RJ, Budhani S, Colledge E, Scott S. Deafness to fear in boys with psychopathic tendencies. Journal of Child Psychology & Psychiatry & Allied Disciplines. 2005;46:327–336. doi: 10.1111/j.1469-7610.2004.00356.x. [DOI] [PubMed] [Google Scholar]
- Blair RJ, Morris JS, Frith CD, Perrett DI, Dolan RJ. Dissociable neural responses to facial expressions of sadness and anger. Brain. 1999;122:883–893. doi: 10.1093/brain/122.5.883. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends Cogn Sci. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Trainor R, Giedd J, Vauss Y, Vaituzis CK, Hamburger S, Kozuch P, Rapoport JL. The role of the anterior cingulate in automatic and controlled processes: a developmental neuroanatomical study. Dev Psychobiol. 1997;30:61–69. [PubMed] [Google Scholar]
- Casey RJ, Schlosser S. Emotional responses to peer praise in children with and without a diagnosed externalizing disorder. Merrill-Palmer Quarterly. 1994;40 [Google Scholar]
- Cimbora DM, McIntosh DN. Emotional responses to antisocial acts in adolescent males with conduct disorder: a link to affective morality. Journal of Clinical Child & Adolescent Psychology. 2003;32:296–301. doi: 10.1207/S15374424JCCP3202_16. [DOI] [PubMed] [Google Scholar]
- Critchley HD. Neural mechanisms of autonomic, affective, and cognitive integration. J Comp Neurol. 2005;493:154–166. doi: 10.1002/cne.20749. [DOI] [PubMed] [Google Scholar]
- Damasio AR. A neural basis for sociopathy. Archives of General Psychiatry. 2000;57:128–129. [Google Scholar]
- Damasio AR, Tranel D, Damasio H. Individuals with sociopathic behavior caused by frontal damage fail to respond autonomically to social stimuli. Behavioural Brain Research. 1990;41:81–94. doi: 10.1016/0166-4328(90)90144-4. [DOI] [PubMed] [Google Scholar]
- Davidson RJ, Putnam KM, Larson CL. Dysfunction in the neural circuitry of emotion regulation--a possible prelude to violence.[see comment] Science. 2000;289:591–594. doi: 10.1126/science.289.5479.591. [DOI] [PubMed] [Google Scholar]
- DeLisi LE, Dauphinais ID, Gershon ES. Perinatal complications and reduced size of brain limbic structures in familial schizophrenia. Schizophr Bull. 1988;14:185–191. doi: 10.1093/schbul/14.2.185. [DOI] [PubMed] [Google Scholar]
- Denckla M. Revised Neurological Examination for Subtle Signs. Psychopharmacol Bull. 1985;21:773–800. [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT, Albert MS, Killiany RJ. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain. 1995;118(Pt 1):279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- Edens JF, Marcus DK, Lilienfeld SO, Poythress NG., Jr. Psychopathic, not psychopath: taxometric evidence for the dimensional structure of psychopathy. J Abnorm Psychol. 2006;115:131–144. doi: 10.1037/0021-843X.115.1.131. [DOI] [PubMed] [Google Scholar]
- Eisenberg N, Fabes RA, Karbon M, Murphy BC, Wosinski M, Polazzi L, Carlo G, Juhnke C. The relations of children's dispositional prosocial behavior to emotionality, regulation, and social functioning. Child Dev. 1996;67:974–992. [PubMed] [Google Scholar]
- Eisenberg N, Sadovsky A, Spinrad TL, Fabes RA, Losoya SH, Valiente C, Reiser M, Cumberland A, Shepard SA. The relations of problem behavior status to children's negative emotionality, effortful control, and impulsivity: concurrent relations and prediction of change. Dev Psychol. 2005;41:193–211. doi: 10.1037/0012-1649.41.1.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberger NI, Way BM, Taylor SE, Welch WT, Lieberman MD. Understanding Genetic Risk for Aggression: Clues From the Brain's Response to Social Exclusion. Biol Psychiatry. 2006 doi: 10.1016/j.biopsych.2006.08.007. [DOI] [PubMed] [Google Scholar]
- Frankle WG, Lombardo I, New AS, Goodman M, Talbot PS, Huang Y, Hwang DR, Slifstein M, Curry S, Abi-Dargham A, Laruelle M, Siever LJ. Brain serotonin transporter distribution in subjects with impulsive aggressivity: a positron emission study with [11C]McN 5652. Am J Psychiatry. 2005;162:915–923. doi: 10.1176/appi.ajp.162.5.915. [DOI] [PubMed] [Google Scholar]
- Frick PJ, Morris AS. Temperament and developmental pathways to conduct problems. J Clin Child Adolesc Psychol. 2004;33:54–68. doi: 10.1207/S15374424JCCP3301_6. [DOI] [PubMed] [Google Scholar]
- Garnefski N, Kraaij V, van Etten M. Specificity of relations between adolescents' cognitive emotion regulation strategies and Internalizing and Externalizing psychopathology. J Adolesc. 2005;28:619–631. doi: 10.1016/j.adolescence.2004.12.009. [DOI] [PubMed] [Google Scholar]
- Ghashghaei HT, Hilgetag CC, Barbas H. Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala. Neuroimage. 2007;34:905–923. doi: 10.1016/j.neuroimage.2006.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grafman J, Schwab K, Warden D, Pridgen A, Brown HR, Salazar AM. Frontal lobe injuries, violence, and aggression: a report of the Vietnam Head Injury Study. Neurology. 1996;46:1231–1238. doi: 10.1212/wnl.46.5.1231. [DOI] [PubMed] [Google Scholar]
- Harris G, Andreasen NC, Cizadlo T, Bailey JM, Bockholt HJ, Magnotta VA, Arndt S. Improving tissue classification in MRI: a three-dimensional multispectral discriminant analysis method with automated training class selection. Journal Of Computer Assisted Tomography. 1999;23:144–154. doi: 10.1097/00004728-199901000-00030. [DOI] [PubMed] [Google Scholar]
- Herpertz SC, Mueller B, Qunaibi M, Lichterfeld C, Konrad K, Herpertz-Dahlmann B. Response to emotional stimuli in boys with conduct disorder. American Journal of Psychiatry. 2005;162:1100–1107. doi: 10.1176/appi.ajp.162.6.1100. [DOI] [PubMed] [Google Scholar]
- Hill AL, Degnan KA, Calkins SD, Keane SP. Profiles of externalizing behavior problems for boys and girls across preschool: the roles of emotion regulation and inattention. Dev Psychol. 2006;42:913–928. doi: 10.1037/0012-1649.42.5.913. [DOI] [PubMed] [Google Scholar]
- Hollingshead A. Four factor index of social skills. New Haven, CT: Yale; 1975. [Google Scholar]
- Izard CE, Fine S, Mostow A, Trentacosta C, Campbell J. Emotion processes in normal and abnormal development and preventive intervention. Dev Psychopathol. 2002;14:761–787. doi: 10.1017/s0954579402004066. [DOI] [PubMed] [Google Scholar]
- Keltner D, Moffitt TE, Stouthamer-Loeber M. Facial expressions of emotion and psychopathology in adolescent boys. J Abnorm Psychol. 1995;104:644–652. doi: 10.1037//0021-843x.104.4.644. [DOI] [PubMed] [Google Scholar]
- Kerns JG, Cohen JD, MacDonald AW, 3rd, Cho RY, Stenger VA, Carter CS. Anterior cingulate conflict monitoring and adjustments in control. Science. 2004;303:1023–1026. doi: 10.1126/science.1089910. [DOI] [PubMed] [Google Scholar]
- Kochanska G, Aksan N. Children's conscience and self-regulation. J Pers. 2006;74:1587–1617. doi: 10.1111/j.1467-6494.2006.00421.x. [DOI] [PubMed] [Google Scholar]
- Kochanska G, Gross JN, Lin MH, Nichols KE. Guilt in young children: development, determinants, and relations with a broader system of standards. Child Dev. 2002;73:461–482. doi: 10.1111/1467-8624.00418. [DOI] [PubMed] [Google Scholar]
- Koenigs M, Tranel D. Irrational economic decision-making after ventromedial prefrontal damage: evidence from the Ultimatum Game. J Neurosci. 2007;27:951–956. doi: 10.1523/JNEUROSCI.4606-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane RD, Reiman EM, Axelrod B, Yun LS, Holmes A, Schwartz GE. Neural correlates of levels of emotional awareness. Evidence of an interaction between emotion and attention in the anterior cingulate cortex. J Cogn Neurosci. 1998;10:525–535. doi: 10.1162/089892998562924. [DOI] [PubMed] [Google Scholar]
- Lindgren S, Koeppl G. Assessing child behavior problems in a medical setting: development of the Pediatric Behavior Scale. Greenwich, CT: JAI Press; 1987. [Google Scholar]
- Lorber MF. Psychophysiology of aggression, psychopathy, and conduct problems: a meta-analysis. Psychol Bull. 2004;130:531–552. doi: 10.1037/0033-2909.130.4.531. [DOI] [PubMed] [Google Scholar]
- Magnotta VA, Harris G, Andreasen NC, O'Leary DS, Yuh WT, Heckel D. Structural MR image processing using the BRAINS2 toolbox. Comput Med Imaging Graph. 2002;26:251–264. doi: 10.1016/s0895-6111(02)00011-3. [DOI] [PubMed] [Google Scholar]
- Melnick SM, Hinshaw SP. Emotion regulation and parenting in AD/HD and comparison boys: linkages with social behaviors and peer preference. J Abnorm Child Psychol. 2000;28:73–86. doi: 10.1023/a:1005174102794. [DOI] [PubMed] [Google Scholar]
- Meyer-Lindenberg A, Buckholtz JW, Kolachana B, A RH, Pezawas L, Blasi G, Wabnitz A, Honea R, Verchinski B, Callicott JH, Egan M, Mattay V, Weinberger DR. Neural mechanisms of genetic risk for impulsivity and violence in humans. Proc Natl Acad Sci U S A. 2006;103:6269–6274. doi: 10.1073/pnas.0511311103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nopoulos P, Langbehn DR, Canady J, Magnotta V, Richman L. Abnormal brain structure in children with isolated clefts of the lip or palate. Arch Pediatr Adolesc Med. 2007;161:753–758. doi: 10.1001/archpedi.161.8.753. [DOI] [PubMed] [Google Scholar]
- Ortiz J, Raine A. Heart rate level and antisocial behavior in children and adolescents: a meta-analysis. J Am Acad Child Adolesc Psychiatry. 2004;43:154–162. doi: 10.1097/00004583-200402000-00010. [DOI] [PubMed] [Google Scholar]
- Paus T. Functional anatomy of arousal and attention systems in the human brain. Prog Brain Res. 2000;126:65–77. doi: 10.1016/S0079-6123(00)26007-X. [DOI] [PubMed] [Google Scholar]
- Pezawas L, Meyer-Lindenberg A, Drabant EM, Verchinski BA, Munoz KE, Kolachana BS, Egan MF, Mattay VS, Hariri AR, Weinberger DR. 5-HTTLPR polymorphism impacts human cingulate-amygdala interactions: a genetic susceptibility mechanism for depression. Nat Neurosci. 2005;8:828–834. doi: 10.1038/nn1463. [DOI] [PubMed] [Google Scholar]
- Posner MI, Rothbart MK. Attention, self-regulation and consciousness. Philos Trans R Soc Lond B Biol Sci. 1998;353:1915–1927. doi: 10.1098/rstb.1998.0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pujol J, Lopez A, Deus J, Cardoner N, Vallejo J, Capdevila A, Paus T. Anatomical variability of the anterior cingulate gyrus and basic dimensions of human personality. Neuroimage. 2002;15:847–855. doi: 10.1006/nimg.2001.1004. [DOI] [PubMed] [Google Scholar]
- Raine A, Lencz T, Bihrle S, LaCasse L, Colletti P. Reduced prefrontal gray matter volume and reduced autonomic activity in antisocial personality disorder.[see comment]. Archives of General Psychiatry. 2000;57:119–127. doi: 10.1001/archpsyc.57.2.119. discussion 128-119. [DOI] [PubMed] [Google Scholar]
- Rothbart MK, Ahadi SA, Hershey KL. Temperament and social behavior in childhood. Merrill-Palmer Quarterly. 1994;40:21–39. [Google Scholar]
- Rothbart MK, Ahadi SA, Evans DE. Temperament and personality: origins and outcomes. J Pers Soc Psychol. 2000;78:122–135. doi: 10.1037//0022-3514.78.1.122. [DOI] [PubMed] [Google Scholar]
- Scarpa A, Raine A. Psychophysiology of anger and violent behavior. Psychiatr Clin North Am. 1997;20:375–394. doi: 10.1016/s0193-953x(05)70318-x. [DOI] [PubMed] [Google Scholar]
- Stadler C, Sterzer P, Schmeck K, Krebs A, Kleinschmidt A, Poustka F. Reduced anterior cingulate activation in aggressive children and adolescents during affective stimulation: Association with temperament traits. J Psychiatr Res. 2007;41:410–417. doi: 10.1016/j.jpsychires.2006.01.006. [DOI] [PubMed] [Google Scholar]
- Sterzer P, Stadler C, Krebs A, Kleinschmidt A, Poustka F. Abnormal neural responses to emotional visual stimuli in adolescents with conduct disorder. Biological Psychiatry. 2005;57:7–15. doi: 10.1016/j.biopsych.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Tonkonogy JM. Violence and temporal lobe lesion: head CT and MRI data. Journal of Neuropsychiatry & Clinical Neurosciences. 1991;3:189–196. doi: 10.1176/jnp.3.2.189. [DOI] [PubMed] [Google Scholar]
- Tranel D. “Acquired sociopathy”: the development of sociopathic behavior following focal brain damage. Progress in Experimental Personality & Psychopathology Research. 1994:285–311. [PubMed] [Google Scholar]
- Tranel D, Damasio H. Neuroanatomical correlates of electrodermal skin conductance responses. Psychophysiology. 1994;31:427–438. doi: 10.1111/j.1469-8986.1994.tb01046.x. [DOI] [PubMed] [Google Scholar]
- Tranel D, Bechara A, Denburg NL. Asymmetric functional roles of right and left ventromedial prefrontal cortices in social conduct, decision-making, and emotional processing. Cortex. 2002;38:589–612. doi: 10.1016/s0010-9452(08)70024-8. [DOI] [PubMed] [Google Scholar]
- Tranel D, Damasio H, Denburg NL, Bechara A. Does gender play a role in functional asymmetry of ventromedial prefrontal cortex? Brain. 2005;128:2872–2881. doi: 10.1093/brain/awh643. [DOI] [PubMed] [Google Scholar]
- Veit R, Flor H, Erb M, Hermann C, Lotza M, Grodd W, Birbaumer N. Brain circuits involved in emotional learning in antisocial behavior and social phobia in humans. Neuroscience Letters. 2002;328:233–236. doi: 10.1016/s0304-3940(02)00519-0. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Intelligence Scale for Children. Fourth Edition. San Antonio, TX: Psychological Corporation; 2003. [Google Scholar]
- Woods RP, Cherry SR, Mazziotta JC. Rapid automated algorithm for aligning and reslicing PET images. J Comput Assist Tomogr. 1992;16:620–633. doi: 10.1097/00004728-199207000-00024. [DOI] [PubMed] [Google Scholar]


