Summary
Murine studies have suggested that a population of CD4+ T cells expressing the alpha chain of the interleukin (IL)-2 receptor (CD25+) are phenotypically anergic in response to T cell receptor stimulation and can suppress the function of CD4+ and CD8+ T cells. Recent studies of peripheral lymphocytes from healthy human volunteers have identified a similar population, although little is known about the presence and activity of these cells in patients with cancer and their possible impact on anticancer immunization strategies. Thus, the authors have undertaken these studies in patients with metastatic melanoma undergoing immunizations with known melanoma antigens. CD4+CD25+, CD4+CD25−, and a 1:1 ratio of these isolated T cells were stimulated with soluble anti-CD3 antibody in the presence of irradiated T cell-depleted PBMCs, and proliferation was assessed by measuring [3H]thymidine incorporation. In 13 patients, isolated CD4+CD25+ T cells proliferated 68% (± 5.8%) less than separately cultured CD4+CD25− T cells. Moreover, CD4+CD25+ T cells suppressed the proliferation of an equal number of cocultured CD4+CD25+ T cells in 11 of 13 patients by an average of 60% (± 4.9%). Suppression was not seen at day three of culture and became apparent at days five through nine. The degree of suppression was proportional to the numbers of CD4+CD25+ T cells. Addition of high-dose IL-2 reversed the hypoproliferative phenotype of the CD4+CD25+ T cells and abrogated their suppressive function. These studies demonstrate that anergic and functionally suppressive CD4+CD25+ T cells exist in patients with melanoma undergoing tumor antigen immunization and thus may play a role in modifying the magnitude of the T cell response to immunization.
Keywords: CD4+CD25+ suppressor lymphocytes, Immunotherapy, Interleukin-2, Metastatic melanoma
The identification of human cancer antigens, the development of techniques for active immunization against these antigens, and the use of adoptive transfer of highly active, antigen-specific T cells have brightened the prospects for the development of effective immunotherapies for the treatment of patients with cancer (1,2). Major efforts focused on increasing the effectiveness of antitumor responses have sought to produce increasingly active and targeted antitumor T cells. Recent murine models have emphasized the potential of suppressor lymphocytes to abrogate anticancer immune responses (3–5).
The expression on CD4+ T cells of CD25, the α-chain of the trimeric interleukin (IL)-2 receptor, has been identified as a marker of suppressor cells in murine models (6–9). These cells have been shown to protect from a variety of autoimmune diseases in vivo, including oophoritis (6,7), gastritis (10), diabetes mellitus (6,11), and thyroiditis (6). Moreover, in vitro studies have characterized these cells as hyporesponsive to polyclonal and antigen-specific T cell receptor (TCR) stimulation and suppressive when cocultured with CD4+ and CD8+ T cells (12,13).
Recently, several papers have identified and isolated CD4+CD25+ T cells from the peripheral blood mononuclear cells (PBMC) of healthy humans (14–21). In response to anti-CD3 mediated or alloantigen-mediated stimulation, purified cells exhibited a hyporesponsive phenotype and suppressive functions similar to murine CD4+CD25+ T cells. These studies of cells from healthy volunteers have led to speculation that CD4+CD25+ T cells may play a role in abrogating effective antitumor responses in patients with cancer. We have previously shown that lymphocytes with antigen-specific antitumor reactivity can be induced in the PBMC of patients with melanoma by immunizing with peptide epitopes in adjuvant, though it is not known if these immunized patients also contain cells with suppressive reactivity as well (22). We have thus initiated studies to resolve this question. If CD4+CD25+ T cells from patients with cancer are demonstrated to possess significant suppressive capabilities, future immunotherapy protocols may need to incorporate methods aimed at minimizing suppressor cell mediated impediments to antitumor responses.
MATERIALS AND METHODS
Patients
Patients with melanoma receiving tumor antigen immunizations at the Surgery Branch underwent apheresis. Patients #1 and #2 had not yet received a course of therapy. Of the remaining 11 patients, 8 were immunized against gp100 and showed evidence of circulating antitumor T cells as previously described (22). The remaining patients were immunized against tyrosinase (n =1), TRP-2 (n =1), or MAGE-12 (n =1). PBMC were obtained from patient apheresis samples and incubated overnight in media before use in assays.
Antibodies
The following antibodies were used: phycoerythrin (PE)-conjugated anti-CD25 (M-A251) from BD Biosciences PharMingen (San Diego, CA, U.S.A.); fluorescein isothiocyanate (FITC)-conjugated anti-CD25 (2A3), FITC-conjugated anti-CD4 (SK3), PE-conjugated anti-CD4 (SK3), and PE-conjugated anti-CD3 (SK7) from BD Biosciences Immunocytometry Systems (San Jose, CA, U.S.A.); and anti-CD3 from Ortho Biotech (Raritan, NJ, U.S.A.).
CD4+ T Cell Subset Isolation
In order to isolate the CD4+CD25− T cell and CD4+CD25+ T cell subsets, a MACS system by Miltenyi Biotec (Auburn, CA, U.S.A.) was used. First, a CD4+ T cell negative selection system with an antibody cocktail against CD8, CD11b, CD16, CD19, CD36, and CD56 was used to isolate untouched CD4+ T cells. Next, the CD25+ MACS isolation kit was used to negatively select CD4+CD25− T cells and positively select CD4+CD25+ T cells. Then, the CD4+CD25− T cells were passed through one depletion column (LD column) for greater efficiency of CD25+ cell depletion, and the CD4+CD25+ T cells were passed through a second isolation column (MS column) to improve purity. Purity of the isolated T cell subpopulations was assessed with flow cytometry.
Accessory Cells
A Dynal (Lake Success, NY, U.S.A.) CD3 depletion system was used to produce T cell-depleted accessory cells. Autologous PBMC were incubated with paramagnetic beads coated with anti-CD3. CD3+ T cells were then removed by applying a magnetic field to the cell suspension. The T cell-depleted accessory cells were then irradiated (3300 rad) before use in cultures.
Coculture Proliferation Assays
Cells were cultured in RPMI complete media (CM) containing RPMI 1640 (Gibco, Grand Island, NY, U.S.A.), supplemented with 100 U/mL penicillin/100 μg/mL streptomycin (Biofluids, Biosource International, Rockville, MD, U.S.A.), 50 μg/mL gentamicin (Biofluids, Biosource International), 5 mmol/L HEPES (Biofluids, Biosource International), 2 mmol/L glutamine (Biofluids, Biosource International), and 10% human AB serum (Gemini Bio-Products, Woodland, CA, U.S.A.). 5 × 103 CD4+CD25− T cells alone, 5 × 103 CD4+CD25+ T cells alone, or a ratio of CD4+CD25−:CD4+CD25+ T cells were incubated per well of a 96-well U-bottom plate (Costar, Corning, NY, U.S.A.) with soluble anti-CD3 antibody added (0.05–10 μg/mL final concentration) for stimulation. Ten times as many T cell-depleted accessory cells (5 × 104) were added to each well. Cells were incubated for a varying number of days. For the final 18 hours of culture, 100 μL of the supernatant from each well was removed and replaced with 100 μL media containing 1 μCi [3H]thymidine (PerkinElmer Life Sciences, Boston, MA, U.S.A.). Cells were harvested, and [3H]thymidine incorporation was measured as an assessment of proliferation. All cultures were done in sextuplicates. In some experiments, human recombinant IL-2 (Chiron Corporation, Emeryville, CA, U.S.A.) was added at the onset of culture to the wells at a final concentration of 50 Cetus units/mL.
FACS Analysis
1–5 × 105 cells were washed in cold FACS buffer (PBS with 2% heat inactivated FCS) and then stained with a fluorochrome-conjugated antibody for 30 minutes on ice in the dark. The cells were then washed in cold FACS buffer and fixed with 1% formaldehyde in PBS for 15 minutes on ice in the dark. A final wash was done after incubation with the fixative. Flow cytometry of samples was performed on FACScan or FACSVantage SE (Becton Dickinson) machines using Cell Quest software for acquisition and analysis of data. Lymphocytes were gated by plotting forward scatter versus side scatter. Appropriate isotype controls were performed for each experiment.
RESULTS
CD4+CD25+ T Cells are Present in PBMC of Patients with Melanoma
Analysis with flow cytometry showed there was a CD4+CD25+ population of cells present in the PBMC from patients with melanoma (Fig. 1). This population of cells was not as distinct as described in murine splenocytes, but instead extended as a smear on flow cytometry. Using PE-conjugated CD25+ antibody to measure PBMC from patients, CD4+CD25+ T cells represented 16.6% (± 5.4, n = 20) of total PBMC and 34.2% (± 8.0, n = 20) of CD4+ T cells. Similarly, using FITC-conjugated CD25+ antibody, the CD4+CD25+ T cell population appeared to be 15.5% (± 8.2, n = 23) of the total PBMC and 32.6% (± 14.3, n = 23) of CD4+ T cells.
FIG. 1.
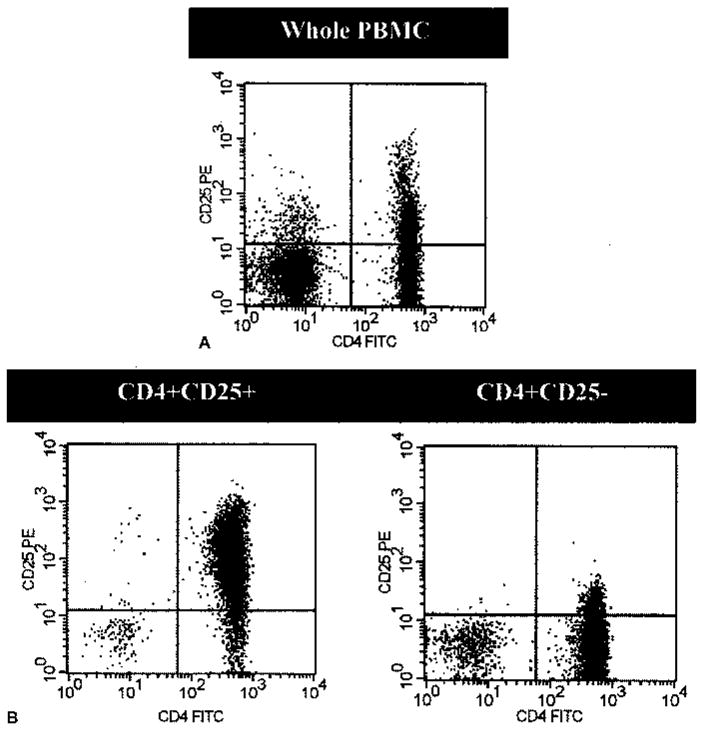
CD4+CD25+ T cells are present in patients with melanoma. Cells were labeled with PE-conjugated CD25 antibody and FITC-conjugated CD4 antibody. Proper isotype controls were also done, but are not presented here. (A) CD4+CD25+ T cells represent a significant portion of total PBMC in patients with melanoma. CD4+CD25+ T cells were 16.6% (± 5.4, n = 20) of total PBMC and 34.2% (± 8.0, n = 20) of CD4+ T cells. (B) Moreover, using the MACS system by Miltenyi Biotec, CD4+CD25+ T cells were purified with 74% purity (SDEV = ± 16.8, n = 16) and CD4+CD25− T cells were purified with 74% purity (SDEV = ± 14.3, n = 16).
Initial attempts at utilizing CD4+CD25+ T cells isolated with the MACS system from cryopreserved PBMC were abandoned due to inconsistent and unreproducible results. Thus, all CD4+CD25+ T cells described in this paper were isolated from fresh apheresis PBMC with a mean purity of 74% (SDEV = ± 16.8, n = 16). CD4+CD25− T cells were isolated with a mean purity of 74% (SDEV ± 14.3%, n = 16).
CD4+CD25+ T cells are Hypoproliferative and Suppressive
CD4+CD25+ T cells alone (5 × 103), CD4+CD25− T cells alone (5 × 103), or a 1:1 ratio of CD4+CD25+: CD4+CD25− T cells (5 × 103:5 × 103) were stimulated in vitro with soluble anti-CD3 in the presence of 1 × 104 autologous, irradiated T cell-depleted accessory cells for 7 days. [3H]thymidine incorporation was measured during the last 18 hours of culture. All cultures were performed in sextuplicate. T cell-depleted accessory cells alone and twice the number of CD4+CD25− T cells per well (1 × 104/well) were performed as controls. Table 1 shows an example of a typical experiment. The incorporation of [3H]thymidine into the CD4+CD25+ T cells was significantly less than into CD4+CD25− T cells (1877 ± 330 vs 9599 ± 1727 cpm, respectively). The CD4+CD25+ T cells exhibited suppressive activity when cocultured with CD4+CD25− T cells. A 1:1 ratio of CD4+CD25+:CD4+CD25− T cells incorporated 2633 ± 210 cpm, thus resulting in a 73% suppression of the proliferation of cocultured CD4+CD25− T cells.
TABLE 1.
Anti-CD3 induced proliferation of CD4+ T cell subsets isolated from a patient with melanoma
| Replicates of [3H]thymidine incorporation (CPM)
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| T cell population | Number of T cells | 1 | 2 | 3 | 4 | 5 | 6 | Mean | SEM |
| CD25+ | 5 × 103 | 3,418 | 1,688 | 1,291 | 1,498 | 2,065 | 1,304 | 1,877 | 330 |
| CD25− | 5 × 103 | 16,714 | 11,121 | 8,330 | 4,195 | 7,240 | 9,990 | 9,599 | 1,727 |
| CD25+:CD25− (1:1) | 5 × 103:5 × 103 | 3,098 | 2,004 | 2,351 | 2,397 | 2,561 | 3,388 | 2,633 | 210 |
| T cell depleted | 5 × 103 | 235 | 329 | 252 | 278 | 195 | 256 | 257 | 18 |
| CD25−/CD25− (1:1) | 5 × 103:5 × 103 | 11,423 | 10,102 | 7,050 | 7,214 | 8,723 | 10,929 | 9,240 | 764 |
Cells were stimulated with 1.5 μg/mL soluble anti-CD3 in the presence of autologous, irradiated T cell-depleted accessory cells for 7 days. CD4+CD25+ T cells were hypoproliferative as compared with the CD4+CD25− T cells, and these cells suppressed the proliferation of cocultured CD4+CD25− T cells by 73%.
% Suppression = 100 × (1 − CD25+:CD25− (1:1)/CD25−)
∴ % Suppression = 73%
Kinetics of Suppression
The kinetics of suppression mediated by the CD4+CD25+ T cells upon an equal number of cocultured CD4+CD25− T cells was evaluated from three to nine days after initiating the culture (Fig. 2A). In three representative patients, CD4+CD25+ T cells proliferated far less than CD4+CD25− T cells, over the entire length of the time course. In patients 1 and 3, the 1:1 ratio of CD4+CD25+:CD4+CD25− T cells remained at a low level of proliferation over the entire time course. The same cocultured cell population in patient 2 increased in proliferation up to day five, after which proliferation declined. Suppression was not seen at day three and exhibited peak suppression at day five to seven after which point suppression plateaued (Fig. 2B). A control using twice the number of CD4+CD25− T cells cultured alone, showed that overcrowding was not a source of suppression. For these three patients, parallel wells were also tested which had half of their medium changed every other day to control for depletion of an essential nutrient or accumulation of an inhibitory toxin, and similar results were obtained (data not shown). Based on these studies, future experiments were cultured for 7 days.
FIG. 2.

Kinetics of suppression. (A) CD4+CD25+ (5 × 103), CD4+CD25− (5 × 103), and a 1:1 ratio of CD4+CD25+:CD4+CD25− (5 × 103:5 × 103) T cells were isolated from three different patients with melanoma. 1.5 μg/mL anti-CD3 and 1 × 104 autologous, irradiated T cell-depleted accessory cells were used to stimulate the cells. A control for overcrowding was included whereby 1 × 104 CD4+CD25− T cells were incubated under identical conditions. [3H]thymidine was added to cultures for the final 18 hours of culture. Cultures in sextuplicate were harvested at days three, five, seven, and nine. CD4+CD25+ T cells proliferated far less than CD4+CD25− T cells over the entire length of the time course. The 1:1 ratio of CD4+CD25+:CD4+CD25− T cells proliferated at a low level over the time course in patients 1 and 3, whereas it increased up to day five and then declined in patient 2. (B) Percent suppression on each day of harvest is shown. Negative % suppression values were denoted as 0% suppression. Suppression was not seen at day three in any patient. Peak suppression was seen at day five to seven. Error bars represent SEM.
Suppression is Dependent on the Number of CD4+CD25+ T cells
We next examined whether a relationship existed between the degree of suppression and the number of CD4+CD25+ T cells added to the coculture. Thus, a 1:1, 1:2, 1:4, 1:8, and 1:16 ratio of CD4+CD25+: CD4+CD25− T cells was cultured with several concentrations (0.5, 1.5, and 5 μg/mL) of soluble anti-CD3 and irradiated T cell-depleted accessory cells. As the number of CD4+CD25+ T cells increased in coculture, the degree of suppression also increased (Fig. 3). Maximal suppression was seen at a 1:1 ratio of suppressors:responders. The degree of proliferation and suppression was similar at all concentrations of anti-CD3 tested.
FIG. 3.
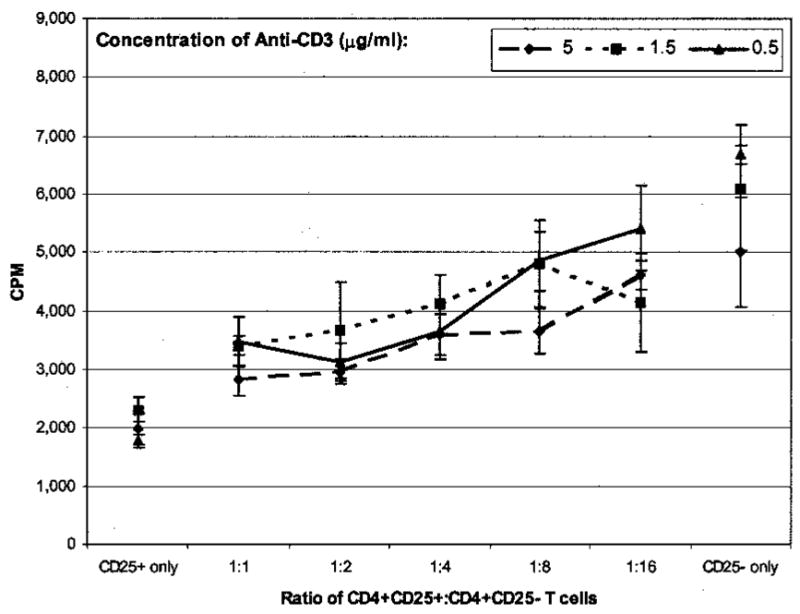
Effect of adding different numbers of CD4+CD25+ T cells upon degree of suppression. Various numbers of CD4+CD25+ T cells were added to a constant number of CD4+CD25− T cells (5 × 103). Cells were stimulated with 1.5 μg/mL anti-CD3 and 1 × 104 T cell-depleted accessory cells for 7 days. [3H]thymidine was added for the final 18 hours of culture. The degree of suppression increased as the number of CD4+CD25+ T cells increased. All points were done in sextuplicate, and error bars represent SEM.
IL-2 Abrogates Suppression and Reverses Hypoproliferation
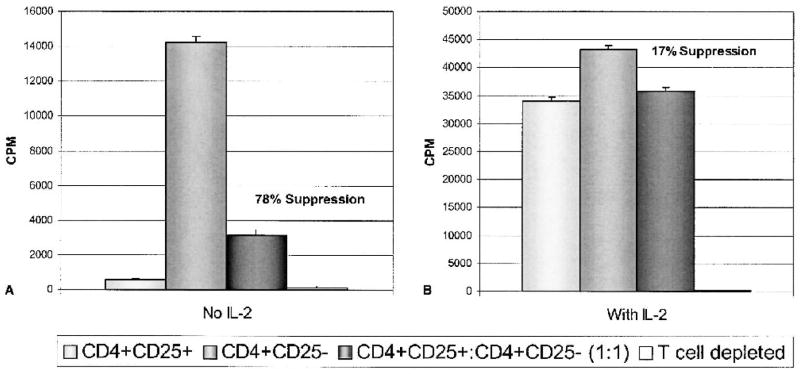
CD4+CD25+ and CD4+CD25− T cells isolated from patients with melanoma were cultured alone and together at a 1:1 ratio with anti-CD3 stimulation and T cell-depleted accessory cells in the presence or absence of 300 IU/mL human recombinant IL-2. Figure 4 shows a representative of three experiments. By day seven of culture in the absence of IL-2, the CD4+CD25+ T cells were significantly hypoproliferative compared with CD4+CD25− T cells cultured alone. Moreover, the CD4+CD25+ T cells suppressed the proliferation of an equal number of cocultured CD4+CD25− T cells by 78%. However, the CD4+CD25+ T cell hyporesponsive state was reversed and the suppression largely abrogated by the addition of IL-2.
FIG. 4.
Effects of IL-2 on CD4+CD25+ T cell-mediated suppression. (A) CD4+CD25+, CD4+CD25−, or a 1:1 ratio of CD4+CD25+:CD4+CD25−T cells were stimulated with 5 μg/mL anti-CD3 and 1 × 104 T cell-depleted accessory cells for 7 days. (B) Identically treated cells were also incubated with 300 IU/mL of human recombinant IL-2. [3H]thymidine was added to culture for the final 18 hours of culture. IL-2 reversed the hyporesponsive state of CD4+CD25+ T cells and largely abrogated suppression mediated by these cells. Conditions were done in sextuplicates, and error bars represent SEM.
Summary of Data from Multiple Patients
Table 2 outlines data gathered from cells isolated from 13 consecutive studied patients with melanoma. Patients 1 and 2 are patients with melanoma before receiving tumor antigen vaccine, whereas the rest are patients who have and currently are receiving vaccinations. In all patients, PBMC were stimulated with 1.5 μg/mL anti-CD3 and 1 × 104 T cell-depleted accessory cells for 7 days. On average, [3H]thymidine incorporation by CD4+CD25+ T cells was 32% (SEM = ± 5.8, n = 13) of the amount incorporated by CD4+CD25− T cells. Moreover, 11 of 13 patients with melanoma exhibited CD4+CD25+ T cells that suppressed cocultured CD4+CD25− T cells. The mean suppression in these 11 patients was 60 ± 4.9%.
TABLE 2.
Summary of patient data
| CPM (Day 7)
|
||||
|---|---|---|---|---|
| Patient | CD4+CD25+ | CD4+CD25− | CD25+:CD25− (1:1) | % Suppression |
| 1 | 1,985 | 5,014 | 2,819 | 44 |
| 2 | 2,383 | 8,006 | 5,549 | 31 |
| 3 | 1,495 | 2,196 | 2,094 | 5 |
| 4 | 1,531 | 2,479 | 2,647 | 0 |
| 5 | 578 | 3,123 | 798 | 74 |
| 6 | 1,218 | 2,455 | 747 | 70 |
| 7 | 718 | 3,336 | 1,294 | 61 |
| 8 | 1,877 | 9,599 | 2,633 | 73 |
| 9 | 1,017 | 6,043 | 3,612 | 40 |
| 10 | 724 | 8,712 | 3,040 | 65 |
| 11 | 1,238 | 2,185 | 1,016 | 54 |
| 12 | 544 | 2,066 | 613 | 70 |
| 13 | 1,320 | 25,390 | 4,441 | 83 |
CD4+CD25+ (5 × 103), CD4+CD25− (5 × 103), or a 1:1 ratio of CD4+CD25+:CD4+CD25− (5 × 103:5 × 103) T cells were stimulated with 1.5 μg/mL anti-CD3 and 1 × 104 irradiated, autologous T cell-depleted accessory cells for 7 days. Negative values for % suppression are denoted as 0% suppression. On average, [3H]thymidine incorporation was 32% (SEM = ±5.8, n = 13) of the amount incorporated by CD4+CD25− T cells. 11 of 13 patients exhibited suppression upon cocultured CD4+CD25− T cells. The mean suppression in these 11 patients was 60% (SEM = ±4.9). All points were done in sextuplicate.
DISCUSSION
Since the discovery by Sakaguchi et al. (6) that CD4+CD25+ T cells were responsible for protecting against organ-specific autoimmunity in day three thymectomized mice and that these cells could protect against autoimmunity mediated by transferred CD4+CD25− T cells to nu/nu mice, numerous studies have aimed at characterizing these suppressor T cells (23–25). These studies identified CD25 as a marker for a population of resting T cells that are anergic to TCR stimulation and can suppress the proliferation of other T cells. CD4+CD25+ T cells are hypothesized to play a critical role in preventing autoimmune responses against “self” antigens.
Studies of CD4+CD25+ T cells in healthy volunteers have corroborated many of the murine results (14–21,26,27). Human CD4+CD25+ T cells share a hyporesponsive phenotype and suppressive activity with their murine counterparts. Woo et al. (26,27) have recently extended these findings to isolated CD4+CD25+ tumor infiltrating lymphocytes (TIL) from non-small cell lung cancers, thus supporting the potential inhibitory activity these cells may have upon an antitumor response.
In the current study, we have attempted to characterize CD4+CD25+ T cells in the PBMC of patients with melanoma who have been actively immunized against melanoma antigens. In these patients, CD4+CD25+ T cells comprise 34.2 ± 8% of CD4+ T cells and 16.6 ± 5.4% of total PBMC. This is somewhat higher than the 5–15% of CD4+ T cells reported in many previous studies (14,16–20,26,27). Although variations in demarcating the quadrants during analysis of flow cytometry data can account for this disparity, this issue is confounded in humans compared with mice due to the absence of a population of CD4+CD25+ T cells that separates distinctly from activated CD4+CD25− T cells. Baecher-Allan et al. have shown that it is the CD4+CD25+high human T cells that have suppressive functions (16).
CD4+CD25+ T cells were isolated from the PBMC of melanoma patients with 74 ± 16.8% purity. These isolated CD4+CD25+ T cells mediated an average of 60 ± 4.9% suppression when cocultured with an equal number of CD4+CD25− T cells. This is similar to the 50–98% suppression reported in other studies (15–17,19,20,27). Addition of IL-2 to cocultures of CD4+CD25+ and CD4+CD25− T cells reversed CD4+CD25+ T cell hypoproliferation and abrogated suppression, similar to other reported studies (15–19), however, the mechanism of this abrogation of suppression is unclear. The CD4+CD25+ T cells may be rapidly proliferating and strongly suppressing the cocultured CD4+CD25− T cells, although the measured incorporation of [3H]thymidine would not change. Conversely, the major contribution could be from CD4+CD25− T cells that have overcome any suppression that might be mediated by the cocultured CD4+CD25+ T cells. If the latter argument is the case, then this mechanism may be a reason why IL-2 immunotherapy can be effective in the treatment of some patients with metastatic kidney cancer or melanoma.
The mechanism of suppression mediated by CD4+CD25+ T cells remains elusive. Other studies with human CD4+CD25+ T cells isolated from human subjects have suggested a cell-cell contact mechanism, since no humoral factor such as TGF-β, IL-10, and IL-4 has been identified as responsible for the suppression (14–20,27). It does not appear that either secretion of a toxic factor, or depletion of essential nutrients caused the apparent suppression since frequent changes of medium during coculture did not impact on the suppression. Recently, McHugh et al. (28) and Shimizu et al. (29) have described, in mice, the increased expression of glucocorticoid-induced tumor necrosis factor receptor (GITR) on CD4+CD25+ T cells and the abrogation of suppression by blocking GITR. CTLA-4 expression is also elevated on CD4+CD25+ T cells. Some studies have highlighted its role in suppression by reporting the induction of organ specific autoimmunity in vivo or the abrogation of suppression in vitro by adding anti-CTLA-4 antibody (30,31). However, human studies have not shown anti-CTLA-4 antibody to be effective in abrogating suppression in vitro (14,16,20). Identification of a cell surface molecule that mediates suppression may not only help to purify these cells, but also facilitate more targeted research into the mechanism of suppression.
The suppression that we observed in cocultures appeared at day five and was not apparent on day three probably due to the requirement for activation of the CD4+CD25+ T cells to mediate suppression. Baecher-Allan et al. (16) report similar findings in addition to suppression greater at day seven compared with day five. In our kinetic experiments, suppression was observed from day five through nine, although variations in individual patients were seen.
Future work is required to further characterize any role that CD4+CD25+ T cells might play in human antitumor responses. Studies relevant to cancer immunotherapy may shed light on whether these suppressor cells mediate decreased proliferation or cytokine release from CD8+ T cells in the context of polyclonal stimulation or antigen-specific immune responses. Importantly, in the PBMC of immunized patients with melanoma, we have identified CD4+CD25+ T cells that have demonstrated suppression upon coculture with CD4+CD25− T cells. In these immunized patients, suppressor cells may be hindering the development of tumor antigen-specific CD8+ T cells in the periphery and inhibiting antitumor activity at the tumor site. Further identification of these CD4+CD25+ suppressor cells from melanoma lesions and determination of any effects of these cells on tumor antigen reactive CD4+ or CD8+ T cells would be important in devising possible future immunotherapy protocols.
References
- 1.Rosenberg SA. A new era for cancer immunotherapy based on the genes that encode cancer antigens. Immunity. 1999;10:281–7. doi: 10.1016/s1074-7613(00)80028-x. [DOI] [PubMed] [Google Scholar]
- 2.Rosenberg SA. Progress in human tumor immunology and immunotherapy. Nature. 2001;411:380–4. doi: 10.1038/35077246. [DOI] [PubMed] [Google Scholar]
- 3.Fu T, Shen Y, Fujimoto S. Tumor-specific CD4(+) suppressor T-cell clone capable of inhibiting rejection of syngeneic sarcoma in A/J mice. Int J Cancer. 2000;87:680–7. [PubMed] [Google Scholar]
- 4.Onizuka S, Tawara I, Shimizu J, et al. Tumor rejection by in vivo administration of anti-CD25 (interleukin-2 receptor alpha) monoclonal antibody. Cancer Res. 1999;59:3128–33. [PubMed] [Google Scholar]
- 5.Shimizu J, Yamazaki S, Sakaguchi S. Induction of tumor immunity by removing CD25+CD4+ T cells: a common basis between tumor immunity and autoimmunity. J Immunol. 1999;163:5211–8. [PubMed] [Google Scholar]
- 6.Sakaguchi S, Sakaguchi N, Asano M, et al. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–64. [PubMed] [Google Scholar]
- 7.Asano M, Toda M, Sakaguchi N, et al. Autoimmune disease as a consequence of developmental abnormality of a T cell subpopulation. J Exp Med. 1996;184:387–96. doi: 10.1084/jem.184.2.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suri-Payer E, Amar AZ, Thornton AM, et al. CD4+CD25+ T cells inhibit both the induction and effector function of autoreactive T cells and represent a unique lineage of immunoregulatory cells. J Immunol. 1998;160:1212–8. [PubMed] [Google Scholar]
- 9.Shevach EM. Regulatory T cells in autoimmmunity. Annu Rev Immunol. 2000;18:423–49. doi: 10.1146/annurev.immunol.18.1.423. [DOI] [PubMed] [Google Scholar]
- 10.Suri-Payer E, Cantor H. Differential cytokine requirements for regulation of autoimmune gastritis and colitis by CD4(+)CD25(+) T cells. J Autoimmun. 2001 Mar;16:115–23. doi: 10.1006/jaut.2000.0473. [DOI] [PubMed] [Google Scholar]
- 11.Salomon B, Lenschow DJ, Rhee L, et al. B7/CD28 costimulation is essential for the homeostasis of the CD4+CD25+ immunoregulatory T cells that control autoimmune diabetes. Immunity. 2000;12:431–40. doi: 10.1016/s1074-7613(00)80195-8. [DOI] [PubMed] [Google Scholar]
- 12.Piccirillo CA, Shevach EM. Cutting edge: control of CD8+ T cell activation by CD4+CD25+ immunoregulatory cells. J Immunol. 2001;167:1137–40. doi: 10.4049/jimmunol.167.3.1137. [DOI] [PubMed] [Google Scholar]
- 13.Thornton AM, Shevach EM. CD4+CD25+ immunoregulatory T cells suppress polyclonal T cell activation in vitro by inhibiting interleukin 2 production. J Exp Med. 1998;188:287–96. doi: 10.1084/jem.188.2.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jonuleit H, Schmitt E, Stassen M, et al. Identification and functional characterization of human CD4(+)CD25(+) T cells with regulatory properties isolated from peripheral blood. J Exp Med. 2001;193:1285–94. doi: 10.1084/jem.193.11.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ng WF, Duggan PJ, Ponchel F, et al. Human CD4(+)CD25(+) cells: a naturally occurring population of regulatory T cells. Blood. 2001;98:2736–44. doi: 10.1182/blood.v98.9.2736. [DOI] [PubMed] [Google Scholar]
- 16.Baecher-Allan C, Brown JA, Freeman GJ, et al. CD4+CD25high regulatory cells in human peripheral blood. J Immunol. 2001;167:1245–53. doi: 10.4049/jimmunol.167.3.1245. [DOI] [PubMed] [Google Scholar]
- 17.Dieckmann D, Plottner H, Berchtold S, et al. Ex vivo isolation and characterization of CD4(+)CD25(+) T cells with regulatory properties from human blood. J Exp Med. 2001;193:1303–10. doi: 10.1084/jem.193.11.1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stephens LA, Mottet C, Mason D, et al. Human CD4(+)CD25(+) thymocytes and peripheral T cells have immune suppressive activity in vitro. Eur J Immunol. 2001;31:1247–54. doi: 10.1002/1521-4141(200104)31:4<1247::aid-immu1247>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 19.Taams LS, Smith J, Rustin MH, et al. Human anergic/suppressive CD4(+)CD25(+) T cells: a highly differentiated and apoptosis-prone population. Eur J Immunol. 2001;31:1122–31. doi: 10.1002/1521-4141(200104)31:4<1122::aid-immu1122>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 20.Levings MK, Sangregorio R, Roncarolo MG. Human cd25(+)cd4(+) t regulatory cells suppress naive and memory T cell proliferation and can be expanded in vitro without loss of function. J Exp Med. 2001;193:1295–1302. doi: 10.1084/jem.193.11.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wing K, Ekmark A, Karlsson H, et al. Characterization of human CD25+ CD4+ T cells in thymus, cord and adult blood. Immunology. 2002;106:190–9. doi: 10.1046/j.1365-2567.2002.01412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosenberg SA, Yang JC, Schwartzentruber DJ, et al. Immunologic and therapeutic evaluation of a synthetic peptide vaccine for the treatment of patients with metastatic melanoma. Nat Med. 1998;4:321–7. doi: 10.1038/nm0398-321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sakaguchi S. Regulatory T cells: key controllers of immunologic self-tolerance. Cell. 2000;101:455–8. doi: 10.1016/s0092-8674(00)80856-9. [DOI] [PubMed] [Google Scholar]
- 24.Shevach EM, McHugh RS, Piccirillo CA, et al. Control of T-cell activation by CD4+ CD25+ suppressor T cells. Immunol Rev. 2001;182:58–67. doi: 10.1034/j.1600-065x.2001.1820104.x. [DOI] [PubMed] [Google Scholar]
- 25.Shevach EM. Certified professionals: CD4(+)CD25(+) suppressor T cells. J Exp Med. 2001;193:F41–6. doi: 10.1084/jem.193.11.f41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Woo EY, Chu CS, Goletz TJ, et al. Regulatory CD4(+)CD25(+) T cells in tumors from patients with early-stage non-small cell lung cancer and late-stage ovarian cancer. Cancer Res. 2001;61:4766–72. [PubMed] [Google Scholar]
- 27.Woo EY, Yeh H, Chu CS, et al. Cutting edge: Regulatory T cells from lung cancer patients directly inhibit autologous T cell proliferation. J Immunol. 2002;168:4272–6. doi: 10.4049/jimmunol.168.9.4272. [DOI] [PubMed] [Google Scholar]
- 28.McHugh RS, Whitters MJ, Piccirillo CA, et al. CD4(+)CD25(+) immunoregulatory T cells: gene expression analysis reveals a functional role for the glucocorticoid-induced TNF receptor. Immunity. 2002;16:311–23. doi: 10.1016/s1074-7613(02)00280-7. [DOI] [PubMed] [Google Scholar]
- 29.Shimizu J, Yamazaki S, Takahashi T, et al. Stimulation of CD25(+)CD4(+) regulatory T cells through GITR breaks immunologic self-tolerance. Nat Immunol. 2002;3:135–42. doi: 10.1038/ni759. [DOI] [PubMed] [Google Scholar]
- 30.Read S, Malmstrom V, Powrie F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25(+)CD4(+) regulatory cells that control intestinal inflammation. J Exp Med. 2000;192:295–302. doi: 10.1084/jem.192.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takahashi T, Tagami T, Yamazaki S, et al. Immunologic self-tolerance maintained by CD25(+)CD4(+) regulatory T cells constitutively expressing cytotoxic T lymphocyte-associated antigen 4. J Exp Med. 2000;192:303–10. doi: 10.1084/jem.192.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]