Abstract
Global environmental change affects the sustained provision of a wide set of ecosystem services. Although the delivery of ecosystem services is strongly affected by abiotic drivers and direct land use effects, it is also modulated by the functional diversity of biological communities (the value, range, and relative abundance of functional traits in a given ecosystem). The focus of this article is on integrating the different possible mechanisms by which functional diversity affects ecosystem properties that are directly relevant to ecosystem services. We propose a systematic way for progressing in understanding how land cover change affects these ecosystem properties through functional diversity modifications. Models on links between ecosystem properties and the local mean, range, and distribution of plant trait values are numerous, but they have been scattered in the literature, with varying degrees of empirical support and varying functional diversity components analyzed. Here we articulate these different components in a single conceptual and methodological framework that allows testing them in combination. We illustrate our approach with examples from the literature and apply the proposed framework to a grassland system in the central French Alps in which functional diversity, by responding to land use change, alters the provision of ecosystem services important to local stakeholders. We claim that our framework contributes to opening a new area of research at the interface of land change science and fundamental ecology.
Keywords: biodiversity, land change, mass ratio hypothesis, plant functional traits
Global environmental changes, including land use and land cover changes, have considerable impacts on the ecological properties of ecosystems and therefore on the ecosystem services (ES) that societies derive from them (1). Although links between ecosystem properties (EP) and ES are not always trivial, many ES and their changes can be reasonably quantified by EP that are routinely measured in ecological studies (2). Global change effects on EP can be direct, through their effects on physical and chemical processes and on the metabolism and behavior of organisms. Global change drivers can also influence EP indirectly through their impacts on biodiversity, either through their effects on local biota or by altering the ability of organisms to disperse through landscapes. Relevant changes in biodiversity are manifested through changes in plant functional diversity (FD), i.e., the value, range, and relative abundance of plant functional traits in a given ecosystem (2). Although often subtler than direct effects of global change drivers (3, 4), these indirect biotic effects remain a major source of uncertainty in predicting the impacts of global change on ES provision.
Conceptual models accounting for links between the functional trait values of local plant communities and EP are scattered in the literature, and the conceptual connections between them are not always clear. We articulate the most important of those models into a generic procedure and propose a small number of logical steps to reduce uncertainty in the prediction of EP and derived ES within the context of land cover change (Fig. 1). Reducing this uncertainty has important theoretical and applied implications. First, although there is increasing consensus on the fact that plant functional traits strongly affect EP and resulting ES (5, 6), very little is known about the relative role of different components of FD, such as the mean and frequency distribution of plant trait values (2, 7). Our generic method allows identification of cases in which EP can be satisfactorily predicted from different FD components and to quantify their relative importance. Second, the reduction of uncertainty will help identify the ES most vulnerable to biodiversity changes (1, 8, 9).
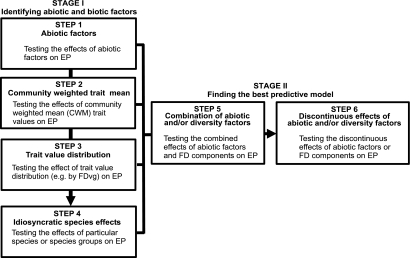
Fig. 1.
Diagrammatic representation of the steps proposed to reduce uncertainty in the prediction of EP and ES on the basis of plant FD. In stage I, the models tested at each step (M1–M4) link EP with driving factors of different nature: abiotic factors (AFi), community-aggregated trait value or CWM of any one functional trait (CWMi), distribution of values of any one trait present in a community (FDvgi), and local abundance of any one species present in the community (Ab spi). At each step, significant factors are identified and conserved for stage II. In stage II, combined models are built by adding statistically significant factors from steps 1–4 and conserving those that significantly improve the model (following a standard criterion, e.g., the Akaike criterion). The process concludes when further information on FD does not reduce uncertainty in EP prediction any further. Generalized models for each step are as follows: M1, EP = ƒ(AFi, AFj, …, AFn); M2, EP = ƒ(CWMi, CWMj, …, CWMn); M3, EP = ƒ(FDvgi, Fdvgj, …, Fdvgn); M4, EP = ƒ(Ab spi, Ab spj, …, Ab spn); M5, EP = ƒ(AFi, CWMj, FDvgk, …, Sp Abn); M6, if CWMi < T then EP = ƒ1(CWMi); if CWMi > T then EP = ƒ2(CWMi); if CWMi = T then EP cannot be predicted from CWMi. T = threshold (see stage II, step 6).
FD at the Interface Between Global Change and EP
FD can affect ES through its effect on EP, notably major biogeochemical processes related to carbon, nutrient and water cycling (2), and disturbance regimes (10). All of the main candidate mechanisms by which diversity is expected to affect EP (mass ratio, selection, niche complementarity, and insurance) (6–8) strongly depend on the functional attributes of local communities that are central to our procedure (11). FD can be quantified by two main components. First, a community weighted mean value (hereafter CWM) can be calculated for each trait as the mean of trait values in the community, weighted by the relative abundance of the species carrying each value (12). This community-aggregated metric represents the expected functional trait value of a random community sample, often understood as the dominant trait value in a community. Second, the distribution of trait values within the community can be expressed through various metrics, among which functional divergence (hereafter FDvg), representing the degree of overlap in trait values within the community (13), is increasingly used.
The Mass Ratio Hypothesis: A Cornerstone of FD–EP Relationships
The mass ratio hypothesis (3) states that ecosystem functioning, at a given point in time, is chiefly determined by trait values of the dominant contributors to plant biomass. According to this hypothesis, EP should be predictable from the CWM of traits with proven links with resource capture, usage, and release at the individual and ecosystem levels.
The mass ratio hypothesis is well supported by both theory and empirical evidence. There are conceptual models linking a relatively small number of plant (and especially leaf) traits with EP such as primary productivity, nutrient cycling, and trophic transfer to herbivores (3, 10, 14–20) that appear consistent across biomes, ecosystems, and floras (15–19). Links of local CWM of such plant traits with biogeochemistry-derived ES have been documented in several cases (21–25). After accounting for such effects, the distribution (e.g., FDvg) of plant trait values may play only a secondary role. Conversely, when the relationship between CWM and EP is poor, other components of FD, such as FDvg, might exert relatively strong effects.
Results
Reducing Uncertainty in Six Steps.
Assuming that, within a given land system, the major land cover types could be sampled and their vegetation described through the relative abundance of species as well as the species-specific values of key traits (e.g., from plant trait databases), we propose a formal procedure for (i) identifying the major abiotic and biotic factors that affect EP relevant to ES and (ii) constructing useful predictive models of these EP (Fig. 1).
The procedure is organized in two stages comprising six steps. Stage I uses four steps to test sequentially for the effects of individual sets of factors. Within a given vegetation type, it starts by testing abiotic factors as drivers of EP (step 1). It then proceeds with community-level functional properties (CWM; step 2) where vegetation is simplified into an average trait value, strongly determined by the functional trait values of the more abundant species. In step 3, additional biotic complexity is taken into account through the degree of overlap in trait values within the community (FDvg; step 3). Finally, the procedure explores remaining idiosyncratic effects of particular species (step 4).
Although taken individually each step in stage I has already received considerable interest in the literature, we propose to go further and examine them in combination and to account for the relative effects of each component. Thus, stage II first combines all significant variables from steps 1–4 using a step-wise procedure (step 5). This bottom-up approach reflects the choice of being hypothesis-driven in our analysis. Finally, step 6 explores the possible discontinuous effects of FD or other variables, thereby accounting for remaining unexplained variance.
Stage I: Identifying Abiotic and Biotic Drivers of EP and ES.
Step 1: Effects of abiotic factors.
Abiotic variables often explain a significant amount of variation in EP (4, 19). This is especially the case when sampled land cover types span substantial variations in climate or soil, e.g., driven by topography, and/or when large spatial scales are considered. The first step in our procedure should thus check for a continuous relationship linking abiotic factors such as temperature, moisture, or soil chemical quality as independent variables against EP as a response variable (Fig. 1, step 1). For our approach, this could be seen as an initial null hypothesis, where biodiversity effects are of no detectable importance to the prediction of EP. There are many examples of abiotic effects on key EP. Water availability affects litter decomposition (14). Topography is an important determinant of soil stability (26). Primary productivity and nutrient cycling are strongly affected by soil fertility and/or water-holding capacity (27), abiotic factors that are in turn affected by land use (28). Many studies have also demonstrated strong legacies of past land use or disturbance regime on soils that carry over to current EP and ES (29, 30).
Step 2: Effects of community functional properties.
In cases where the variations in EP cannot be satisfactorily explained by abiotic factors alone, one can assess whether FD exerts nonnegligible effects on EP. On that basis, we propose to start the process by testing for a continuous relationship based on the mass ratio hypothesis (Fig. 1, step 2). Model 2 (Fig. 1 legend) accounts for the relationship between EP and CWM for key plant functional traits. Traits are considered one at a time or may be combined to reflect trait syndromes. As a special case, the relative proportion of different growth forms (i.e., tussock grasses) can be used as CWM. Some examples of CWM strongly influencing EP and ES have been given in The Mass Ratio Hypothesis: A Cornerstone of FD–EP Relationships. Other examples include the often dramatic alteration of EP and ES through changes in CWM due to the expansion of invasive species with functional traits different from those of the native flora (31). CWM of single traits may be sufficient to explain the variance in EP as in specific leaf area and leaf tensile strength explaining specific productivity (22) and decomposition rate, respectively (14). However, it is common for multiple traits to affect a single EP (32). Here uncertainty in the prediction of EP from CWM is reduced by the inclusion of one or more traits (such as whole-plant and leaf traits or aboveground and root traits), but not by adding information on the distribution of trait values (next step).
Step 3: Effects of the distribution of trait values.
In cases where abiotic factors or CWM of a single trait or multiple traits do not satisfactorily explain the variations in EP, the effects of the distribution of trait values (measured for example by FDvg) should be explored (Fig. 1, step 3). Examples of ecosystem effects attributed to differences in trait values among coexisting species include, for instance, enhanced biomass production and soil fertility by mixtures of species with and without nitrogen-fixing symbionts (23), with different leafing phenology (21), or with different rooting depths (33). However, most of these examples have been assessed as the effect of functional group richness rather than by quantifying FDvg. Another example is the alteration of litter decomposition and thus soil nutrient availability by litter mixing effects associated to the joint presence of fast-decomposing and slow-decomposing plants (34). Pollination of a particular species is influenced by the distribution of trait values in the surrounding community (9). Where the inclusion of FDvg significantly improves the predictive model (model 3), consideration of the distribution across species of values of one or more plant traits reduces uncertainty.
Step 4: Idiosyncratic effects of particular species.
If EP cannot be satisfactorily explained by the weighted mean or distribution of the plant traits within land cover types, then it is possible that EP is associated in a significant and continuous way to the abundance of particular plant species (Fig. 1, step 4). Such effects could, among other reasons, be due to functional traits whose links with the EP of interest are not obvious from first principles or the literature and thus were not measured. For example, biomass production and nutrient retention in a grassland soil have been best explained by the colonization ability rather than by traits associated with nutrient use strategies (35). In the absence of data on colonization ability, the local abundance of certain species could assist in reducing uncertainty in predicting EP. Idiosyncratic effects could also be due to unknown species interactions with other plants, animals, or microorganisms (36). Idiosyncratic species effects can also be examined in an exploratory mode focusing on species known to strongly respond to specific global change drivers. Combined effects of several species may be tested after individual effects are demonstrated (32).
Stage II: Finding the Best Predictive Model.
Step 5: Combining abiotic factors and FD components.
Individual factors tested in steps 1–4 may provide significant predictions of EP, yet combining these models might further decrease the uncertainty in EP. It is therefore necessary to test various combinations of significant abiotic and biotic factors and to retain the final model that explains the largest amount of variance in the most parsimonious manner (step 5). This combination may lead to the rejection of some effects of FD components that are significant when tested individually (steps 2 and 3), i.e., because of collinearity with abiotic factor(s) affecting EP (step 1). In this case one can conclude that reducing the uncertainty in the prediction of EP does not strictly require additional information on plant traits. Conversely, FD effects may override abiotic effects on EP, e.g., when land use decisions lead to contrasting FD in otherwise homogeneous abiotic environments. Individual factors that do not significantly improve the explanatory power of the model can be considered as irrelevant for practical purposes.
There are only few examples in the literature where the effects of abiotic factors and several FD components have been explored simultaneously. As a notable exception, litter decomposition is often best explained by a combination of climatic variables and CWM of leaf traits such as nutrient concentration (14). As a result, the effects of nitrogen addition (e.g., by atmospheric deposition or through management) often depend on CWM of those leaf traits (37). Productivity is also the complex outcome of climate, management (e.g., fertilization), trait values of dominant species, and sometimes various effects of species richness or trait distribution (21, 23, 25). Most studies, however, especially the majority of those considering effects of species richness on productivity, have not conducted a systematic analysis of the different FD components.
Although our framework is flexible and can incorporate various procedures for model construction, we suggest building the model with those variables identified as significant in steps 1–4 using a step-wise process whereby complexity is progressively added to the model until a satisfactory level of predictive power is reached (model 5). The default option presented here implies a hierarchy of drivers of EP starting with abiotic factors, but reordering is possible depending on the particularities of the study at hand (e.g., spatial scale of interest).
Step 6: Discontinuous effect of CWM or trait distribution on EP.
In all models discussed so far, we have assumed that single abiotic factors and/or FD components, or their combinations, explained EP. There can be cases, however, in which none of steps 1–5 yields a satisfactory model of EP. A plausible alternative explanation here could be that EP is strongly driven by a biotic or abiotic factor, but their relationship is discontinuous (model 6 in Fig. 1). For example, in some grasslands soil nitrogen losses depend in a discontinuous way on the C:N ratio of dominant grasses (38). Flammability of ecosystems also has a strongly discontinuous relationship with the abundance of flammable species (39). The presence of key species (step 4) can further result in discontinuous biotic effects on EP. For EP where continuous relationships prove not to be satisfactory, more intensive sampling of the gradient(s) or additional experimental approaches are needed to identify thresholds (T in model 6, Fig. 1 legend).
Case Study: FD and ES in Subalpine Grasslands.
We used a previously published data set depicting changes in FD and EP in subalpine grasslands under different combinations of past and present land use (24, 40, 41). Key ES of importance to regional stakeholders, including farmers, other local residents, and tourists, were related to grassland EP (Table 1) (41). The data set was submitted to the six-step analysis to search for the best model explaining ES delivery from abiotic factors and FD components. Results on the significant factors identified via general linear models are presented in Table 1. Below we highlight three cases where different abiotic or FD factors explain variations in EP relevant to the ES identified in the land system.
Table 1.
Summary of significant results (P < 0.05) for general linear models of EP following steps 1–6 in Fig. 1
| ES | EP | Stage I: Individual effects of abiotic and biotic factors |
Stage II: Combining significant effects into the best predictive model (Step 5: Final model) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Step 1: Abiotic factors |
Step 2: CWM traits |
Step 3: Trait distribution |
|||||||
| Variable | P value | Variable | P value | Variable | P value | Model | % var | ||
| Case 1: Fodder production | ANPP | NNI | 0.004 | — | — | FDvg_VH | 0.040 | ANPP = −0.028 + 0.0014 NNI | 44 |
| FDvg_LNC | 0.091 | ||||||||
| SANPP | NNI | 0.045 | — | — | — | — | SANPP = −0.012 + 0.00085 NNI | 22 | |
| Standing AGB | — | — | — | — | FDvg_LHS | 0.089 | No suitable model; step 6: suitable model is nonlinear, depending on past ploughing (49) | <15 | |
| Case 2: Snow gliding prevention, cultural heritage, and habitat for butterfliesStanding litter biomass | Standing litter biomass | — | — | CWM_VH | 0.002 | FDvg_VH | 0.10 | Standing litter biomass = 9.4 + 0.35 CWM_VH − 7.54 CWM_LNC − 0.00063 CWM_LTS | 75 |
| CWM_LNC | 0.012 | ||||||||
| CWM_LTS | 0.046 | ||||||||
| Litter decomposability | — | — | CWM_LNC | <0.001 | — | — | Litter decomposability = 13.5 + 3.02 CWM_LNC − 0.39 CWM_Lig:N | 91 | |
| CWM_Lig:N | <0.001 | ||||||||
| Case 3: Maintenance of soil fertility | Available NO3 | — | — | CWM_LNC | <0.001 | — | — | NO3 = −25.8 + 21.2 CWM_LNC + 0.0085 CWM_RL | 76 |
| CWM_RL | 0.044 | ||||||||
| Denitrification potential | — | — | CWM_LNC | 0.001 | FDvg_LHS | 0.026 | Denitrification potential = −59.5 + 80.6 CWM_LNC + 0.042 CWM_RL | 67 | |
| CWM_RL | 0.034 | ||||||||
| Case 4: Sustained green fodder production through summer | Soil water content | WHC | 0.003 | CWM_LA | 0.043 | — | — | Soil water content = 2.07 + 1.55 WHC − 1.58 AGB + 0.00083 CWM_LA − 0.0014 CWM_RL | 85 |
| AGB | <0.001 | CWM_RL | 0.001 | ||||||
Numerical results are presented for steps 1–3 and step 5. Although tested, no significant relationship was found between any of the EP examined and the abundance of F. paniculata; hence, step 4 is not presented in the table. Relevant EP were elicited through stakeholder interviews and complemented by expert scientific knowledge (in italics). —, no significant effect of any variable tested within the step. Acronyms after CWM_ and FDvg_ refer to measured plant traits. See Methods for the full name of each trait and for details on the statistical methods.
Case 1.
Two EP relevant to fodder production, aboveground net primary productivity (ANPP) and specific ANPP (SANPP) (see Methods), were best explained by a single abiotic variable, the nitrogen nutrition index (NNI) (step 1 in Table 1). Although ANPP was significantly related to light acquisition by plants through within-community variation in vegetative height (FDvg_VH) and leaf nitrogen content (FDvg_LNC; marginally significant) (step 3), these terms were no longer significant after effects of NNI were included (step 5). Overall, models explained <50% of the total variance and were not improved by the inclusion of idiosyncratic effects such as the abundance of a single species, i.e., Festuca paniculata (step 4), a species that sequesters nutrients in large quantities of slowly decomposing litter (24). No discontinuity was detected by visual inspection of EP–CWM or EP–FDvg graphs (step 6; results not shown). Standing green aboveground biomass (AGB) was an extreme case, where no suitable model could be found across the five steps. However, when considering mown fields alone, AGB was linearly related to the CWM of leaf nitrogen content (LNC); i.e., there was a discontinuous relationship conditional on current management (step 6). Hence, for fodder production FD components could largely be ignored.
Cases 2 and 3.
Litter accumulation was identified by stakeholders as negatively affecting a range of ES including prevention of snow gliding, cultural heritage, aesthetic value, and provision of habitat for butterflies. In contrast to fodder production, abiotic factors did not influence EP associated with litter accumulation, which were best explained by CWM of leaf traits (leaf nitrogen and lignin contents; leaf tensile strength) and CWM of VH (in the case of total accumulated litter in the spring) (step 2). A marginally significant relationship was also found with the distribution of VH within communities (step 3), but for parsimony it was eliminated from the final model. Both for standing litter (the pool) and litter decomposability (the flux), FD components explained a very high proportion of the total variance. Similarly, CWM of leaf and root traits explained a very high proportion of the total variance in the maintenance of soil fertility (step 2). Hence, services associated with litter accumulation and soil fertility could be strongly related to average functional properties of the vegetation, providing support for the mass ratio hypothesis.
Case 4.
Finally, sustained fodder production of green fodder through summer, which was associated with soil water content (SWC) in the summer period, illustrated the case of a complex model associating both abiotic and FD factors. SWC was explained by local soil conditions determining water-holding capacity and by an additional AGB term that accounted for direct biophysical effects of AGB on soil evaporation (water conservation) and plant transpiration (water demand). However, as shown in case 1, no significant model was found relating AGB to either local abiotic conditions or FD components. Effects of community-level means of leaf area (net positive effect representing reduction of soil evaporation) and root length (RL; negative effect representing water uptake) were significant and were included in the final model, with a very high proportion of the total variance explained. Hence, the prolonged delivery of green fodder depended on the combination of local abiotic factors, their effects on aboveground standing biomass, and average functional properties of the vegetation.
Discussion
ES are the key conceptual link between social evaluations of ecosystems and their EP. Although a wealth of information is available on land cover change both in the form of current vegetation maps and as scenario-based projections, few tools exist for translating this information into relevant indicators of EP or ES provision. Moreover, those studies that do address this question often do so for individual components of FD on specific EP. Here we propose a way to address the relative effect of distinct FD components in combination with abiotic factors on the variability of different EP. Applying the procedure presented here permits a quantitative assessment of the links between land cover changes and the services provided by affected ecosystems, thus reducing uncertainties on EP predictability. In this way it contributes to opening a new area of research at the interface of land change science and biodiversity research.
Reducing Uncertainty in the Prediction of ES Delivery in Response to Environmental Change.
Our case study illustrates the variety of ways in which EP underlying the delivery of ES can be related to abiotic factors and different components of FD. The quantitative models produced can also be applied to make projections of the future delivery of ES under contrasting land cover change scenarios (41). The links between CWM of plant traits and EP are strong and widespread, as shown by theory and accumulating empirical evidence under contrasting ecological and biogeographical contexts. In the specific example of the subalpine grasslands, when FD was involved, there was support for the mass ratio hypothesis, because community-level mean (CWM), rather than the distribution (quantified here by FDvg) of traits, ultimately explained EP, but other components of FD have been found to reduce uncertainty in predictions of EP (2, 8). Nevertheless, our case study also showed that the knowledge of FD does not always decrease uncertainty with respect to EP and that abiotic factors can sometimes be sufficient for practical purposes. Our objective here is therefore to highlight the fact that predicting ES from FD is a tractable yet complex task and that measuring several components of FD can be useful. In this context, the main strength of our method is to provide a generic procedure for selecting models and ranking FD components by their importance to local EP and ES in different land systems.
Although we have applied the proposed framework to plants for demonstration, we expect it to be applicable to other organisms and associated ES (e.g., insect diversity and pollination or crop protection; soil organism diversity and soil fertility). Some ES (e.g., pollination and nutrient cycling) will further require the combined consideration of FD across trophic levels (9, 32), short of which uncertainty in EP cannot be reduced from plant FD alone. This should make it possible to gain a more precise understanding of the extent to which biodiversity needs to be taken into account to predict changes in ES and human well-being under different scenarios.
It should be noted that, although it can rank different FD components in terms of explained variation in EP and ES, our method is not designed for the critical testing of different mechanisms underlying such FD effects. The mass ratio and idiosyncratic species effect models have been theoretically linked to the “selection” mechanism, whereas the model that focuses on FDvg, by stressing the presence of a variety of functional trait values, can be seen as linked to the “niche complementarity effect” (which also includes facilitation) (6, 7). Rather than providing a crucial test for these mechanisms, our method explores whether there are recurrent combinations of abiotic and FD factors that explain EP for different ES.
Practical Implications and Perspectives.
To link land cover changes, whether through land use or other drivers, to changes in ES, our procedure requires data on abiotic and FD factors. For the latter, information is needed on both the traits and the relative abundance of species in the different land cover types under investigation. Recording species lists is often easier than measuring their relative abundance and trait values, yet our approach provides one more reason for compiling such databases on the basis of the rich yet scattered information available (2). Projections of EP and ES consequences of CWM shifts are currently well advanced (42), but projections for other factors, such as FDvg or discontinuous effects, are still in their infancy.
Adequate societal response to global change require quantitative assessments of the ES consequences of different land change alternatives (1). In this context, our approach offers an innovative way to develop quantitative predictive models of land cover change impacts on ES. By explicitly including FD effects on EP and ES in these models, our procedure also allows for a formal incorporation of biodiversity as a driving factor in the sensitivity of ES to global change.
Methods
Study Site and Field Measurements.
The Lautaret study site is located on a south-facing slope of the central French Alps (45°2′24″ N, 6°82′24″ E). Climate is subalpine, and the soil is sandy-loam over a calc-shale bedrock. Past and current land use were analyzed to identify dominant combinations, referred to as “land use states.” The five most common land use states were characterized by the presence (terraces) or absence (traditional grasslands) of past plowing and current grassland management: mown and fertilized terraces, mown but unfertilized terraces, unmown and lightly grazed terraces, mown grasslands, and unmown and lightly grazed grasslands (24). Fifteen grasslands representative of the five states (three grasslands per state) were surveyed in June and July 2003 for floristic composition (species relative abundances), plant functional traits at the population level, and soil parameters (16). Community-level means for root biomass, RL, specific RL, and root nitrogen content, stratified by depth, were measured monthly during 2005 (40). Soil water-holding capacity was calculated from soil texture and structural data by using the SPAW model (43). Fertility was assessed by the NNI, which quantifies actual nitrogen availability for plant growth through the dilution of nitrogen in closed canopies (44). Disturbance regimes were quantified by using disturbance date (expressed in degree days) and the percentage of biomass harvested or alternatively the percentage height loss as measures of disturbance intensity (24).
EP.
Biomass stocks, primary productivity (ANPP and SANPP, the logarithm of ANPP per unit of production time) (22), litter accumulation, and decomposition were quantified during the 2004 season following standardized protocols (22, 24). The leaf lignin:nitrogen ratio and the decomposability of litter (14) were determined for samples collected in July 2003. Components of the nitrogen cycle including total nitrogen pools, available nitrates (estimated with resin bags), and microbial nitrification and denitrification potentials were quantified throughout the 2004 and 2005 seasons (30). Finally, effects of vegetation on soil volumetric water content were assessed experimentally by a vegetation removal experiment in 2005 and repeated measurements through the growing season in vegetated versus unvegetated plots (40).
Quantification of FD.
Plant FD within each grassland was quantified by using two metrics: CWM trait values calculated by using species' relative abundance (22) and FDvg quantified by using a modification of Rao's index that takes into account intraspecific trait variability (45). These two metrics were calculated for vegetative height (VH), specific leaf area, leaf dry matter content, LNC, leaf nitrogen to phosphorus ratio (N:P), leaf tensile strength, and flowering phenology. Root traits and aboveground lignin:nitrogen ratio were available only as aggregated values. Finally we combined specific leaf area, VH, and seed mass as a single FD index representative of species functional strategies (LHS, sensu ref. 46) by averaging FDvg across these three traits.
Statistical Analysis.
We tested the relationships between EP and FD using general linear models (GenStat 9.0) in the six steps of our proposed procedure. For each of the six steps we proceeded preferentially based on expert knowledge about abiotic factors and traits relevant to the EP of interest.
Step 1.
Effects of fertility (represented by NNI) and soil water-holding capacity were first tested individually, then in combination. Effects of disturbance date or intensity on EP were tested in ref. 24 but were not significant. We thus chose not to include them in this analysis.
Step 2.
We used an ascending procedure (i) testing CWM for individual traits and then (ii) combining significant traits.
Step 3.
We included FDvg of individual traits into previously selected models (significant terms only) for either traits already significant or additional traits.
Step 4.
If no significant effects could be found in steps 2 and 3 we included the abundance of F. paniculata, the grass species that strongly dominates unmown grasslands (24). Previous analyses highlighted the effects of this species' abundance on EP and ES identified by local stakeholders (41).
Steps 1–4 were completed sequentially, then in step 5 all significant terms from steps 1–4 were combined in a step-wise ascending procedure. The most parsimonious model was selected by using the Akaike criterion.
Step 6.
If none of the linear models from steps 1–4 (involving FD metrics, abiotic factors, and abundance of F. paniculata) were satisfactory, we reinspected data for possible discontinuities.
ACKNOWLEDGMENTS.
We thank the organizers of the Missillac workshop, hosted by the W. A. Beling Family and funded by the Borchard Foundation, for stimulating discussions. This work was supported by the Inter-American Institute for Global Change Research (IAI CRN II 2015, which is supported by U.S. National Science Foundation Grant GEO-0452325); the European Commission Projects RUBICODE (GOCE-2006-036890) and VISTA (EVK2-2001-000356); the UFR de Biologie, Université Joseph Fourier, Grenoble, through a visiting professorship to S.D.; and FONCyT (BID 1428 OC-AR PICT 20441) and CONICET (PIP 6091), Argentina.
Abbreviations
- AGB
aboveground biomass
- ANPP
aboveground net primary productivity
- SANPP
specific ANPP
- CWM
community weighted mean value
- EP
ecosystem property
- ES
ecosystem service
- FD
functional diversity
- FDvg
functional divergence
- LNC
leaf nitrogen content
- NNI
nitrogen nutrition index
- RL
root length
- VH
vegetative height.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Millennium Ecosystem Assessment. Ecosystems and Human Well-Being: Biodiversity Synthesis. Washington, DC: World Resources Institute; 2005. p. 86. [Google Scholar]
- 2.Díaz S, Lavorel S, Chapin FS, Tecco PA, Gurvich DE, Grigulis K. In: Terrestrial Ecosystem in a Changing World. Canadell J, Pataki DE, Pitelka LF, editors. Berlin: Springer; 2007. pp. 79–91. [Google Scholar]
- 3.Grime JP. J Ecol. 1998;86:902–910. [Google Scholar]
- 4.Loreau M, Naeem S, Inchausti P, Bengtsson J, Grime JP, Hector A, Hooper DU, Huston MA, Raffaelli D, Schmid B, et al. Science. 2001;294:804–808. doi: 10.1126/science.1064088. [DOI] [PubMed] [Google Scholar]
- 5.Hooper DU, Chapin FS, Ewel JJ, Hector A, Inchausti P, Lavorel S, Lawton JH, Lodge DM, Loreau M, Naeem S, et al. Ecol Monogr. 2005;75:3–35. [Google Scholar]
- 6.Díaz S, Fargione J, Chapin FS, Tilman D. PLoS Biol. 2006;4:1300–1305. doi: 10.1371/journal.pbio.0040277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Petchey OL, Gaston KJ. Ecol Lett. 2006;9:741–758. doi: 10.1111/j.1461-0248.2006.00924.x. [DOI] [PubMed] [Google Scholar]
- 8.Balvanera P, Pfisterer AB, Buchmann N, He JS, Nakashizuka T, Raffaelli D, Schmid B. Ecol Lett. 2006;9:1146–1156. doi: 10.1111/j.1461-0248.2006.00963.x. [DOI] [PubMed] [Google Scholar]
- 9.Kremen C, Williams NM, Aizen MA, Gemmill-Herren B, LeBuhn G, Minckley R, Packer L, Potts SG, Roulston T, Steffan-Dewenter I, et al. Ecol Lett. 2007;10:299–314. doi: 10.1111/j.1461-0248.2007.01018.x. [DOI] [PubMed] [Google Scholar]
- 10.Lavorel S, Garnier E. Funct Ecol. 2002;16:545–556. [Google Scholar]
- 11.Díaz S, Cabido M. Trends Ecol Evol. 2001;16:646–655. doi: 10.1016/s0169-5347(01)02181-4. [DOI] [PubMed] [Google Scholar]
- 12.Violle C, Navas ML, Vile D, Kazakou E, Fortunel C, Hummel I, Garnier E. Oikos. 2007;116:882–892. [Google Scholar]
- 13.Mason NWH, Mouillot D, Lee WG, Wilson JB. Oikos. 2005;111:112–118. [Google Scholar]
- 14.Aerts R. Oikos. 1997;79:439–449. [Google Scholar]
- 15.Bardgett RD, Wardle DA. Ecology. 2003;84:2258–2268. [Google Scholar]
- 16.Garnier E, Lavorel S, Ansquer P, Castro H, Cruz P, Dolezal J, Eriksson O, Fortunel C, Freitas H, Golodets C, et al. Ann Bot. 2007;99:967–985. doi: 10.1093/aob/mcl215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Díaz S, Hodgson JG, Thompson K, Cabido M, Cornelissen JHC, Jalili A, Montserrat-Marti G, Grime JP, Zarrinkamar F, Asri Y, et al. J Veg Sci. 2004;15:295–304. [Google Scholar]
- 18.Coley PD, Barone JA. Annu Rev Ecol Evol Syst. 1996;27:305–335. [Google Scholar]
- 19.Grime JP. Plant Strategies, Vegetation Processes and Ecosystem Properties. Chichester, UK: Wiley; 2001. p. 416. [Google Scholar]
- 20.Leps J, Osbornovakosinova J, Rejmanek M. Vegetatio. 1982;50:53–63. [Google Scholar]
- 21.Hooper D, Vitousek P. Science. 1997;277:1302–1305. [Google Scholar]
- 22.Garnier E, Cortez J, Billes G, Navas ML, Roumet C, Debussche M, Laurent G, Blanchard A, Aubry D, Bellmann A, et al. Ecology. 2004;85:2630–2637. [Google Scholar]
- 23.Kirwan L, Luscher A, Sebastia MT, Finn JA, Collins RP, Porqueddu C, Helgadottir A, Baadshaug OH, Brophy C, Coran C, et al. J Ecol. 2007;95:530–539. [Google Scholar]
- 24.Quetier F, Thebault A, Lavorel S. Ecol Monogr. 2007;77:33–52. [Google Scholar]
- 25.Vilà M, Vayreda J, Comas L, Ibañez JJ, Mata T, Obon B. Ecol Lett. 2007;10:241–250. doi: 10.1111/j.1461-0248.2007.01016.x. [DOI] [PubMed] [Google Scholar]
- 26.Tasser E, Mader M, Tappeiner U. Basic Appl Ecol. 2003;4:271–280. [Google Scholar]
- 27.Personeni E, Luscher A, Loiseau P. Soil Biol Biochem. 2005;37:819–827. [Google Scholar]
- 28.Dimitrakopoulos PG, Siamantziouras ASD, Galanidis A, Mprezetou I, Troumbis AY. Environ Manage. 2006;37:826–839. doi: 10.1007/s00267-004-0179-6. [DOI] [PubMed] [Google Scholar]
- 29.Fraterrigo JM, Turner MG, Pearson SM, Dixon P. Ecol Monogr. 2005;75:215–230. [Google Scholar]
- 30.Robson TM, Lavorel S, Clement JC, Le Roux X. Soil Biol Biogeochem. 2007;39:930–941. [Google Scholar]
- 31.Levine JM, Vila M, D'Antonio CM, Dukes JS, Grigulis K, Lavorel S. Proc R Soc London Ser B. 2003;270:775–781. doi: 10.1098/rspb.2003.2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eviner VT, Chapin FS. Annu Rev Ecol Evol Syst. 2003;34:455–485. [Google Scholar]
- 33.Dimitrakopoulos PG, Schmid B. Ecol Lett. 2004;7:574–583. [Google Scholar]
- 34.Smith VC, Bradford MA. Oikos. 2003;102:235–242. [Google Scholar]
- 35.Symstad AJ, Tilman D. Oikos. 2001;92:424–435. [Google Scholar]
- 36.Shachak M, Jones CG, Granot Y. Science. 1987;236:1098–1099. doi: 10.1126/science.236.4805.1098. [DOI] [PubMed] [Google Scholar]
- 37.Knorr M, Frey SD, Curtis PS. Ecology. 2005;86:3252–3257. [Google Scholar]
- 38.Wedin D, Tilman D. Science. 1996;274:1720–1723. doi: 10.1126/science.274.5293.1720. [DOI] [PubMed] [Google Scholar]
- 39.Grigulis K, Lavorel S, Davies ID, Dossantos A, Lloret F, Vila M. Global Change Biol. 2005;11:1042–1053. [Google Scholar]
- 40.Gross N. Grenoble, France: Université Joseph Fourier; 2007. PhD thesis. [Google Scholar]
- 41.Quétier F, Lavorel S, Thuiller W, Davies I. Ecol Appl. 2007;17:2377–2386. doi: 10.1890/06-0750.1. [DOI] [PubMed] [Google Scholar]
- 42.Cornelissen J, Lavorel S, Garnier E, Díaz S, Buchmann N, Gurvich D, Reich P, ter Steege H, Morgan H, van der Heijden M, et al. Austr J Bot. 2003;51:335–380. [Google Scholar]
- 43.Saxton KE, Rawls WJ, Romberger JS, Papendick RI. Soil Sci Soc Am J. 1986;50:1031–1036. [Google Scholar]
- 44.Duru M. J Sci Food Agric. 1997;74:175–185. [Google Scholar]
- 45.Leps J, de Bello F, Lavorel S, Berman S. Preslia. 2006;78:481–501. [Google Scholar]
- 46.Westoby M. Plant Soil. 1998;199:213–227. [Google Scholar]