Abstract
Objective
To measure brain serotonin-1A (5HT1A) receptor binding potential (BP) in healthy and depressed postpartum women
Design
5HT1A receptor BP was measured with positron emission tomography using [11C]WAY100635 a single time. MANOVA was used to determine depression effects on 5HT1A receptor BP in related brain regions.
Setting
Academic research environment.
Patients
Seven postpartum healthy controls and nine postpartum depressed(PD) subjects with perinatal (antepartum or postpartum) depression onset. Of the 9 PD subjects, 5 had unipolar depression and 4 had bipolar disorder.
Interventions
None
Main Outcome Measures
5HT1A receptor binding potential.
Results
Age, time since delivery, and reproductive hormones did not differ between groups. Postsynaptic 5HT1A receptor binding in PD was reduced 20–28% relative to controls, with most significant reductions in anterior cingulate and mesiotemporal cortices.
Conclusions
Postsynaptic 5HT1A receptor binding is reduced in PD by a similar magnitude as has been shown in other depression samples. The postpartum hormonal milieu and the large proportion of bipolar spectrum subjects in the PD group may have accentuated this finding in this small sample. Recognition of this neurobiological deficit in PD may be useful in the development of treatments and prevention strategies for this disabling disorder.
Key words (6): postpartum depression, serotonin, 5HT1A receptor, PET, estradiol
INTRODUCTION
Postpartum depression is a critical public health problem because it affects at least 580,000 American women annually (1, 2), impedes healthy infant and child development (3–6), and disrupts families (7). Existing treatments, although effective for postpartum mood disorders (8–10), are often rejected by puerperal women who prefer no medication (because of lactation) or who minimize/deny mental illness in the context of hormonal fluctuations of childbearing (11). Discovery of altered central neurobiological processes in postpartum mood disorders has the potential to increase treatment acceptability for women with this disorder, raise the importance of postpartum depression treatment among practitioners, and decrease the stigma of postpartum depression.
While there is evidence of heightened mood sensitivity to estrogen and progesterone fluctuations in women with past postpartum depression (12), little is known about specific neurobiological systems that are disrupted by the hormonal changes of childbearing. The serotonin (5HT) system has been proposed as an important mediator of perinatal depression because depression in women (13) and premenstrual dysphoric disorder (14, 15) are highly responsive to serotonergic antidepressants. Furthermore, the perinatal hormones estradiol, progesterone (16), and cortisol (17) have been consistently demonstrated to have neuroregulatory effects on the central 5HT system.
The 5HT1A receptor is a well-established molecular target for the action of serotonin. In experimental animals, serotonin-increasing antidepressants inducetonic activation of postsynaptic 5HT1A receptors (18) and the complete absence of 5HT1A receptors (19) is associated with depression-like behavior. 5HT1A receptor reductions (binding potential in imaging studies; mRNA and/or density in postmortem studies) have been demonstrated in a majority of neuroimaging (20–25) and postmortem (17, 20, 22) studies of major depressive disorder, although several studies have shown increases (26, 27).
We evaluated the central 5HT1A receptor system with positron emission tomography (PET) and the selective 5HT1A receptor radioligand [11C]WAY100635. We focused on limbic brain regions noted for postsynaptic 5HT1A receptor binding decreases in depression: mesiotemporal cortex (includes amygdala and hippocampus), left lateral orbitofrontal cortex, and subgenual anterior cingulate cortex. We hypothesized that perinatally depressed women (PD is defined as prevalent cases of depression in the 16 weeks following childbirth) would have reduced postsynaptic 5HT1A receptor binding relative to postpartum controls.
MATERIALS AND METHODS
Enrollment occurred between October 2002 and December 2006 as part of a larger study exploring 5HT1A receptor status in postpartum depression. Subjects provided written informed consent as approved by the University of Pittsburgh Biomedical Institutional Review Board. Subjects were women who delivered a healthy, term infant in the preceding 16 weeks. Multiparous women were included. Breast and bottlefeeders were included. All subjects were interviewed with the Structured Clinical Interview for DSM-IV (28). Depression was defined by DSM IV criteria for unipolar or bipolar major depression and a 25-item Hamilton rating scale for depression score (HAM25) ≥ 14. Prevalent rather than incident cases of postpartum depression were included because postpartum depression commonly begins antenatally (29) and to maximize the generalizability of the research. Women with bipolar illness were included, because these women are particularly sensitive to depressive recurrence postpartum (30, 31). Postpartum control subjects had no personal history of a major Axis I disorder and had no family history of a mood or psychotic disorder.
Subjects were excluded if they had medical or neurological illnesses likely to affect cerebral physiology or anatomy, gross abnormalities of brain structure evident by magnetic resonance images (MRI), suicidal intent, substance abuse within one year, lifetime history of substance dependence (other than nicotine), eating disorders, use of hormonal contraception, or exposure to psychotropic or other medications likely to alter cerebral physiology or monoamine function within 3 weeks (5 weeks for fluoxetine) in PD subjects.
Nine PD and seven control subjects were enrolled and imaged. Endogenous reproductive hormone exposures were characterized through menstrual cycle charting and through measurement of morning serum concentrations of estradiol, progesterone, follicle stimulating hormone (FSH), luteinizing hormone (LH), and prolactin on the day of the scan. Scan day reproductive hormones blood specimens were drawn between 9:45 am – 12:15 pm, with mean (SD) blood draw time of 10:24 (0:38). Reproductive hormones were analyzed in duplicate. Estradiol and progesterone were measured by radioimmunoassay (RIA) (Coat-A-Count, DPC, Los Angeles, CA), as previously described (32). LH, and FSH were measured using time resolved immunofluorescence (Delfia, Finland), as previously described (32). Intra and inter-assay coefficients of variation (CVs) for each of these assays are less than 10% and less than 5%, respectively. Prolactin was measured using time resolved immunofluorescence (Delfia, Finland), as described previously (32). All specimens from a given participant were analyzed in duplicate and in the same assay run to reduce variability. Between and within assay CVs were less than 10%.
PET scans were acquired on an ECAT HR+ PET scanner (Siemens, Erlangen, Germany) in three-dimensional (3D) mode [63 transaxial planes [2.4-mm thickness; in-plane resolution = 4.1 mm full-width at half-maximum (FWHM) over a 15.2-cm field of view], as previously described (24). Radiosynthesis of [carbonyl-11C]WAY100635 ([11C]WAY) was performed as previously described (33). A transmission scan was obtained to correct the PET data for attenuation effects. A dynamic emission scan (34 frames of increasing length over 90 min) was then initiated following IV bolus administration of 11.7 to 16.2 mCi (mean ± SD = 14.6 ± 1.2) of high specific activity [11C]WAY (1.58 ± 0.71 mCi/nmol at time of injection). Arterial blood was sampled during scanning and corrected for radiolabeled metabolites to compute the plasma input function of [11C]WAY.
There were 9 breastfeeders (4 PD and 5 controls) in the sample. Approximately 15 minutes after the conclusion of the scan, study participants expressed their milk with a Medela multi-use, electronic, double breast pump (Medela, Inc., McHenry, IL) for 10–30 minutes to reduce discomfort of breast milk engorgement and for analysis of the total radioactivity of total and WAY concentrations in milk (34).
To provide an anatomical framework for analysis of the PET data, magnetic resonance images (MRI) were obtained using a 1.5 T Signa Scanner (GE Healthcare, Milwaukee, WI) and a 3-dimensional spoiled gradient recalled (SPGR) sequence (TE = 5, TR = 25, flip angle = 40°, NEX = 1, section thickness = 1.5 mm with no intersection gap). PET images were aligned with MR images using automated image registration (35). Regions of interest (ROIs) were manually traced on the MR image using a modified version of the IDL-based (Interactive Data Language, Boulder, CO) computer program, ROITOOL, of CTI PET Systems (Knoxville, TN) according to guidelines previously published (23).
We a priori hypothesized 5HT1A receptor reductions in the PD relative to control group in the following regions of interest (ROI): mesiotemporal cortex, left lateral orbitofrontal cortex, and subgenual anterior cingulate cortex. These ROI are associated with mood, emotion expression, and emotion regulation (36) and are associated with 5HT1A receptor reductions in major depression (25, 37). A reference region for assessing nonspecifically bound and free radioligand was defined in the cerebellum using guidelines that excluded the vermis (38) and minimized the spill-in effects from neighboring cortex (23, 24, 39–41). ROI analyzed in an exploratory fashion included pregenual anterior cingulate cortex, right lateral orbitofrontal cortex, postcentral gyrus, occipital cortex, and raphe nucleus.
Regional tissue time-activity concentrations were obtained from the dynamic PET image for each ROI. Logan graphical analysis with generalized linear least squares smoothing (41–43) was applied to the arterial input function and regional tissue time-activity concentrations to derive [11C]WAY distribution volume (DV). [11C]WAY BP (5HT1A receptor binding) was calculated as [(regional DV/cerebellar DV) − 1] (44, 45). Because young individuals with major depressive disorder have evidence of regional brain volume reductions (46, 47), partial volume correction (48, 49) was employed to control for the possible dilutional effect of expanded CSF spaces on brain radioactivity concentrations.
Statistical inference for depression effects on 5HT1A receptor BP was conducted with multivariate analysis of variance (MANOVA) using STATA software, version 8 (Stata Corp, College Station, Tex). MANOVA was the preferred statistical test based on our prediction that 5HT1A receptor binding among distinct ROI is related (23, 25, 26). The assumption of equal variance in 5HT1A receptor binding between groups was satisfied.
To evaluate the effect of other variables on 5HT1A receptor BP in this small sample, we performed exploratory univariate regressions for all primary ROIs with the independent variables age, BMI, breastfeeding, duration post-birth, and hormone concentrations. Variables that were significantly associated with the dependent measure at p≥0.15 were then added to the MANCOVA. We set α = 0.01 for the individual covariates in the MANCOVA to determine if any of these variables had important effects on 5HT1A receptor BP.
Hormonal values did not satisfy tests for normality and were therefore transformed to natural log (1 + hormone of interest). A MANOVA of lactation status on hormone concentrations was conducted. Statistical tests on group differences in demographic, clinical, and hormone data were performed with Pearson chi-square for categorical and Mann-Whitney U exact tests for continuous variables. Spearman correlations were performed to explore the relationship between 5HT1A receptor BP and estradiol concentrations.
Results
Subject Characteristics
Demographic and clinical data for the control and PD subjects are presented in table 1. Subjects were 4 to 13 weeks postpartum at the time of the scan. Six of nine PD subjects experienced depression onset during pregnancy; three subjects had depression onset by 3 weeks postpartum. Two depressed subjects had a history of premenstrual dysphoric disorder, one of whom also had a prior postpartum depressive episode. Four of nine PD subjects had a lifetime diagnosis of bipolar disorder [bipolar I (n=1), bipolar II (n=2), bipolar NOS (n=1)]. Seven of nine PD subjects were psychotropic drug naïve. Mean depression symptom scores (HAM25 and EPDS) at enrollment (25.3 ± 8.5) and on the scan day (mean ± SD HAM25 = 21.7 ± 7.9) indicated mild to moderate depression in the PD group (Table 1). Clinical evaluation on the scan day confirmed absence of hypomania, mania, and mixed episodes in all subjects. Comorbid disorders in the depressed group included past substance/alcohol use disorders (n=5), panic disorder (n=1), social phobia (n=2), generalized anxiety (n=1), and obsessive compulsive disorder (n=3).
Table 1.
Subject characteristics [mean (SD)]
| CONTROL | PD | Statistics | |
|---|---|---|---|
| n | 7 | 9 | |
| INTAKE DATA | |||
| Age | 33.0 (3.9) | 26.9 (7.9) | MWUexact = 13.0; p=0.06 |
| Bipolar number (%) | 0 (0%) | 4 (44%) | n/a |
| Antidepressant naïve (%) | 7 (100%) | 7 (77.8%) | n/a |
| Breastfeeder number (%) | 5 (71%) | 4 (44%) | Pearson chi2 = 1.2; p = 0.28 |
| HAM25* | 3.1 (2.3) | 21.7 (7.9) | MWUexact = 4.5; p=0.003 |
| EPDS* | 1.4 (1.4) | 13.3 (3.1) | MWUexact = 0.0; p=0.000 |
| Weeks post-birth | 11.1 (2.2) | 9.6 (2.9) | MWUexact = 19.0; p=0.21 |
| BMI | 26.1 (3.7) | 27.6 (3.8) | MWUexact = 25.5; p=0.55 |
| LH (IU/L) | 5.0 (6.7) | 2.9 (2.4) | MWUexact = 25.0; p=0.54 |
| FSH† (IU/L) | 10.4 (12.8) | 6.4 (1.9) | MWUexact = 31.0; p=1.00 |
| Estradiol (pg/ml) | 36.1 (20.5) | 45.5 (18.6) | MWUexact = 21.0; p=0.30 |
| Progesterone (ng/ml) | 0.4 (0.3) | 2.2 (4.6) | MWUexact = 20.0; p=0.24 |
| Prolactin (ng/ml) | 23.9 (22.3) | 19.6 (26.0) | MWUexact = 26.0; p=0.61 |
p(MWU,2-tailed) < 0.02.
At 4 months post-scan, 3 PD subjects were remitted in their depression, 1 PD subject experienced depression improvement short of remission, and 5 PD subjects remained depressed. Depression symptom scores in the control group remained unchanged at four months post-scan, which confirmed a stable diagnosis of normal mood in the control sample.
Hormone and breastfeeding data
In 13 out of 16 subjects, estradiol, progesterone, LH and FSH concentrations on the scan day were consistent with early follicular phase or anovulation, as would be expected in women who are 4–13 weeks postpartum (Table 1). One PD subject had progesterone concentrations of 7.96 ng/ml at intake and 14.38 ng/ml on the scan day which suggested 2 possible menstrual cycles prior to her scan, although she denied menstrual bleeding. Pregnancy was excluded by urine pregnancy test on the scan day. One PD subject was scanned at mid-cycle (on the basis of high estradiol concentration on the day preceding the PET scan). One control subject had unusually high FSH (39.3 IU/L), potentially suggestive of perimenopause or premature ovarian failure. Hormone concentrations were not significantly different between groups. Two control and two PD subjects reported menstrual bleeding prior to the scan day.
There was a significant effect of breastfeeding status on the hypothalamic-pituitary-ovarian axis hormone concentrations estradiol, progesterone, LH, FSH, and prolactin [Wilks’ lambda=0.2056; F(5,10)=7.73, p=0.003]. Post-hoc testing indicated that breastfeeding was significantly associated with lower estradiol [F(1,14)=8.31, p=0.01], progesterone [F(1,14)=4.33, p=0.06], and FSH concentrations [F(1,14)=5.18, p=0.04] and higher prolactin concentrations [F(1,14)=26.25, p=0.0002], as would be expected (50).
PET data
Mean [11C]WAY BP values (5HT1A receptor binding) for PD and control groups are presented in table 2. MANOVA of [11C]WAY BP in the three a priori regions of interest indicated a significant main effect of depression [F(3,12)=13.67, Wilks’ lambda=0.23, p=0.0004] (Figure 1). Post hoc ANOVA tests detected significant depression effects on reducing [11C]WAY BP in mesiotemporal cortex [21.6% mean decrease; F(1,14)=22.5, p= 0.0003], subgenual cingulate cortex [27.6% mean decrease; F(1,14)=23.4, p=0.0002], and left lateral orbitofrontal cortex [17.9% mean decrease; F(1,14)=7.13, p=0.018]. Perinatal depression was also associated with reduced [11C]WAY BP in secondary ROI [F(5,10)= 3.24, Wilks’ lambda=.38, p=0.054], with the most significant decreases present in right lateral orbitofrontal cortex [23.4% mean decrease; F(1,14)=8.72, p=0.011) and pregenual anterior cingulate cortex [23.4% mean decrease; F(1,14)=17.2, p=0.001). [11C]WAY BP was not significantly different between subjects with unipolar versus bipolar depression (Figure 2).
Table 2.
5HT1A receptor BP [mean (SD)] measured with [11C]WAY100635 derived with 90 minutes of Logan graphical analysis, corrected for partial volume effects.
| PP-C | PP-D | % DIFF | |
|---|---|---|---|
| A priori regions of interest * [F(3,12)=13.67, Wilks’ lambda=0.23, p=0.0004] | |||
| Mesiotemporal cortex | 9.69 (0.95) | 7.60 (0.82) | 21.66 |
| L. lateral orbitofrontal cortex | 5.00 (0.48) | 4.11 (0.77) | 17.85 |
| Subgenual anterior cingulate | 7.00 (0.97) | 5.07 (0.61) | 27.60 |
| Post hoc regions of interest * [F(5,10)= 3.24, Wilks’ lambda=.38, p=0.054] | |||
| Pregenual anterior cingulate | 5.80 (0.68) | 4.44 (0.62) | 23.38 |
| R. lateral orbitofrontal cortex | 5.24 (0.64) | 4.01 (0.94) | 23.41 |
| Postcentral gyrus | 4.06 (0.67) | 3.29 (0.56) | 19.13 |
| Occipital cortex | 2.20 (0.26) | 1.78 (0.32) | 19.04 |
| Raphe nucleus (Presynaptic region) | 4.09 (0.45) | 3.66 (0.62) | 10.58 |
Multivariate analysis of variance (MANOVA) test of the main effect of depression on 5HT1A receptor BP.
Figure 1.
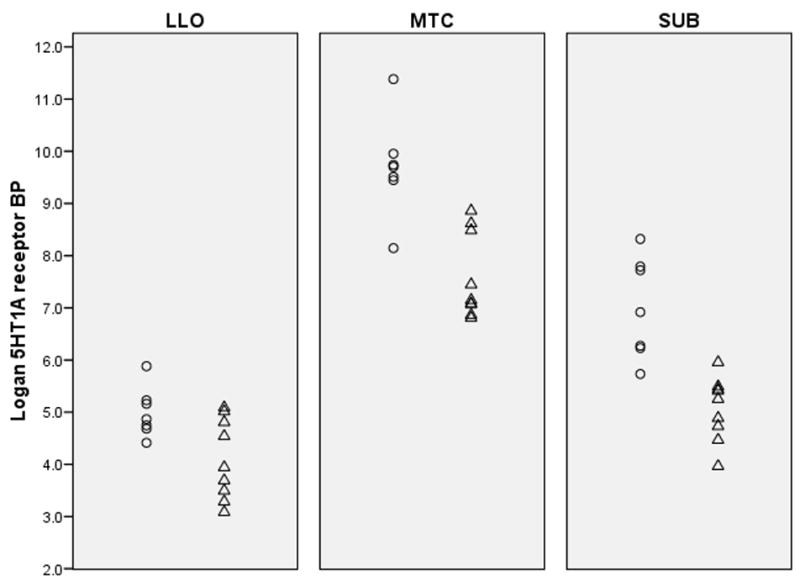
Dot plots indicate that 5HT1A receptor BP is lower in postpartum depressed (△) relative to postpartum control subjects (○) in primary regions of interest. Abbreviations: LLO=left lateral orbitofrontal cortex, MTC=mesiotemporal cortex, SUB=subgenual anterior cingulate.
Figure 2.
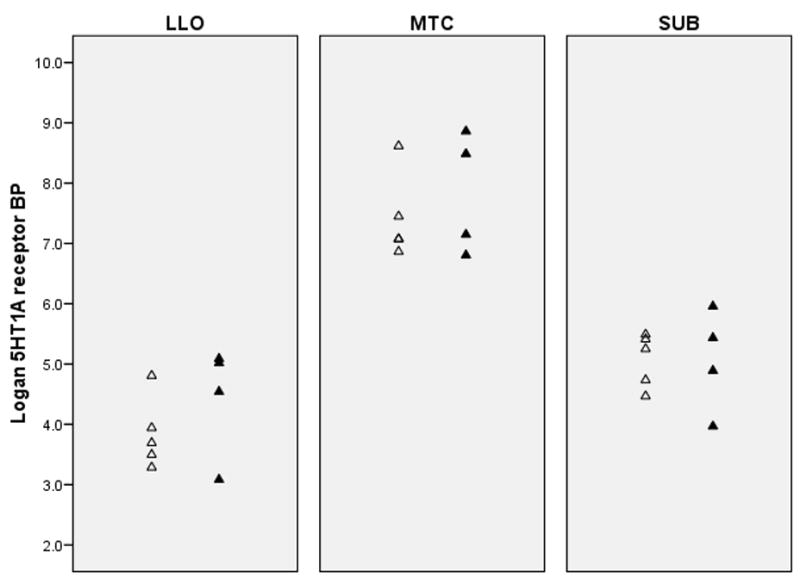
Dot plots indicate that 5HT1A receptor BP is not different between unipolar (△) and bipolar (▲) depressed subjects in all primary regions of interest. Abbreviations as per Figure 1.
In our exploratory analyses, age, BMI, and breastfeeding status reached the critical threshold of p ≤ 0.15 in univariate regressions. When entered with depression status into the MANCOVA, only depression reached the critical threshold of p ≤ 0.01.
Injected [11C]WAY dose, metabolism of [11C]WAY over time, and protein binding of [11C]WAY was not different between groups. [11C]WAY BP was not correlated with estradiol concentrations in the entire sample or to HAM25 score in the depressed subgroup.
The distribution volume (DV) of free and non-specifically bound [11C]WAY as estimated by the cerebellum (CER) was 0.73 ± 0.17 in the control group and 0.87 ± 0.31 in the PD group [p(MWU,2-tailed, exact) = 0.47]. Notably, one subject in the PD group had a CER DV value that was nearly 2 standard deviations greater that the PD CER DV mean. When the analysis excluded this subject, mean ± SD CER DV of control and PD groups were similar [control = 0.73 ± .17, PD = 0.79 ± .20] and the main findings of reduced [11C]WAY BP in the PD group in mesiotemporal, lateral orbitofrontal, and anterior cingulate cortices remained significant.
Discussion
These preliminary findings demonstrated that postsynaptic 5HT1A receptor BP was reduced in women with PD versus control by a similar magnitude (>20%) as shown previously in non-postpartum samples. Our findings suggest that the previously-described association between depression andreduced 5HT1A receptor binding also extends to the puerperium, a time of multiple physiologic disruptions (metabolic, hormonal, sleep deprivation). Anterior cingulate, mesiotemporal, and lateral orbitofrontal cortices were the brain regions of greatest 5HT1A receptor differences between groups. Because postsynaptic 5HT1A receptors in these brain regions modulate neuronal circuits that regulate emotion, 5HT1A receptor decreases could mediate the heightened anxiety, depressed mood, and suboptimal infant attunement associated with PD. Other roles for postsynaptic 5HT1A receptors through their localization on astrocytes and other glia (51) include release of trophic factors that promote 5HT neuronal outgrowth (52). Reductions of 5HT1A receptors in PD, therefore, could impede the neuroprotection of the broader central nervous system that is needed for optimal mental health.
Because these PD subjects were largely psychotropic drug naïve and could participate in the study unmedicated, the sample may have been less severely depressed or functionally impaired than other PD women. While this is a conservative bias, it is noteworthy that 5HT1A receptor reductions were apparent in a potentially milder sample of PD women. The detection of a significant effect of depression on 5HT1A receptor BP in this small cohort may be attributable to the homogeneous demographic and reproductive characteristics of the sample. The effect does not appear to be a result of the high rate of women with bipolar disorder in the depressed sample, since there didn’t appear to be a difference in 5HT1A receptor BP between the unipolar and bipolar subjects. The ability to delineate unipolar versus bipolar neurobiological differences in the puerperium isan important future research direction. Bipolar I disorder is usually a more severe and persistent disorder than unipolar disorder and may be associated with greater neuropathology (36), including 5HT1A receptor deficits (23); however, bipolar spectrum disorder has been less studied.
Because the majority of women in this study experienced depression onset during pregnancy (n=6 out of 9), it is not possible to determine whether timing of childbearing-related depression onset has an effect on 5HT1A receptor binding in postpartum depressed women. In addition, whether the 5HT1A receptor deficit in PD represents a trait abnormality related to depression vulnerability or a state-related change that emerges in women with mood disturbance during the hormonal shifts of childbearing remains an intriguing question that requires additional study. Prior research in a male sample suggested that 5HT1A receptor deficits persist after MDD remission (53). Similar research in postpartum depression would be worthwhile.
Although 5HT1A receptor binding was not related to estradiol concentration, 5HT1A receptor binding may be related to other components of the postpartum endocrine milieu. A possible mechanism for 5HT1A receptor reductions in PD is the known hypercortisolemia of the puerperium, which is less responsive to negative feedback in postpartum depressed relative to postpartum control women (17, 54). Whether breastfeeding, associated with attenuated glucocorticoid responses to stress (55, 56), modulates 5HT1A receptor binding is another area for further exploration. Breastfeeding was associated with a trend toward increased 5HT1A receptor BP in subgenual cingulate gyrus in exploratory analyses.
It is noteworthy that 5HT1A receptor BP increases in MDD have been reported in individuals with enrichment of the G allele distribution of the C(−1019)G 5-HT1A promoter polymorphism (26, 57). It would be interesting to examine genetic factors in future studies of 5HT1A receptor BP in unipolar and bipolar PD.
Pregnancy and the puerperium present a unique multidimensional challenge to the homeostasis of women. Biological, psychological, and social states change dramatically over the course of childbearing. This is the first study to measure neuroreceptor changes in the early puerperium which may underlie susceptibility to depression. Recognition of this neurobiological deficit in PD suggests that development of 5HT1A receptor directed treatments (including, perhaps, encouragement of breastfeeding or administration of oxytocin analogues) may be highly beneficial for the treatment and prevention of this disabling disorder.
Acknowledgments
We would like to thank members of the PET Facility Staff who carried out the acquisition of PET data and care of all subjects during PET procedures; Andrea Confer, Carl Becker, Danielle Mullen, Julie Giombetti, Wendy Bogers, and Alicia Corominal who assisted with study recruitment, data entry, image analysis, and endocrine analyses; NIMH, NARSAD for supporting this research; Magee-Womens Clinical Research Center, and Womens Behavioral HealthCARE for providing infrastructure to run this project, and Peter Schmidt for his helpful comments on this manuscript.
Financial Support: MH64561 to Dr. Moses; M01-RR000056 to University of Pittsburgh; Dr. Meltzer’s time was supported by K24 MH64625; Dr. Wisner’s time was supported by MH 57102 and MH 53735.
Footnotes
Capsule: Postsynaptic 5HT1A receptor binding is reduced in postpartum depression by a similar magnitude as shown in other depression samples in brain regions critical to mood regulation.
References
- 1.Gaynes BN, Gavin NSM-B, et al. Quality AfHRa. Vol. 119. AHRQ; 2005. Perinatal Depression: Prevalence, Screening Accuracy, and Screening Outcomes. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sit D. Women and bipolar disorder across the life span. JAMWA. 2004;59:91–100. [PMC free article] [PubMed] [Google Scholar]
- 3.Grace SL, Evindar A, Stewart DE. The effect of postpartum depression on child cognitive development and behavior: a review and critical analysis of the literature. Archives of Women’s Mental Health. 2003 Nov;6(4):263–274. doi: 10.1007/s00737-003-0024-6. [DOI] [PubMed] [Google Scholar]
- 4.Murray L. The impact of postnatal depression on infant development. J Child Psychol Psychiatry. 1992;33:543–561. doi: 10.1111/j.1469-7610.1992.tb00890.x. [DOI] [PubMed] [Google Scholar]
- 5.Goodman SH, Gotlib IH. Risk for psychopathology in the children of depressed mothers: a developmental model for understanding mechanisms of transmission. Psychological Review. 1999 Jul;106(3):458–490. doi: 10.1037/0033-295x.106.3.458. [DOI] [PubMed] [Google Scholar]
- 6.Misri S, Reebye P, Kendrick K, Carter D, Ryan D, Grunau RE, et al. Internalizing behaviors in 4-year-old children exposed in utero to psychotropic medications.[see comment] American Journal of Psychiatry. 2006 Jun;163(6):1026–1032. doi: 10.1176/ajp.2006.163.6.1026. [DOI] [PubMed] [Google Scholar]
- 7.Goodman JH. Paternal postpartum depression, its relationship to maternal postpartum depression, and implications for family health. Journal of Advanced Nursing. 2004 Jan;45(1):26–35. doi: 10.1046/j.1365-2648.2003.02857.x. [DOI] [PubMed] [Google Scholar]
- 8.Cohen LS, Viguera AC, Bouffard SM, Nonacs RM, Morabito C, Collins MH, et al. Venlafaxine in the treatment of postpartum depression. Journal of Clinical Psychiatry. 2001 Aug;62(8):592–596. doi: 10.4088/jcp.v62n0803. [DOI] [PubMed] [Google Scholar]
- 9.Yonkers K, Wisner K, Stowe Z, Leibenluft E, Cohen L, Miller L, et al. Management of bipolar disorder during pregnancy and the postpartum period. Am J Psych. 2004;161:608–620. doi: 10.1176/appi.ajp.161.4.608. [DOI] [PubMed] [Google Scholar]
- 10.Wisner KL, Hanusa BH, Perel JM, Peindl KS, Piontek CM, Sit DKY, et al. Postpartum Depression: A randomized trial of Sertraline vs. Nortriptyline. Journal of Clinical Psychopharmacology. 2006;26:353–360. doi: 10.1097/01.jcp.0000227706.56870.dd. [DOI] [PubMed] [Google Scholar]
- 11.Scholle SH, Kelleher KJ. Assessing primary care among young, low-income women. Women and Health. 2003;37(1):15–30. doi: 10.1300/J013v37n01_02. [DOI] [PubMed] [Google Scholar]
- 12.Bloch M, Schmidt P, Danaceau M, Murphy J, Neiman L, Rubinow D. Effects of gonadal steroids in women with a history of postpartum depression. American Journal of Psychiatry. 2000;157:924–930. doi: 10.1176/appi.ajp.157.6.924. [DOI] [PubMed] [Google Scholar]
- 13.Kornstein SG, Schatzberg AF, Thase ME, Yonkers KA, McCullough JP, Keitner GI, et al. Gender differences in treatment response to sertraline versus imipramine in chronic depression.[see comment] American Journal of Psychiatry. 2000 Sep;157(9):1445–1452. doi: 10.1176/appi.ajp.157.9.1445. [DOI] [PubMed] [Google Scholar]
- 14.Wikander I, Sundblad C, Andersch B, Dagnell I, Zylberstein D, Bengtsson F, et al. Citalopram in premenstrual dysphoria:is intermittent treatment during luteal phases more effective than continuous medication throughout the menstrual cycle? Journal of Clinical Psychopharmacology. 1998;18(5):390–398. doi: 10.1097/00004714-199810000-00007. [DOI] [PubMed] [Google Scholar]
- 15.Freeman EW, Rickels K, Sondheimer SJ, Polansky M. Differerential response to antidepressants in women with premenstrual syndrome/premenstrual dysphoric disorder. Arch Gen Psychiatry. 1999;56:932–939. doi: 10.1001/archpsyc.56.10.932. [DOI] [PubMed] [Google Scholar]
- 16.Bethea CL, Lu NZ, Gundlah C, Streicher JM. Diverse actions of ovarian steroids in the serotonin neural system. Front Neuroendocrinol. 2002;23(1):41–100. doi: 10.1006/frne.2001.0225. [DOI] [PubMed] [Google Scholar]
- 17.Lopez JF, Chalmers DT, Little KY, Watson SJ. Regulation of serotonin 1A, glucocorticoid, and mineralocorticoid receptor in rat and human hippocampus: Implications for the neurobiology of depression. Biological Psychiatry. 1998;43:547–573. doi: 10.1016/s0006-3223(97)00484-8. [DOI] [PubMed] [Google Scholar]
- 18.Haddjeri N, Blier P, De Montigny C. Long-term antidepressant treatments result in tonic activation of forebrain 5HT1A receptors. J Neuroscience. 1998;18(23):10150–10156. doi: 10.1523/JNEUROSCI.18-23-10150.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramboz S, Oosting R, Amara DA, Kung HF, Blier P, Mendelsohn M, et al. Serotonin receptor 1A knockout: An animal model of anxiety-related disorder. Neurobiology. 1998 November 1998;95:14476–14481. doi: 10.1073/pnas.95.24.14476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Arango V, Underwood MD, Boldrini M, Tamir H, Kassir SA, Hsiung S, et al. Serotonin 1A receptors, serotonin transporter binding and serotonin transporter mRNA expression in the brainstem of depressed suicide victims. Neuropsychopharmacology. 2001;25:892–903. doi: 10.1016/S0893-133X(01)00310-4. [DOI] [PubMed] [Google Scholar]
- 21.Mann JJ, Malone K, Diehl D, Perel J, Cooper T, Mintun MA. Demonstration in vivo of reduced serotonin responsivity in the brain of untreated depressed patients. Americal Journal of Psychiatry. 1996;153:174–182. doi: 10.1176/ajp.153.2.174. [DOI] [PubMed] [Google Scholar]
- 22.Bowen DM, Najlerahim A, Procter AW, Francis PT, Murphy E. Circumscribed changes of the cerebral cortex in neuropsychiatric disorders of late life. Proc Natl Acad Sci. 1989;86:9504–9508. doi: 10.1073/pnas.86.23.9504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Drevets WC, Frank E, Price JC, Kupfer DJ, Holt D, Greer PJ, et al. PET imaging of serotonin 1A receptor binding in depression. Biol Psychiatry. 1999;46:1375–1387. doi: 10.1016/s0006-3223(99)00189-4. [DOI] [PubMed] [Google Scholar]
- 24.Meltzer CC, Price JC, Mathis CA, Butters MA, Ziolko SK, Moses-Kolko E, et al. Serotonin 1A receptor binding and treatment response in late-life depression. Neuropsychopharmacology. 2004 Dec;29(12):2258–2265. doi: 10.1038/sj.npp.1300556. [DOI] [PubMed] [Google Scholar]
- 25.Sargent PA, Kjaer KH, Bench CJ, Rabiner EA, Messa C, Meyer J, et al. Brain serotonin 1A receptor binding measured by positron emission tomography with [11C]WAY-100635: Effects of depression and antidepressant treatment. Arch Gen Psychiatry. 2000;57:174–180. doi: 10.1001/archpsyc.57.2.174. [DOI] [PubMed] [Google Scholar]
- 26.Parsey RV, Oquendo MA, Ogden RT, Olvet DM, Simpson N, Huang Y, et al. Altered serotonin 1A binding in major depression:a {carbonyl-C-11]WAY 100635 positron emission tomography study. Biol Psychiatry. 2006 January 15;59(2):106–113. doi: 10.1016/j.biopsych.2005.06.016. [DOI] [PubMed] [Google Scholar]
- 27.Stockmeier CA, Shapiro LA, Dilley GE, Kolli TN, Friedman L, Rajkowska G. Increase in serotonin-1A autoreceptors in the midbrain of suicide victims with major depression-Postmortem evidence for decreased serotonin activity. J Neuroscience. 1998;18(18):7394–7401. doi: 10.1523/JNEUROSCI.18-18-07394.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM-IV Axis I Disorders - Patient Edition. New York: New York State Psychiatric Institute, Biometrics Research Department; 1998. [Google Scholar]
- 29.Stowe ZN, Hostetter AL, Newport DJ. The onset of postpartum depression: Implications for clinical screening in obstetrical and primary care. Americal Journal of Obstetrics and Gynecology. 2005;192:522–526. doi: 10.1016/j.ajog.2004.07.054. [DOI] [PubMed] [Google Scholar]
- 30.Blehar MC, DePaulo JR, Jr, Gershon ES, Reich T, Simpson SG, Nurnberger JI., Jr Women with bipolar disorder: findings from the NIMH Genetics Initiative sample. Psychopharmacology Bulletin. 1998;34(3):239–243. [PubMed] [Google Scholar]
- 31.Viguera A, Nonacs R, Cohen L, Tondo L, Murray A, Baldessarini R. Risk of recurrence of bipolar disorder in pregnant and nonpregnant women. Am J Psych. 2000;157:179–184. doi: 10.1176/appi.ajp.157.2.179. [DOI] [PubMed] [Google Scholar]
- 32.Berga SL, Daniels TL, Giles DE. Women with functional hypothalamic amenorrhea but not other forms of anovulation display amplified cortisol concentrations. Fertility & Sterility. 1997;67:1024–1030. doi: 10.1016/s0015-0282(97)81434-3. [DOI] [PubMed] [Google Scholar]
- 33.McCarron JA, Turton D, Pike VW, Poole K. Remotely controlled production of the 5HT1A receptor radioligand, [carbonyl-11C]WAY-100635, via 11C-carboxylation of an immobilized Grignard reagent. J Labelled Comp Radiopharm. 1996;38:941–953. [Google Scholar]
- 34.Moses-Kolko E, Meltzer CC, Helsel JC, Sheetz M, Mathis C, Ruszkiewicz J, et al. No interruption of lactation is needed after [11C]WAY 100635 or [11C]Raclopride PET. Journal of Nuclear Medicine. 2005;46(10):1765. [PubMed] [Google Scholar]
- 35.Woods RP, Mazziotta JC, Cherry SR. MRI-PET registration with automated algorithm. J Comput Assist Tomogr. 1993;17:536–546. doi: 10.1097/00004728-199307000-00004. [DOI] [PubMed] [Google Scholar]
- 36.Drevets WC, Gadde KM, Krishnan KRR. The Neurobiological Foundation of Mental Illness. In: Charney D, Nestler E, editors. Neurobiology of Mental Illness. Oxford: Oxford Press; 2004. [Google Scholar]
- 37.Arango V, Underwood MD, Gubbi AV, Mann JJ. Localized alterations in pre- and postsynaptic serotonin binding sites in the ventrolateral prefrontial cortex of suicide victims. Brain Research. 1995;688:121–133. doi: 10.1016/0006-8993(95)00523-s. [DOI] [PubMed] [Google Scholar]
- 38.Parsey RV, Arango V, Olvet DM, Oquendo MA, Van Heertum RL, Mann JJ. Regional heterogeneity of 5-HT1A receptors in human cerebellum as assessed by positron emission tomography. J Cereb Blood Flow Metab. 2005;25:785–793. doi: 10.1038/sj.jcbfm.9600072. [DOI] [PubMed] [Google Scholar]
- 39.Meltzer CC, Drevets WC, Price JC, Mathis CA, Lopresti B, Greer PJ, et al. Gender-Specific Aging Effects on the Serotonin 1A Receptor. Brain Research. 2001 23 March;895(1–2):9–17. doi: 10.1016/s0006-8993(00)03211-x. [DOI] [PubMed] [Google Scholar]
- 40.Price JC, Kelley DE, Ryan CM, Meltzer CC, Drevets WC, Mathis CA, et al. Evidence of Increased Serotonin-1A Receptor Binding in Type 2 Diabetes: A Positron Emission Tomography Study. Brain Res. 2002b;927(1):97–103. doi: 10.1016/s0006-8993(01)03297-8. [DOI] [PubMed] [Google Scholar]
- 41.Bailer UF, Frank GK, Henry SE, Price JC, Meltzer CC, Weissfeld L, et al. Altered brain serotonin 5-HT1A receptor binding after recovery from anorexia nervosa measures by positron emission tomography and [11C]WAY 100635. Arch Gen Psychiatry. 2005;62:1032–1041. doi: 10.1001/archpsyc.62.9.1032. [DOI] [PubMed] [Google Scholar]
- 42.Logan J, Fowler JS, Volkow ND, Ding YS, Wang G-J, Alexoff DL. A strategy for removing the bias in the graphical analysis method. J Cereb Blood Flow Metab. 2001;21:307–320. doi: 10.1097/00004647-200103000-00014. [DOI] [PubMed] [Google Scholar]
- 43.Price JC, Xu L, Mazumdar S, Meltzer CC, Drevets WC, Mathis CA, et al. Impact of Graphical Analysis Analysis Bias on Group Comparisons of Regional [carbonyl-11C]WAY Binding Potential Measures. Neuroimage. 2002a;16(3):S72. [Google Scholar]
- 44.Lammertsma A, Bench C, Hume S, et al. Comparison of methods for analysis of clinical [11C]raclopride studies. J Cereb Blood Flow Metab. 1996;16:42–52. doi: 10.1097/00004647-199601000-00005. [DOI] [PubMed] [Google Scholar]
- 45.Mintun MA, Raichle ME, Kilbourn MR, Wooten GF, Welch MJ. A quantitative model for the in vivo assessment of drug binding sites with positron emission tomography. Ann Neurol. 1984;15:217–227. doi: 10.1002/ana.410150302. [DOI] [PubMed] [Google Scholar]
- 46.Drevets WC, Price JL, Simpson JR, Todd RD, Reich T, Vannier M, et al. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997;386:824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- 47.Sheline YI. 3D MRI studies of neuroanatomic changes in unipolar major depression: the role of stress and medical comorbidity. Biological Psychiatry. 2000;48(8):791–800. doi: 10.1016/s0006-3223(00)00994-x. [DOI] [PubMed] [Google Scholar]
- 48.Meltzer CC, Leal JP, Mayberg HS, Wagner HJ, Frost JJ. Correction of PET data for partial volume effects in human cerebral cortex by MR imaging. J Comput Assist Tomogr. 1990;14:561–570. doi: 10.1097/00004728-199007000-00011. [DOI] [PubMed] [Google Scholar]
- 49.Meltzer C, Kinahan P, Nichols T, Greer P, Comtat C, Cantwell M, et al. Comparative evaluation of MR-based partial volume correction schemes for PET. J Nucl Med. 1999;40:2053–2065. [PubMed] [Google Scholar]
- 50.Battin DA, Marrs RP, Fleiss PM, Mishell DR., Jr Effect of suckling on serum prolactin, luteinizing hormone, follicle-stimulating hormone, and estradiol during prolonged lactation. Obstetrics & Gynecology. 1985 Jun;65(6):785–788. [PubMed] [Google Scholar]
- 51.Azmitia EC, Gannon PJ, Kheck NM, Whitaker-Azmitia PM. Cellular localization of the 5HT1A receptor in primate brain neurons and glial cells. Neuropsychopharmacology. 1996;14:35–46. doi: 10.1016/S0893-133X(96)80057-1. [DOI] [PubMed] [Google Scholar]
- 52.Azmitia EC. Serotonin Neurons, Neuroplasticity, and Homeostasis of Neural Tissue. Neuropsychopharmacology. 1999;21:2S. doi: 10.1016/S0893-133X(99)00022-6. [DOI] [PubMed] [Google Scholar]
- 53.Bhagwagar Z, Rabiner EA, Sargent PA, Grasby PM, Cowen PJ. Persistent reduction in brain serotonin1A receptor binding in recovered depressed men measured by positron emission tomography with [11C]WAY-100635. Mol Psychiatry. 2004;9:386–392. doi: 10.1038/sj.mp.4001401. [DOI] [PubMed] [Google Scholar]
- 54.Mastorakos G, Ilias I. Maternal Hypothalamic-Pituitary-Adrenal Axis in Pregnancy and the Postpartum Period. Annals New York Academy of Sciences. 2000;900:95–106. doi: 10.1111/j.1749-6632.2000.tb06220.x. [DOI] [PubMed] [Google Scholar]
- 55.Altemus M, Deuster P, Galliven E, Carter S, Gold PW. Suppression of hypothalamic-pituitary-adrenal axis responses to stress in lactating women. J Clin Endo Metab. 1995;80:2954–2959. doi: 10.1210/jcem.80.10.7559880. [DOI] [PubMed] [Google Scholar]
- 56.Neumann ID, Torner L, Wigger A. Brain oxytocin: Differential inhibition of neuroendocrine stress responses and anxiety-related behaviour in virgin, pregnant and lactating rats. Neuroscience. 2000;95:567–575. doi: 10.1016/s0306-4522(99)00433-9. [DOI] [PubMed] [Google Scholar]
- 57.Lemonde S, Turecki G, Bakish D, Du L, Hrdina PD, Bown CD, et al. Impaired repression at a 5-hydroxytryptamine 1A receptor gene polymorphism associated with major depression and suicide. Journal of Neuroscience. 2003 Sep 24;23(25):8788–8799. doi: 10.1523/JNEUROSCI.23-25-08788.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cox J, Holden J, Sagovsky R. Detection of postnatal depression: Development of the 10-item Edinburgh Postnatal Depression Scale. British Journal of Psychiatry. 1987;150:782–786. doi: 10.1192/bjp.150.6.782. [DOI] [PubMed] [Google Scholar]
- 59.Hamilton M. A rating scale for depression. J Neurol Neurosurg Psychiatry. 1960;23:56–62. doi: 10.1136/jnnp.23.1.56. [DOI] [PMC free article] [PubMed] [Google Scholar]


