Abstract
Feline immunodeficiency virus (FIV) is an immunosuppressive lentivirus of domestic cats that serves as an animal model for the pathogenesis of CD4+ lymphopenia and thymus dysfunction in HIV infected humans. During most cases of adult and pediatric HIV infection, naïve CD4+ T lymphocytes recognized by the expression of the RA isoform of the leukocyte common antigen (CD45RA) are infected at a lower level than memory CD4+ T-lymphocytes identified by that lack the RA isoform; however, children with rapidly progressive disease due to thymic insufficiency harbor high levels of HIV within the naïve subpopulation. In FIV infected cats, the infection status and fate of naïve CD4 lymphocytes is unknown. Recently, Gengozian et al. described a monoclonal antibody (mAb 755) that recognizes the feline homologue to CD45RA, allowing the enumeration of naïve CD4 and CD8 lymphocytes in cats. The purpose of this study was to enumerate CD45RA expression on CD4+ and CD8+ lymphocytes in the blood of normal and FIV-infected cats. One-day-old kittens (n=4) were infected with virions either from a wild type molecular clone of FIV (JSY3; n=1) or a mutant clone lacking an intact open reading frame ORF-A (JSY3-ΔORFA; n=3) at equivalent reverse transcriptase units and compared with data from age-matched uninfected cats. At biweekly intervals, the percentages of CD4+ and CD8+ cells belonging to the CD45RA+ subpopulation were measured by two-color flow cytometry. At 12 weeks post-inoculation both FIV inocula were associated with a reduction in total CD4+ lymphocytes from a median of 22% in controls to 8% in infected cats (P=), contributing to a reduction in the CD4:CD8 ratio from 5.5 in controls to 0.76 in infected cats (P=). The decline in CD4+ lymphocytes was attributable to a disproportionate loss of CD4+CD45RA+ cells: 13% of CD4+ cells were naive in controls, as compared to 7% in FIV infected cats (P=0.004). In contrast, naïve CD8+ lymphocytes did not change significantly with FIV infection (67% of CD8+ cells were CD45RA+ in FIV infected cats as compared to 8% in controls). Therefore, within the context of acute pediatric infection, FIV is associated with a rapid depletion of naïve CD4 lymphocytes from the blood. The pathogenesis of this loss with respect to lytic infection, thymus insufficiency, or transition to a memory phenotype warrants further study.
Keywords: FIV, HIV, naïve CD4 lymphocytes, CD45RA
1. Introduction
Feline immunodeficiency virus (FIV) infection is associated with depletion of CD4 T cells from the peripheral blood, and an increase in CD8 cytotoxic T lymphocytes, resulting in a decreased CD4:CD8 ratio. The pathogenesis of these changes is similar to that of HIV infection, and FIV has become a well established model for HIV immunopathogenesis.
Changes within the CD8 T cell population include an expansion of activated memory CD8 lymphocytes (CD44hi, CD8betalow, CD62Llow), which progressively accumulate within the lungs and peripheral lymph nodes during the course of adult infection (Gebhard et al., 1999). These cells are the principal mediators of non-cytotoxic cell-mediated suppression of FIV replication in vitro. The phenotypic and functional heterogeneity of the CD4 T cell population has not been fully characterized to date. Here we report the characterization of peripheral blood lymphocyte changes at biweekly intervals following neonatal inoculation with FIV. CD45RA is one of four isoforms of the leukocyte common antigen, and is the predominant form expressed on the surface of naïve T cells. Using a previously characterized mAb recognizing CD45RA on feline leukocytes (Gengozian et al., 2005), we have identified the presence of CD45RA in both CD4 and CD8 T cell populations, indicating naïve or memory status of these populations in FIV infected and uninfected feline neonates and adult cats. We report a significant decline in naïve CD4 T cells, but not naïve CD8 T cells, following pediatric FIV infection.
2. Materials and Methods
Six specific pathogen free (SPF) cats ranging in age from birth to 12 weeks were were assigned to protocols approved by the Institutional Animal Care and Use Committees at the University of Florida or Auburn University.
Blood was drawn from cats for pre-inoculation analysis within 24 hours of birth. After random assignment to groups, one-day-old kittens (n=4) were injected intraperitoneally with 200μl of cell culture supernatant containing virions from either the wild type clone JSY3 (n=1) or the ORF-A mutant JSY3ΔORF-A/2 (n=3) at a dose of approximately 104 TCID50. Two cats were administered a sham inoculum consisting of 200μl sterile saline solution. Blood was collected at biweekly intervals and processed for flow cytometry.
The blood was centrifuged at 800-1000 RPM for 5 minutes at room temperature and the serum was withdrawn and frozen for later analysis. Fetal bovine serum (FBS) (Gibco, Carlsbad, CA) was added to the cellular fraction in an equal amount to the withdrawn serum and resuspended for a complete blood count (CBC) and the remainder was used for flow cytometric analysis.
One hundred to 500 μl of EDTA-treated blood was used for whole blood lysis. Fourteen mL of room temperature 1X lysis solution was made from 10X lysis solution (22.5g ammonium chloride, 2.5g potassium bicarbonate, 92.5mg tetrasodium EDTA for 250mL) and at least 100 μl of whole blood was added and mixed by inversion. The solution was incubated at room temperature for 3 minutes and was centrifuged at 300 g for 6 minutes. The supernatant was decanted and 10mL of flow buffer (1X PBS, 2% FBS, 0.1% Sodium Azide) was added to the cells. The solution was mixed gently and spun again under the same conditions. The supernatant was decanted and 1mL of flow buffer was added to the cells. The cells were gently mixed and filtered through a 35μm cell strainer cap into a 5 ml round bottom tube.
Monoclonal mouse anti-feline CD4 antibody (clone #vpg34), conjugated to fluorescein isothiocyanate (FITC) was prepared at an optimal concentration based on a preliminary study and a mouse IgG monoclonal antibody of irrelevant specificity conjugated to FITC was used at similar concentration (Serotec, Inc., Raleigh, NC) as a negative control. The monoclonal mouse anti-feline CD8 conjugated to R-Phycoerythrin (RPE) was used at the manufacturer's recommended dilution. A mouse anti-feline CD4 antibody conjugated to RPE (clone #3-4F4) (Southern Biotech, Birmingham, AL) was used at a 1:400 dilution of the manufacturer's preparation. The monoclonal mouse anti-feline CD45RA (mAb 755) antibody was conjugated to Alexa 488 using the Zenon® Mouse IgG1 Labeling Kit (Molecular Probes, Invitrogen, Inc., Carlsbad, CA) according the manufacturer's instructions. Tubes were placed on ice throughout the procedure, and were incubated in the dark.
Dual fluorescence of the following combinations of antibodies was used: feline anti-CD4-FITC vs. feline anti-CD8-RPE; feline anti-CD4-RPE vs. feline anti-CD45RA-Alexa 488; feline anti-CD8-RPE, feline anti-CD45RA-Alexa 488. After antibodies were added to 5 ml polystyrene tubes, 2 × 105 cells were added in 100 μl and incubated on ice in the dark for 30 minutes. The cells were washed twice with flow buffer at 1000 RPM for 5 minutes at 4°C. Cells were then washed twice and resuspended in isotonic 0.5% paraformaldehyde. Samples were analyzed either with a DakoCytomation MoFlo flow cytometer and Summit software (DakoCytomation, Inc., Fort Collins, CO) or with a FACScan flow cytometer and Consort-32 computer system and LYSYS-II software (Becton Dickinson, Inc.).
Naïve cells were expressed as the percentage of CD4 or CD8 lymphocytes that co-labeled with CD45RA, or as absolute numbers calculated as the product of the percentage and the absolute lymphocyte count. The general linear model of analysis of variance was used to determine significance between infected and uninfected groups. All statistical analyses were performed using SAS statistical software (SAS Institute, Inc., Cary, NC).
3. Results and Discussion
Naïve CD4 T cell percentages and absolute numbers were significantly decreased over a 12 week period in FIV-infected cats when compared with uninfected cats. (P=0.001 for each; Figures 1A and 1C, respectively). This is in agreement with Roederer et al. who showed that absolute naïve CD4 T cell numbers are lost in HIV-infected individuals (Roederer et al., 1995; Spina et al., 1997)). This is also in agreement with Delobel et al. who found that in HIV-infected poor immunological responders, there was a significant loss of naïve T cells in both percentages and absolute numbers (Delobel et al., 2006). We did not see significant reductions in either naïve CD8 T cell percentages (P=0.042[ac3]) (Figure 1B) or absolute naïve CD8 T cell numbers (P=0.109) (Figure 1D) in the same time period. This finding is not in agreement with Roederer et al. who reported that absolute CD8 T cell counts decreased with HIV infection (Roederer et al., 1995).
Figure 1.
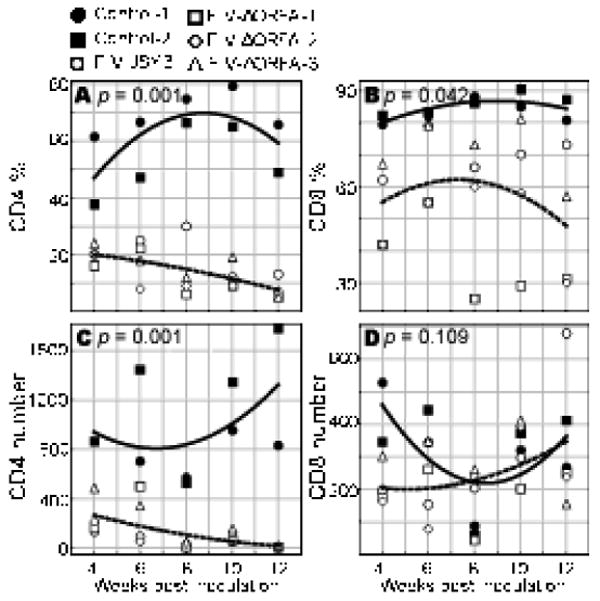
CD4 T cell and CD8 T cell percentages and absolute numbers with FIV infection. A. CD4 T cell percentage throughout 12 weeks of FIV infection or control (sham infection). B. CD8 T percentage throughout 12 weeks of FIV infection or control (sham infection). C. CD4 T cell numbers throughout 12 weeks of FIV infection or control (sham infection). D. CD4 T cell numbers throughout 12 weeks of FIV infection or control (sham infection).
At 12 weeks post-inoculation the FIV-infected cats (both JSY3 and JSY3-ORF-A/2) had reduced CD4+ lymphocytes, from a median of 22% in controls to 8% in infected cats (P=0.004), contributing to a reduction in the CD4:CD8 ratio from 5.5 [ac5]in controls to 0.76 in infected cats (P=0.007). The decline in CD4+ lymphocytes was attributable to a disproportionate loss of CD4+CD45RA+ cells: 58% of CD4+ cells were CD45RA+ in controls, as compared to 7% in FIV infected cats (P=0.004). In contrast, naïve CD8+ lymphocytes did not change significantly with FIV infection (67% of CD8+ cells were CD45RA+ in FIV infected cats as compared to 84% in controls). Representative results of the flow cytometric analyses with dual antibody labeling of anti-CD4/anti-CD45RA and anti-CD8/anti-CD45RA antibodies are shown in Figure 2 and 3 respectively.
Figure 2.
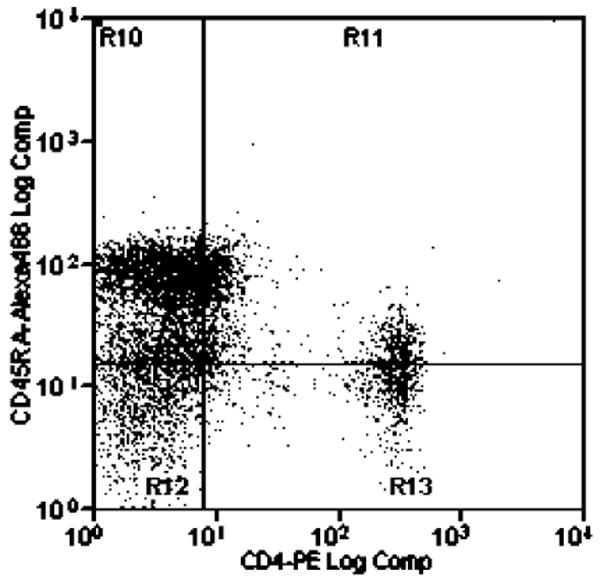
Flow cytometric analysis of dual antibody labeling with anti-CD4-Alexa 488 and anti-CD45RA (755)-PE in control and FIV-infected cats.
Figure 3.
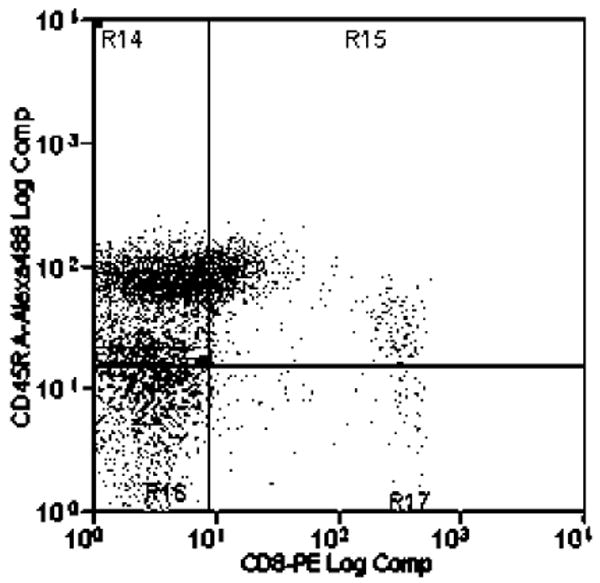
Flow cytometric analysis of dual antibody labeling with anti-CD8-Alexa 488 and anti-CD45RA (755)-PE in control and FIV-infected cats.
Four isoforms of the CD45 (leukocyte common antigen) family of highly glycosolated transmembrane proteins have been identified in humans: CD45RA, CD45RB, CD45RC, and CD45RO (Serra-Pages, 1995). The relative molecular weights of the corresponding antigens range from 180kDa for CD45RO to 200-220kDa for CD45RA (Thomas, 1989). The CD45 cytoplasmic domain has been identified as a protein tyrosine kinase, which is involved in signaling events in B and T cell activation (Altin and Sloan, 1997; Dahlke et al., 2004). For T cells the pattern of CD45 expression varies with the stage of development and activation by antigenic stimulation. Initial studies showed naïve T cells (CD4 or CD8) to express CD45RA, and memory cells expressed CD45RO (Clement et al., 1988; Rudd et al., 1987; Sanders et al., 1988). However, cells can convert phenotypically from CD45RA to CD45RO following 14 days in vitro activation by mitogens (Geginat et al., 2001; Poppema et al., 1996), and there is cyclic expression of these surface markers in 30 day in vitro culture (Warren and Skipsey, 1991).
In normal human neonates, the majority of CD4 T cells express CD45RA (naïve cells), and a progressive age related transition occurs so that by late infancy, most CD4 T cells are expressing CD45RO (memory cells) (Bradley et al., 1989; De Paoli et al., 1988). In adults, CD45RA-expressing T cells are long lived proliferating T cell population, with immunologic memory maintained by CD4 CD45RO-expressing T cells (Michie et al., 1992).
During the course of HIV infection there is an absolute reduction in CD4 T cells (Hengel et al., 1999). It has been shown that phenotypically both CD4+ CD45RA+ cells and CD4+ CD45RO+ cells are reduced with HIV infection (Roederer et al., 1995; Spina et al., 1997). This finding could be skewed though because it is possible for CD45RO+ (Spina et al., 1997) cells to revert back to CD45RA+ phenotype after becoming quiescent (Spina et al., 1997). HIV viral reservoirs may have a role in the depletion of CD4 T cells. Both memory (Saavedra-Lozano et al., 2004; Sleasman et al., 1996) and naïve T cells have been implicated as viral reservoirs (Blaak et al., 2000; Ostrowski et al., 1999).
Another mechanism of depletion of naïve CD4 T cells that has been implicated is thymic insufficiency (dysfunction) (Delobel et al., 2006; McBreen et al., 2001). The findings of Delobel, et al. did not support this, but rather that an excessive destruction of mature CD4 T cells in the periphery is the cause of the depletion (Delobel et al., 2006). CD4+ CD8+ double positive thymocytes can be infected by HIV and therefore may contribute to the pool of infected naïve T cells and eventual death of these cells. Results have shown that developing thymocytes can be infected, but they do not become infected naïve T cells, eliminating the possibility that they contribute to the supply of HIV-infected naïve T cells. They do, however, then play a significant role in depleting the supply of new naïve T cells (Brenchley et al., 2004). In addition, no naïve CD8 T cells could be found in HIV-infected patients further supporting the idea that infected thymocytes do not contribute to the supply of HIV-infected naïve T cells (Brenchley et al., 2004).
Finally, T cell activation by HIV infection has been proposed as a mechanism leading to the depletion of CD4 T cells (Grossman et al., 2002; Hunt et al., 2003). Mechanisms other than direct killing of infected CD4 T cells may play a role in CD4 T cell depletion considering HIV mainly infects activated CD4 T cells (Grossman et al., 2002). Activation of resting T cells by HIV can occur several ways including directly through antigen presenting cells presenting HIV antigens in the context of MHCII (Weissman et al., 1996), indirectly through inflammatory cytokines (Matsuyama et al., 1991), or through exposure to gp120 (Capobianchi, 1996). These cells proliferate and some die by activation-induced cell death (AICD), virus specific CTLs, or by virus itself (Grossman et al., 2002; Hellerstein et al., 1999). Normally, after activation, some T cells with become long lived memory cells with withdrawal of antigenic stimulation. But with HIV infection which causes chronic activation of T cells, there seems to be an imbalance between cell production and cell loss within a given population of resting T cells mediated by several different pathways (Grossman et al., 2002).
L-selectin (CD62L) expression has been shown to be a marker of either naïve/memory or activated (effector) CD8 cells (Hamann et al., 1997; Zimmerman et al., 1996). In addition, reduced expression of the CD8 β chain defines activated CD8 cells (Schmitz et al., 1998). The persistent high levels of activated CD8 cells is an atypical profile associated with HIV and FIV infection; healthy individuals usually have very few circulating activated T cells, except during active immune responses (Picker et al., 1993). Gebhard, et al. (1999) reported that FIV infection is characterized by a loss of naïve CD8 cells and an increase in activated CD8 cells as shown with the activation marker CD62L, the adhesion marker CD44, and the integrins CD49d and CD18. Also, within this population of CD8 activated T cells there is a phenotype with strong anti-FIV suppressor activity. This indicates that the naïve CD8 population is being replaced with activated CD8 T cells (Gebhard et al., 1999), and the CD45RA mAb reported here should be helpful in defining these changes with a single marker.
The recent identification of the feline equivalent for CD45RA as a means to identify naïve and memory CD4 and CD8 T cell populations has extended the utility of the FIV model into the investigation of naïve T-cell depletion (Gengozian et al., 2005). The loss of naïve CD4 T cells in FIV-infected cats supports FIV as a useful model for this aspect of HIV immunopathogenesis, and warrants further study to define the mechanisms responsible for naïve CD4 T cell loss during FIV infection.
References
- Altin JG, Sloan EK. The role of CD45 and CD45-associated molecules in T cell activation. Immunol Cell Biol. 1997;75:430–445. doi: 10.1038/icb.1997.68. [DOI] [PubMed] [Google Scholar]
- Blaak H, van't Wout AB, Brouwer M, Hooibrink B, Hovenkamp E, Schuitemaker H. In vivo HIV-1 infection of CD45RA(+)CD4(+) T cells is established primarily by syncytium-inducing variants and correlates with the rate of CD4(+) T cell decline. Proc Natl Acad Sci U S A. 2000;97:1269–1274. doi: 10.1073/pnas.97.3.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley LM, Bradley JS, Ching DL, Shiigi SM. Predominance of T cells that express CD45R in the CD4+ helper/inducer lymphocyte subset of neonates. Clin Immunol Immunopathol. 1989;51:426–435. doi: 10.1016/0090-1229(89)90041-x. [DOI] [PubMed] [Google Scholar]
- Brenchley JM, Hill BJ, Ambrozak DR, Price DA, Guenaga FJ, Casazza JP, Kuruppu J, Yazdani J, Migueles SA, Connors M, Roederer M, Douek DC, Koup RA. T-cell subsets that harbor human immunodeficiency virus (HIV) in vivo: implications for HIV pathogenesis. J Virol. 2004;78:1160–1168. doi: 10.1128/JVI.78.3.1160-1168.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capobianchi MR. Induction of lymphomonocyte activation by HIV-1 glycoprotein gp120. Possible role in AIDS pathogenesis. J Biol Regul Homeost Agents. 1996;10:83–91. [PubMed] [Google Scholar]
- Clement LT, Yamashita N, Martin AM. The functionally distinct subpopulations of human CD4+ helper/inducer T lymphocytes defined by anti-CD45R antibodies derive sequentially from a differentiation pathway that is regulated by activation-dependent post-thymic differentiation. J Immunol. 1988;141:1464–1470. [PubMed] [Google Scholar]
- Dahlke MH, Larsen SR, Rasko JE, Schlitt HJ. The biology of CD45 and its use as a therapeutic target. Leuk Lymphoma. 2004;45:229–236. doi: 10.1080/1042819031000151932. [DOI] [PubMed] [Google Scholar]
- De Paoli P, Battistin S, Santini GF. Age-related changes in human lymphocyte subsets: progressive reduction of the CD4 CD45R (suppressor inducer) population. Clin Immunol Immunopathol. 1988;48:290–296. doi: 10.1016/0090-1229(88)90022-0. [DOI] [PubMed] [Google Scholar]
- Delobel P, Nugeyre MT, Cazabat M, Sandres-Saune K, Pasquier C, Cuzin L, Marchou B, Massip P, Cheynier R, Barre-Sinoussi F, Izopet J, Israel N. Naive T-cell depletion related to infection by X4 human immunodeficiency virus type 1 in poor immunological responders to highly active antiretroviral therapy. J Virol. 2006;80:10229–10236. doi: 10.1128/JVI.00965-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebhard DH, Dow JL, Childers TA, Alvelo JI, Tompkins MB, Tompkins WA. Progressive expansion of an L-selectin-negative CD8 cell with anti-feline immunodeficiency virus (FIV) suppressor function in the circulation of FIV-infected cats. J Infect Dis. 1999;180:1503–1513. doi: 10.1086/315089. [DOI] [PubMed] [Google Scholar]
- Geginat J, Sallusto F, Lanzavecchia A. Cytokine-driven proliferation and differentiation of human naive, central memory, and effector memory CD4(+) T cells. J Exp Med. 2001;194:1711–1719. doi: 10.1084/jem.194.12.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gengozian N, Foster JS, Kestler DP. Characterization of a monoclonal antibody identifying a CD45RA antigen on feline leukocytes. Vet Immunol Immunopathol. 2005;108:253–264. doi: 10.1016/j.vetimm.2005.05.012. [DOI] [PubMed] [Google Scholar]
- Grossman Z, Meier-Schellersheim M, Sousa AE, Victorino RM, Paul WE. CD4+ T-cell depletion in HIV infection: are we closer to understanding the cause? Nat Med. 2002;8:319–323. doi: 10.1038/nm0402-319. [DOI] [PubMed] [Google Scholar]
- Hamann D, Baars PA, Rep MH, Hooibrink B, Kerkhof-Garde SR, Klein MR, van Lier RA. Phenotypic and functional separation of memory and effector human CD8+ T cells. J Exp Med. 1997;186:1407–1418. doi: 10.1084/jem.186.9.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellerstein M, Hanley MB, Cesar D, Siler S, Papageorgopoulos C, Wieder E, Schmidt D, Hoh R, Neese R, Macallan D, Deeks S, McCune JM. Directly measured kinetics of circulating T lymphocytes in normal and HIV-1-infected humans. Nat Med. 1999;5:83–89. doi: 10.1038/4772. [DOI] [PubMed] [Google Scholar]
- Hengel RL, Jones BM, Kennedy MS, Hubbard MR, McDougal JS. Markers of lymphocyte homing distinguish CD4 T cell subsets that turn over in response to HIV-1 infection in humans. J Immunol. 1999;163:3539–3548. [PubMed] [Google Scholar]
- Hunt PW, Martin JN, Sinclair E, Bredt B, Hagos E, Lampiris H, Deeks SG. T cell activation is associated with lower CD4+ T cell gains in human immunodeficiency virus-infected patients with sustained viral suppression during antiretroviral therapy. J Infect Dis. 2003;187:1534–1543. doi: 10.1086/374786. [DOI] [PubMed] [Google Scholar]
- Matsuyama T, Kobayashi N, Yamamoto N. Cytokines and HIV infection: is AIDS a tumor necrosis factor disease? Aids. 1991;5:1405–1417. doi: 10.1097/00002030-199112000-00001. [DOI] [PubMed] [Google Scholar]
- McBreen S, Imlach S, Shirafuji T, Scott GR, Leen C, Bell JE, Simmonds P. Infection of the CD45RA+ (naive) subset of peripheral CD8+ lymphocytes by human immunodeficiency virus type 1 in vivo. J Virol. 2001;75:4091–4102. doi: 10.1128/JVI.75.9.4091-4102.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michie CA, McLean A, Alcock C, Beverley PC. Lifespan of human lymphocyte subsets defined by CD45 isoforms. Nature. 1992;360:264–265. doi: 10.1038/360264a0. [DOI] [PubMed] [Google Scholar]
- Ostrowski MA, Chun TW, Justement SJ, Motola I, Spinelli MA, Adelsberger J, Ehler LA, Mizell SB, Hallahan CW, Fauci AS. Both memory and CD45RA+/CD62L+ naive CD4(+) T cells are infected in human immunodeficiency virus type 1-infected individuals. J Virol. 1999;73:6430–6435. doi: 10.1128/jvi.73.8.6430-6435.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picker LJ, Treer JR, Ferguson-Darnell B, Collins PA, Bergstresser PR, Terstappen LW. Control of lymphocyte recirculation in man. II. Differential regulation of the cutaneous lymphocyte-associated antigen, a tissue-selective homing receptor for skin-homing T cells. J Immunol. 1993;150:1122–1136. [PubMed] [Google Scholar]
- Poppema S, Lai R, Visser L, Yan XJ. CD45 (leucocyte common antigen) expression in T and B lymphocyte subsets. Leuk Lymphoma. 1996;20:217–222. doi: 10.3109/10428199609051610. [DOI] [PubMed] [Google Scholar]
- Roederer M, Dubs JG, Anderson MT, Raju PA, Herzenberg LA, Herzenberg LA. CD8 naive T cell counts decrease progressively in HIV-infected adults. J Clin Invest. 1995;95:2061–2066. doi: 10.1172/JCI117892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudd CE, Morimoto C, Wong LL, Schlossman SF. The subdivision of the T4 (CD4) subset on the basis of the differential expression of L-C/T200 antigens. J Exp Med. 1987;166:1758–1773. doi: 10.1084/jem.166.6.1758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saavedra-Lozano J, Cao Y, Callison J, Sarode R, Sodora D, Edgar J, Hatfield J, Picker L, Peterson D, Ramilo O, Vitetta ES. An anti-CD45RO immunotoxin kills HIV-latently infected cells from individuals on HAART with little effect on CD8 memory. Proc Natl Acad Sci U S A. 2004;101:2494–2499. doi: 10.1073/pnas.0308381100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders ME, Makgoba MW, Shaw S. Human naive and memory T cells: reinterpretation of helper-inducer and suppressor-inducer subsets. Immunol Today. 1988;9:195–199. doi: 10.1016/0167-5699(88)91212-1. [DOI] [PubMed] [Google Scholar]
- Schmitz JE, Forman MA, Lifton MA, Concepcion O, Reimann KA, Jr, Crumpacker CS, Daley JF, Gelman RS, Letvin NL. Expression of the CD8alpha beta-heterodimer on CD8(+) T lymphocytes in peripheral blood lymphocytes of human immunodeficiency virus- and human immunodeficiency virus+ individuals. Blood. 1998;92:198–206. [PubMed] [Google Scholar]
- Serra-Pages C, Morimoto C, Schlossman SF, Saito H, Streuli M. White Cell Differentiation Antigens. 5. Vol. 1. Oxford Press; 1995. [Google Scholar]
- Sleasman JW, Aleixo LF, Morton A, Skoda-Smith S, Goodenow MM. CD4+ memory T cells are the predominant population of HIV-1-infected lymphocytes in neonates and children. Aids. 1996;10:1477–1484. doi: 10.1097/00002030-199611000-00004. [DOI] [PubMed] [Google Scholar]
- Spina CA, Prince HE, Richman DD. Preferential replication of HIV-1 in the CD45RO memory cell subset of primary CD4 lymphocytes in vitro. J Clin Invest. 1997;99:1774–1785. doi: 10.1172/JCI119342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas ML. The leukocyte common antigen family. Annu Rev Immunol. 1989;7:339–369. doi: 10.1146/annurev.iy.07.040189.002011. [DOI] [PubMed] [Google Scholar]
- Warren HS, Skipsey LJ. Loss of activation-induced CD45RO with maintenance of CD45RA expression during prolonged culture of T cells and NK cells. Immunology. 1991;74:78–85. [PMC free article] [PubMed] [Google Scholar]
- Weissman D, Barker TD, Fauci AS. The efficiency of acute infection of CD4+ T cells is markedly enhanced in the setting of antigen-specific immune activation. J Exp Med. 1996;183:687–692. doi: 10.1084/jem.183.2.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman C, Brduscha-Riem K, Blaser C, Zinkernagel RM, Pircher H. Visualization, characterization, and turnover of CD8+ memory T cells in virus-infected hosts. J Exp Med. 1996;183:1367–1375. doi: 10.1084/jem.183.4.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]


