Abstract
There has been little work on the specificity and mechanisms underlying the appetite of potassium (K+) deprived rats, and there are conflicting results. To investigate the contribution of oral factors to changes in intake induced by K+ deficiency, we conducted two experiments using 20-s “brief access” tests. In Experiment 1, K+-deprived rats licked less for water than did replete rats. After adjusting for this difference, K+-deprived rats exhibited increased licking for 100 mM CaCl2, 100 mM MgCl2, and 100 mM FeCl2 compared with K+-replete rats. In Experiment 2, which used larger rats, the K+-deprived and replete groups licked equally for water, 500 mM Na·Gluconate, 350 mM KCl, 500 mM KHCO3, and 1 mM quinine·HCl, but the K+-deprived rats licked more for 500 mM KCl, 500 mM CsCl, and 500 mM NaCl than did the replete rats. Licking was unaffected by addition to NaCl of 200 μM amiloride, an epithelial Na+ channel (ENaC) blocker, or 100 μM ruthenium red, a vanilloid receptor 1 (VR-1) antagonist, or by addition to KCl of 50 μM 4-aminopyridine, a K+ channel blocker. These findings suggest that K+-deprivation produces a non-specific appetite that is guided by oral factors. We found no evidence that this response was mediated by ENaC, VR-1, or K+ channels in taste receptor cells.
Keywords: Mineral, Intake, Palatability, Brief access test
1. Introduction
Several minerals, including potassium (K+), are essential for rats and other animals to maintain normal physiology. Surprisingly, there is not clear evidence that K+-deprived rats develop a compensatory appetite that helps to relieve their deficit, as they do when deprived of sodium [1], calcium [2], magnesium [3], phosphorus [4,5], iron [6], zinc [7], copper [8], or selenium [9]. Blake and Jurf [10] reported that K+-deprived rats increased consumption of NaCl solutions but decreased or did not adjust intake of KCl solutions in 24-h two-bottle preference tests (versus water). In contrast, Adam and Dawborn [11] found that K+-deprived rats increased intakes of NaCl, KCl, and CaCl2 in 24-h tests with water. Disruption of Na+/K+ balance via the sodium-pump inhibitor, strophanthin, was also reported to increase the preference for KCl-glucose solutions over equimolar glucose solutions [12]. Tordoff [13] reported that K+ deprivation had a complex effect on long-term NaCl intake, with deprived rats showing elevated consumption relative to controls on treatment days 3–6, but then significantly reduced NaCl intakes after day 15. These studies are difficult to interpret for many reasons, including the fact that K+ deprivation has been reported to induce thirst and polydipsia [14–16].
Most appetites are the result of a combination of unlearned processes, such as taste, and learned ones, such as the association of orosensory and environmental cues with the postingestive consequences of nutrient consumption (e.g. amino acids [17]; calcium [18]). Long-term tests can be strongly influenced by postingestive consequences but brief access licking tests minimize consumption and thus the opportunity for postingestive feedback signals to be generated for learning to occur. This paradigm therefore allows the orosensory controls of appetite expression to be better evaluated. To date, short-term lick tests have supported an unlearned component for the appetites induced by sodium and calcium deficiencies [19–21]. Although some work has addressed this issue in K+-deprived rats, the available data do not resolve how K+-deprived animals change their ingestive behavior, or the nature of the underlying mechanisms. In two-choice preference tests lasting 0.5–5.0 min, more K+-deprived than replete rats preferred 100 mM KCl over either water or a MgSO4 solution [22]. However, in 5-min two-choice preference tests, K+-deprived rats consumed 17–690 mM NaCl solutions in preference to 1000 mM KCl than did replete rats [23]. Taken together, these studies raise the question of whether K+-deprived rats seek a source of K+ or whether the appetite for KCl is simply part of a more generalized mineral appetite.
It has been proposed that gustatory transduction of K+ involves passage through basolateral K+ channels in taste receptor cells that can be blocked by 4-aminopyridine (4-AP; [24]), though definitive evidence has not been obtained. While electrophysiological and behavioral data suggest that KCl has a bitter component to its taste [25–29], there is also behavioral evidence that KCl can be distinguished easily from bitter stimuli such as quinine [27–29]. Rats are able to distinguish KCl from NaCl [30,31], which is thought to taste primarily salty, though there is also evidence that K+ and sodium share a transduction pathway [32]. Sodium taste transduction relies on an apical epithelial sodium channel (ENaC) that can be blocked by amiloride, as well as on an amiloride-insensitive, vanilloid receptor-1 (VR-1) variant that is also permeable to K+ and that is blocked by the VR-1 antagonists SB-366791 [32] and, in non-taste tissues, ruthenium red [33–35]. Rats are unable to discriminate KCl solutions from NaCl solutions mixed with amiloride [36], and amiloride alters NaCl-induced neural responses in the rat nucleus of the solitary tract to a response profile that is more characteristic of that for KCl [37], suggesting that amiloride-NaCl mixtures taste like KCl solutions. The permeability of VR-1 channels to both sodium and K+ may provide the basis for this similarity in the taste of the two compounds following amiloride application.
The purpose of this study was to determine how licking for a diverse array of taste solutions is affected by K+ deprivation, and whether taste plays a role in mediating these responses. In addition, we evaluated the roles of ENaC, VR-1, and 4-AP-sensitive K+ channels in mediating the K+ deprivation-induced increases in licking for NaCl and KCl solutions. In Experiment 1, we recorded licking of replete and K+-deprived rats for an array of taste solutions in 20-s access tests. Because rats licked maximally for several solutions used in Experiment 1, potentially masking group differences, in Experiment 2 we used a similar paradigm with more concentrated solutions and older rats that were less likely to be as severely affected by dietary deficiency.
2. Materials and methods
2.1. Subjects
Thirty-two male Sprague-Dawley rats (Charles River, Wilmington, MA) weighing 201 ± 15 g (Experiment 1) or 357 ± 63 g (Experiment 2) at the start of the experiments served as subjects. Rats were individually housed in Plexiglas cages (47 × 26 × 20.5 cm) in a temperature- and humidity-controlled room on a 12-h:12-h light-dark cycle. Prior to the beginning of the experiments, rats were allowed ad libitum access to food (Purina Lab Chow No. 5001, Lab Diets, St. Louis, MO) and tap water. All testing occurred during the dark cycle at approximately the same time each day. Body weight and daily food and water consumption were recorded throughout the experiments.
2.2. Taste solutions
Taste solutions were mixed before each experiment using distilled water and reagent grade chemicals (Sigma-Aldrich, St. Louis, MO; Fisher Scientific, Pittsburgh, PA; Avocado Research Chemicals, Ward Hill, MA). Solutions were refrigerated and returned to room temperature 30 min prior to testing. Sucrose solutions were re-mixed every 48–72 h. The concentrations listed for 4-aminopyridine (4-AP), ruthenium red (RR), amiloride, and SB-366791 (Sigma) mixtures reflect the final concentrations of the stimulus and the blocker after mixing. Due to the light sensitivity of amiloride, all reagent bottles and drinking tubes containing amiloride solutions were wrapped in tin foil.
2.3. Apparatus
Behavioral testing was conducted in a room separate from the vivarium in two MS-160 Davis Rig lickometers (DiLog Instruments, Tallahassee, FL) interfaced to computers. This apparatus, described elsewhere in detail [38], consists of a 29 × 14.5 × 19 cm metal and opaque plastic cage with a wire mesh floor. On one side of the cage a 35 × 14 mm vertical slot was centered 7 cm above the floor. This slot allowed access to a single drinking spout attached to an inverted 70-mL bottle housed on an external motorized rack. Prior to each trial the rack, which held up to 16 bottles, moved the specified bottle into position and a motorized shutter that blocked access to the drinking spout opened. Tongue contacts with the drinking spout allowed individual licks to be recorded with a 1 ms resolution. All tube presentation sequences were computer controlled, and licking data were stored for offline analysis. A white noise generator (HoMedics, Inc., Commerce Township, MI) masked background noise throughout all training and testing sessions.
2.4. Procedure
2.4.1. Potassium deprivation
At 2–4 days before the start of Davis Rig training, the standard chow was removed and small hard plastic or metal dishes containing either AIN-76A (Dyets, Inc; Bethlehem, PA) purified rodent diet (n = 8 for each experiment; replete group) or a K+-deficient version of the same diet (n = 8 for each experiment; K+-deprived group) were secured to the bottom of each rat’s cage. On the same day, tap water was replaced with distilled water. Diets were available ad libitum throughout the experiment (19 to 21 days). All subjects’ cages were replaced daily with new cages and bedding.
2.4.2. Training
Rats were deprived of water for approximately 23 h before the start of all training and testing sessions. On training days 1 and 2, rats were offered a single 15-min presentation (clock beginning with the first lick) of one bottle of distilled water (dH2O) in the Davis Rig. Following these sessions, rats were given 30-min access to water in their home cages before starting water deprivation for the next day’s session. On the next 3–6 training days, rats were offered two 20-s presentations (clock beginning with the first lick) of each of 12 (Experiment 1) or 13 (Experiment 2) bottles of dH2O. If a rat failed to lick the tube within 20 s of the start of a presentation, the 20 s clock was not started, the shutter closed, and the next tube was presented. There was a 5-s interval between consecutive presentations. Rats were not allowed 30-min access to water in their home cages afterwards if they failed to sample each test bottle at least once during the training session. Rats were trained until they sampled at least once from each bottle during the training session. Bottles were rinsed and refilled several times daily, spouts were rinsed between every session, and the Davis Rigs were cleaned thoroughly between testing of replete and K+-deprived rats.
2.4.3. Brief access testing
Brief access testing of an array of 12 (Experiment 1) or 14 (Experiment 2) taste solutions commenced as soon as rats sampled reliably during training sessions. The stimulus arrays for each experiment are listed in Table 1. In both experiments, arrays were chosen that included compounds with diverse taste qualities, in order to examine broadly how K+ deprivation affects short-term licking. For both experiments, each solution was presented in random order twice during each daily testing session (i.e., 2 trials per taste solution per day). Rats were tested daily until they had sampled each stimulus at least 10 times, over 6–10 days under conditions of approximately 23-h water deprivation with 30-min access to dH2O in their home cages after test sessions in which they sampled each taste solution at least once. The results of Experiment 1 indicated that the concentrations of KCl, NaCl, and KHCO3 tested were not strong enough to suppress licking relative to that for water, which caused difficulties in interpreting the results. Therefore, stronger concentrations were selected for Experiment 2. In addition, in Experiment 1 SB-366791 rapidly precipitated from the solution, so a different VR-1 channel blocker, ruthenium red (RR), was used for Experiment 2. Concentrations of the channel blockers amiloride, RR, and 4-AP in Experiment 2 were chosen based on pilot work (data not shown), which indicated that the solutions at the tested concentrations were not avoided relative to water and presumably did not have a clear bitter or aversive taste by themselves. Presentations of dH2O immediately followed presentations of solutions containing amiloride, RR, or 4-AP in an attempt to reduce the likelihood that blockers were bound during subsequent presentations.
Table 1.
Stimuli presented to rats during brief access tests in Experiments 1 and 2
| Experiment 1 | Experiment 2 |
|---|---|
| Deionized water | Deionized water |
| 100 mM KCl | 350 mM KCl |
| 200 mM KCl | 500 mM KCl |
| 200 mM NaCl | 500 mM KCl+50 μM 4-AP |
| 200 mM KHCO3 | 500 mM KHCO3 |
| 200 mM NaCl+1 μM SB-366791 | 500 mM NaCl |
| 200 mM NaCl+100 μM amiloride | 500 mM NaCl+200 μM amiloride |
| 10 mM citric acid | 500 mM NaCl+100 μM ruthenium red |
| 100 mM FeCl2 | 500 mM NaGluconate |
| 100 mM CaCl2 | 500 mM CsCl |
| 100 mM MgCl2 | 1 mM quinine-HCl |
| 100 mM sucrose | 50 μM 4-AP |
| 200 μM amiloride | |
| 100 μM ruthenium red |
2.4.4. Plasma and urine K+ concentrations
Variables related to K+ balance were measured to confirm that the K+-free diet effectively induced K+ deficiency by the time of testing with taste solutions, and that the access to small amounts of K+ during testing did not replete the K+-deprived rats. In Experiment 1, tail blood (100–300 μL) was collected into heparinized Natelson capillary tubes (Fisher Scientific) after the last training day and after the last brief access test, following water repletion. Care was taken to avoid hemolysis, but three samples had to be discarded after such contamination was apparent. Samples were transferred to pre-chilled microfuge tubes and spun at 10,000 × g for 1 min. Plasma was drawn off each sample and stored at −20 °C. K+ concentrations of plasma were determined from 50 to 100 μL aliquots using a colorimetric assay (Pointe Scientific, Canton, MI).
Although the plasma analysis revealed statistically significant differences between the replete and K+-deprived groups, the procedure was sensitive to contamination by red blood cells, and the absolute size of the group difference was modest. These problems were avoided in Experiment 2 by switching to analysis of urinary K+ concentration. Urine samples were collected overnight after the last training session and the last testing session. Rats were placed in hanging wire cages over pans that were tilted so that urine ran to one corner, where it flowed through a hole into a covered plastic container. Food dishes were secured to the cage bottoms over shallow plastic weigh boats to minimize food spillage into the pan. Samples were stored at −20 °C until determination of K+ concentration using a VITROS 350 chemistry analyzer (Ortho-Clinical Diagnostics, Rochester, NY).
2.4.5. Twenty-four hour Two-bottle Test
We measured overnight KCl intake to gain insight into the conflicting reports as to whether K+-deprived rats show an appetite for KCl in long-term tests [10,11], and to confirm that the dietary deprivation procedure was effective. After urine or blood collection at the end of each experiment, rats were returned to their home cages, where they were given a 24-h two-bottle choice test with dH2O and 200 mM KCl. The relative positions of the two bottles were counterbalanced across rats. All bottles were weighed to the nearest 0.1 g before and after the test.
2.4.6. Data analysis
For brief access taste testing, the first 10 sampled presenations of each taste solution were averaged for each rat, as all rats had sampled each taste solution at least 10 times over the course of each experiment. Brief access trials with 2 licks or less were disregarded to control for the possibility of falsely registered licks or a failure to sample the taste solution. Group licking averages for each taste solution were determined by averaging individual means. Brief access testing was analyzed using a two-way ANOVA with taste solution as a within-subjects factor and with diet group (K+-deprived vs. replete) as a between-subjects factor. Post-hoc t-tests were used to evaluate between-group differences in licking for each taste solution. Water and KCl intakes in the 24-h two-bottle tests were analyzed using 2 × 2 ANOVA (Group × Taste Solution) to compare water intake, 200 mM KCl intake, percent KCl:water preference, and total fluid intake. Plasma and urine K+ concentrations were analyzed using two-way ANOVAs with the time the sample was collected (post-training or post-testing) as a within-subjects factor and with group as a between-subjects factor. Group differences in K+ concentrations for each sampling time were evaluated with post-hoc t-tests. The K+ concentrations in urine samples collected from all K+-deprived rats after brief access testing were below the detection threshold of the assay (2.5 mM/L); therefore we conservatively assigned these animals K+ concentrations of 2.5 mM/L to permit a parametric statistical analysis. Licking for water in training trials was also examined using a two-way ANOVAwith training day as a within-subjects factor and with depletion group as a between-subjects factor, followed by post-hoc t-tests to compare each training day. Body weights and weight gain over the course of the experiment were compared between groups of rats using t-tests.
All analyses were performed using SPSS 11.5 (SPSS, Inc., Chicago, IL). The threshold for significance was p < 0.05, but values between 0.05 and 0.1 were considered to indicate a “trend” that approached significance and are labeled clearly as such.
3. Results
3.1. Experiment 1
Three findings indicated that the K+-deficient diet had its intended effects. Relative to controls, the K+-deprived rats (a) gained less weight over the course of the experiment (replete: 99 ± 4 g; K+-deprived: 34 ± 5 g; t(14) = 9.96, p < 0.001) and weighed less after brief access testing (replete: 294 ± 5 g; K+-deprived: 196 ± 5 g; t(14) = 13.32, p < 0.001), (b) had significantly lower plasma K+ concentrations and (c) drank significantly more 200 mM KCl in the final 24-h choice test. Plasma K+ concentrations were lower in the K+-deprived group [Fig. 1A; F(1,11) = 6.89; p = 0.02] and were unaffected by the time the samples were collected (i.e., training versus post-testing) or the interaction of diet group with time [n.s.]. In the 24-h two-bottle choice test with water and KCl, K+-deprived rats drank significantly more KCl and significantly less water than did replete controls [Fig. 1B; taste solution × group interaction [F (1,13) = 62.4; p < 0.001] and the ratio of KCl consumption to water consumption was significantly greater in the K+-deprived than replete rats [Fig. 1B inset; t(13) = 3.47; p = 0.004]. Total fluid intakes of the two groups did not differ (Fig. 1B).
Fig. 1.
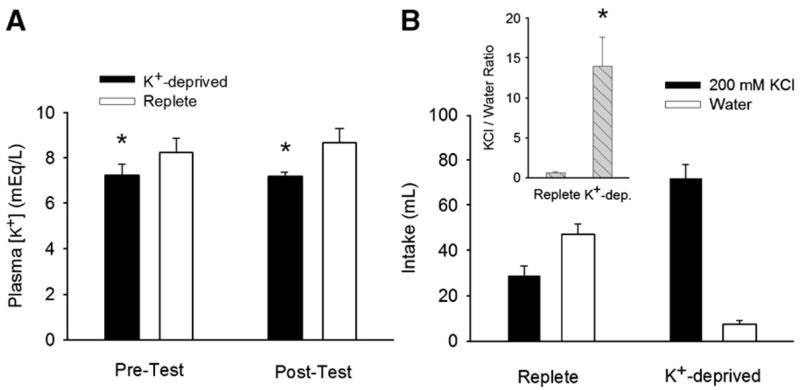
A. Mean (+SEM) plasma K+ concentrations of K+-deprived and replete rats on the fourth day of water training (Pre-Test) and following the last brief access testing session in Experiment 1 (Post-Test). K+-deprived rats had significantly lower plasma K+-concentrations as indicated by a statistically significant main effect of deprivation group, with no group × test-phase interaction. B. Mean (+SEM) 24-h intakes of 200 mM KCl and dH2O by K+-deprived and replete rats following brief access testing. The lack of an effect of group indicates total fluid consumption did not differ between groups, but the statistically significant taste solution × group interaction indicates that K+-deprived and replete rats differed in their solution preferences. B, inset. Mean (+SEM) ratios of 24-h 200 mM KCl intake: 24-h dH2O intake in the two-bottle preference tests. K+-deprived rats exhibited a robust preference for KCl that was not observed in replete rats. (*, significantly different from replete rats, p < 0.05)
Mean licks for the 12 stimuli were compared between the groups (Fig. 2), and they differed for some stimuli (significant taste solution × group interaction [F(11,154) = 15.8; p < 0.001]), but they did not differ in total licks exhibited (effect of group, n. s.), suggesting that the K+-deprived rats were not in malaise. Post-hoc tests revealed that, relative to replete rats, K+-deprived rats licked significantly more 100 mM CaCl2 and 100 mM MgCl2 and significantly less dH2O, 10 mM citric acid, 100 mM sucrose, and 200 mM KHCO3 [t(14) ≥ 2.8, p < 0.02 in all cases].
Fig. 2.
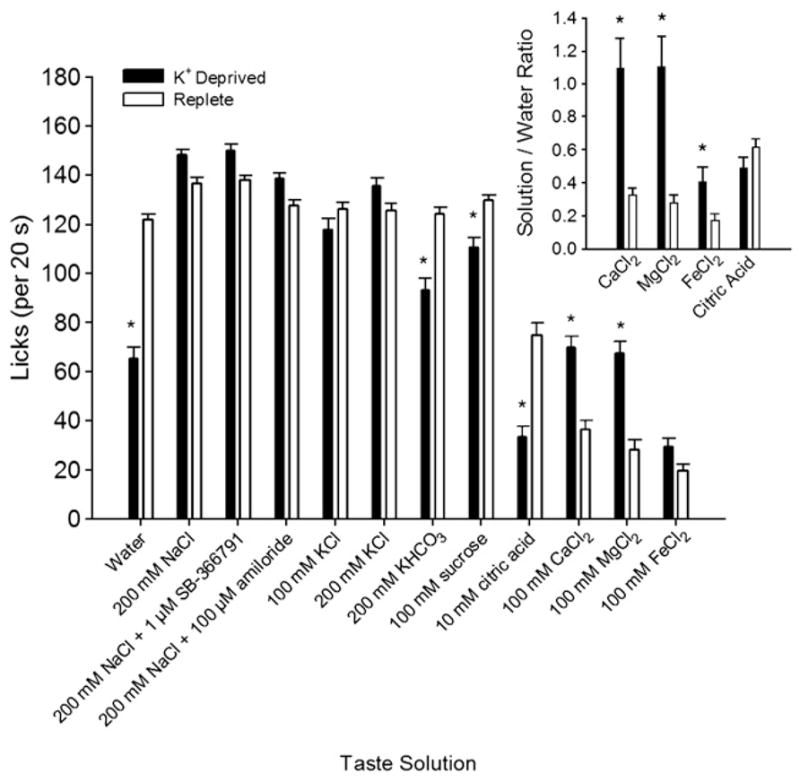
Mean (+SEM) licks (in 20-s trials) for an array of taste solutions by K+-deprived and replete rats in Experiment 1. Relative to replete rats, K+-deprived rats licked more 100 mM CaCl2 and 100 mM MgCl2 but less dH2O, 10 mM citric acid, 100 mM sucrose, and 200 mM KHCO3. Group differences in taste evaluation of NaCl and KCl solutions may have been masked by the fact that both K+-deprived and replete rats licked maximally for these solutions. Inset. Analysis of taste solutions for which lick responses were well below the maximal lick rate. Mean (+SEM) ratios of brief access licking for 100 mM CaCl2, 100 mM MgCl2, 100 mM FeCl2 and 10 mM citric acid relative to the number of licks expressed for water. Relative to replete rats, K+-deprived rats showed significant increases in licking for the chloride-containing salt solutions. (*, significantly different from replete rats, p < 0.05)
The two groups also differed on water intake during training trials (Fig. 3A). We found a significant effect of training day [F (4,52) = 20.8, p < 0.001] and of group [F(1,13) = 532.4, p < 0.001] on water licking in training trials and a significant day × group interaction [F(4,52) = 3.62, p < 0.011], suggesting that group differences in water licking were apparent before rats were exposed to taste solutions. K+-deprived rats also grew more slowly than replete rats; after correcting for body weight, the group differences in water licking in training trials were attenuated (Fig. 3B).
Fig. 3.
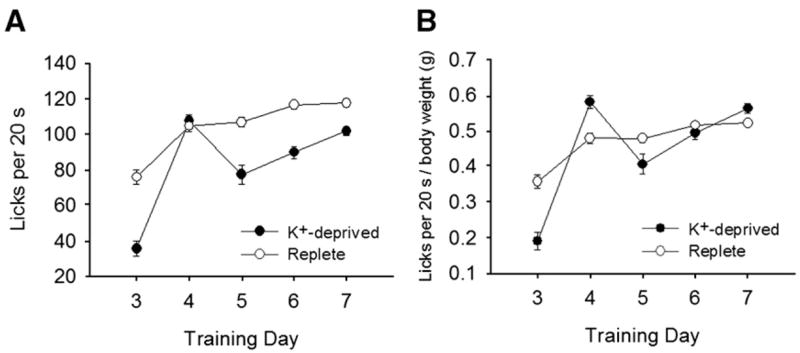
A. Mean (+SEM) licks (in 20-s trials) for dH2O by K+-deprived and replete rats during days 3–7 of training of Experiment 1. A significant effect of group suggests that K+-deprived rats licked less than replete rats during water training trials. B. Mean (+SEM) ratios of licking to body weight for dH2O training trials. No group differences were apparent, suggesting that the reduction in thirst in the K+-deprived rats relative to the replete rats may have been due to group differences in weight rather than to a more specific effect of K+-deprivation.
The group difference in water licking during brief access testing suggests that K+ deprivation may have had a generalized effect on the motivation of the animals to drink, and thus it may be inappropriate to compare groups directly on mean licks to taste solutions. One approach to negate this problem is to express licking to solutions relative to each animal’s water score. In the current study, though, this approach is problematic, because licking to water was near maximal in the replete group, making it difficult to gauge whether a stimulus that was licked to the same extent as water (e.g., 100 mM KCl) either matched water in palatability, or was more palatable than water but the replete rats were constrained by their maximum lick rate [39]. Nonetheless, this complication does not apply to CaCl2, MgCl2, FeCl2, and citric acid, because mean licking in both groups was submaximal for these taste solutions. We therefore expressed licking scores for these four stimuli relative to water licks, but did not calculate relative licks for the other seven taste compounds, for the reasons described above, nor did we draw any conclusions based on absolute lick scores for the seven stimuli. Relative licking did not differ for citric acid, but was significantly higher in K+-deprived rats for CaCl2, MgCl2, and FeCl2 [Fig. 2 inset; t(14) ≥ 2.35, p < 0.04 in all cases], indicating that these solutions tasted more palatable to them than they did to replete animals.
3.2. Experiment 2
As was the case in Experiment 1, several results confirmed the effectiveness of the K+-free diet to induce K+ deficiency. (a) K+-deprived rats gained significantly less body weight over the course of the experiment (replete: 115 ± 7 g, K+-deprived: 64 ± 9 g; t(14) = 4.64, p < 0.001). However, the mean body weights for the groups were closer than those found in Experiment 1 and were not significantly different (replete 373 ± 7 g; K+-deprived 343 ± 14 g; t(14) = 1.91, p = 0.076) at the end of brief access testing. (b) K+-deprived rats exhibited significantly lower urinary K+ concentrations than did replete rats (Fig. 4A; effect of group [F(1,14) = 58.22; p < 0.001]; group × day interaction [F(1,14) = 45.85, p < 0.001]). There was also a significant effect of day [F(1,14) = 67.77; p < 0.001], with higher urinary K+ concentrations prior to short-term lick testing in both groups. This result is not surprising, because the rats would have been more severely water-deprived before testing, and fluid deprivation increases urinary K+ concentration [40], presumably due to elevated aldosterone levels. (c) In the 24-h two-bottle test, K+-deprived rats drank significantly more 200 mM KCl and significantly less water than did replete controls [Fig. 4B; taste solution × group interaction F (1,14) = 22.1; p < 0.001] and the ratio of 200 mM KCl intake to water intake was significantly greater in K+-deprived than in replete rats [t(14) = 4.75; p < 0.001]. The two groups did not differ in total fluid consumption [effect of group, n. s.]. In contrast to Experiment 1, the groups did not differ in licking for water during training trials [Fig. 5A; group main effect and training day × group interaction [n.s.]] even when consumption was expressed as a ratio of licks to body weight (Fig. 5B; [n.s]).
Fig. 4.
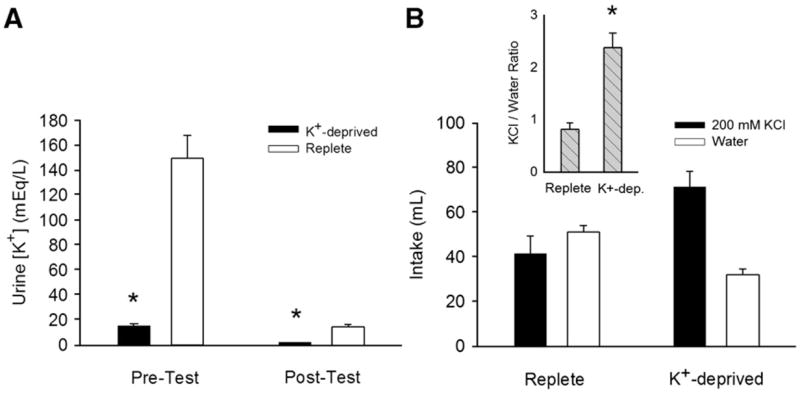
A. Mean (+SEM) K+ concentrations in urine samples collected overnight before the first (Pre-Test) and after the last (Post-Test) brief access testing session from K+-deprived and replete rats in Experiment 2. K+-deprived rats had significantly lower urine K+ concentrations than replete rats both before and after testing, confirming that the K+ deprivation protocol effectively induced K+ deficiency. B. Mean (+SEM) 24-h intakes of 200 mM KCl and dH2O by K-deprived and replete rats following brief access testing. B, inset. Mean (+SEM) ratios of 24-hr 200 mM KCl intake: 24-h dH2O intake in the two-bottle preference tests. K+-deprived rats preferred 200 mM KCl to dH2O (ratio < 1), whereas replete rats exhibited the opposite preference (ratio > 1). (*, significantly different from replete rats, p < .05)
Fig. 5.
A. Mean (+SEM) licks (in 20-s trials) for dH2O by K+-deprived and replete rats during days 3–5 of training of Experiment 2. No group differences were apparent. B. Mean (+SEM) ratios of licking to body weight for dH2O training trials. Again, K+-deprived rats did not differ from replete rats.
There was a significant effect of K+-deprivation on taste solution licking [Fig. 6; F(1,14) = 10.10; p = 0.007], which was more distinct for some solutions than others [taste solution × group interaction, F (13,182) = 5.39; p < 0.001]. Relative to controls, the K+-deprived rats licked significantly more for 500 mM KCl, 500 mM CsCl, 500 mM KCl+4-AP, 500 mM NaCl, and 500 mM NaCl+100 μM RR [t(14)≥2.42, p≤0.03 in these cases]. They also tended to lick more 500 mM NaCl+200 μM amiloride but this was not significant [t(14) = 1.83; p = 0.09]. No significant group differences were found for the other stimuli, including water or any of the water “washes” after the blocker solutions.
Fig. 6.
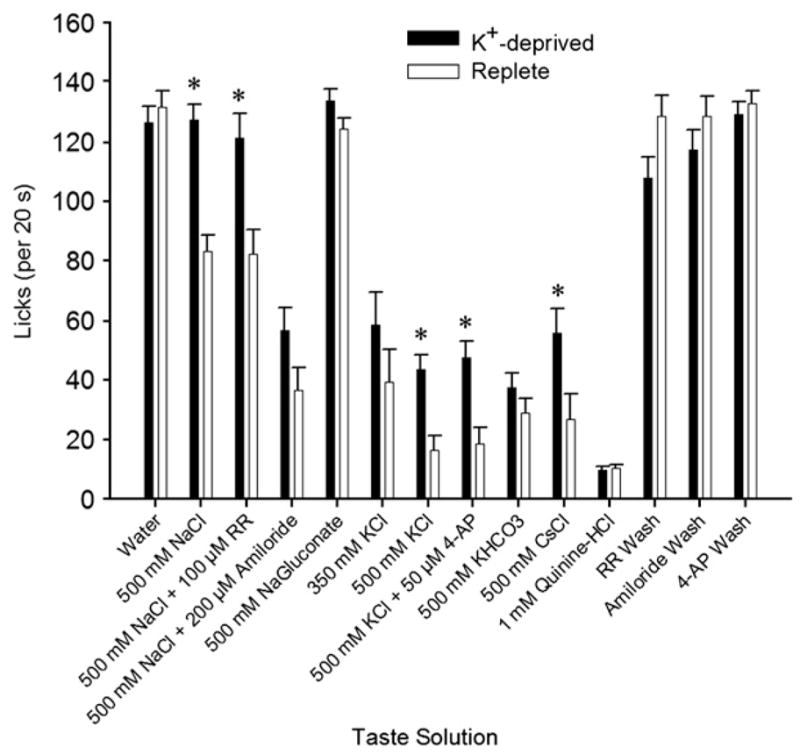
Mean (+SEM) licks (in 20 s trials) for an array of taste solutions by K+-deprived and replete rats in Experiment 2. K+-deprived rats licked significantly more for 500 mM KCl, 500 mM CsCl, 500 mM KCl+50 μM 4-aminopyridine (4-AP), 500 mM NaCl, and 500 mM NaCl+100 μM Ruthernium Red (RR) than did deprived rats.
4. Discussion
4.1. Appetite specificity
K+-deprived rats showed elevated long- and short-term intake of KCl, compared with replete rats. Postingestive satiety and learning factors would have been negligible in the short-term tests, therefore it is likely that immediate orosensory factors were sufficient to guide the responses to the taste solutions. We found that K+ deprivation increased short-term licking for several chloride salts (NaCl, KCl, CaCl2, FeCl2, MgCl2 and CsCl), many of which did not contain K+. Furthermore, K+ deprivation did not enhance licking for KHCO3. These results show that K+ deprivation does not produce an unlearned appetite specifically for K+ salts.
The increased licking for KCl, NaCl, and CaCl2 by the K+-deprived rats relative to replete rats is consistent with previous findings of increased consumption of these same salt solutions in long-term (24 h; [11]) and less systematic shorter-term (30–300 s; [22]) preference tests (the other compounds that we used were not tested in these studies). The current results also confirm reports of increased K+ consumption after K+ deprivation in 24 h two-bottle tests, which was not a consistent finding in previous studies [11]. Adam and Dawborn [11] reported that K+-deprived rats consumed equal volumes of KCl and KHCO3 when they were presented independently with water in 5-h tests, and that the amounts of both solutions consumed were correlated with the severity of K+ deficiency. These results are in contrast with our finding that, when compared with replete rats, K+-deprived rats licked the same for 500 mM KHCO3 in brief access tests. The increased ingestion of KHCO3 reported by Adam and Dawborn likely reflects an influence of K+ deprivation through post-ingestive learning rather than through an effect on unlearned sensory (e.g., taste) processing. That is, their K+-deprived rats may have associated KHCO3 consumption with relief from K+-deficiency, which drove further intake before the experimenters took their first measurement.
The sense of taste should have been a major determinant of our animals’ behavior, and so the fact that the K+-deprived rats’ behavior was related more closely to an anion than a cation was surprising. The taste quality of minerals is thought to be determined primarily by their cation [41,42], and rats deprived of sodium and calcium show appetites for multiple compounds that contain whichever cation is needed [2,20,43]. Furthermore, sodium-deprived rats showed a blunted appetite for NaCl when it is mixed with amiloride, which is thought to interfere with the passage of sodium cations into taste receptor cells [44–46]. Nevertheless, anions do exert some influence on perceived taste quality in humans [47] and on taste preferences for minerals in rodents. For example, rats and mice prefer sodium to a greater extent versus water when paired with pyrophosphate anions than when paired with chloride, and these results cannot be explained simply by differences in perceived intensity of the compounds [48]. We also note that mineral appetite experiments rarely include compounds with anions other than chloride, and so in these studies it is impossible to know the relative contributions of the cation versus chloride in guiding the animals’ intake.
4.2. Transduction mechanisms
We not only tested licking responses for a diverse array of taste compounds, but we also attempted to investigate the contribution of specific gustatory transduction mechanisms to the results by mixing various channel blockers with NaCl or KCl. Concentrations of the blockers that were chosen were licked to the same extent as water, and thus it is more likely that any reduction in licking caused by adding them to a NaCl or KCl solution was due to action of the blockers on ion channels, rather than due to other factors, such as strong bitter taste. These blockers are thought to interfere with the actions of sodium or K+ cations, but they had no discernible effects on K+-deprivation-induced appetites for KCl and NaCl. While this outcome is consistent with K+-deprived rats seeking chloride anions rather than K+ or other cations, these tests are only initial probes that may provide a useful starting point for future dose–response studies that would be required to confirm this hypothesis.
Our results indicate that K+-deprivation produced an unlearned appetite for several chloride salts, raising the possibility that the appetite induced by K+-deficiency is directed toward the chloride ion. Recent evidence suggests that apical inward-rectifying chloride channels are involved in KCl taste transduction. In patch-clamp experiments on undissociated mouse taste cells, Miyamoto et al. [49] found that responses to 500 mM KCl were blocked by the Cl− channel blocker niflumic acid. It is tempting to speculate that the mineral appetite induced by K+-deprivation may be mediated by this chloride channel or by another one present in taste receptor cells. Alternatively, the appetite for chloride salts that we observed could instead be due to a non-direct action of chloride ions on the paracellular action of different cations (i.e., diffusion across tight junctions; [50]). Cations paired with chloride would be able to access basolateral taste-receptor cell channels by passing through tight junctions, bringing chloride anions with them to maintain electroneutrality, whereas cations paired with larger, less permeable anions would have more restricted access to basolateral channels.
Paracellular pathways are also relevant to our sodium gluconate data, given that gluconate is a large organic anion that cannot cross tight junctions. As such, taste responses to NaGlu are believed to be mediated primarily by apical amiloride-sensitive Na+ channels rather than by basolateral amiloride-insensitive Na+ channels [50]. In Experiment 2, the difference between groups in licking for NaGlu was not as pronounced as the group difference in licking for NaCl, suggesting a basolateral contribution to the K+-deprived rats’ licking response to NaCl. However, the lesser increase in NaGlu licking by K+-deprived relative to replete rats may have been masked by a ceiling effect. Thus, our data for NaGlu do not provide clear evidence about the role of the basolateral sodium channels in the expression of NaCl appetite in K+-deprived rats.
We found that the addition of 50 μM 4-AP to the 500 mM KCl solution did not alter licking in either K+-deprived or replete rats relative to 500 mM KCl alone. Although 4-AP effectively blocks K+ channels in various preparations at concentrations ranging from 10 μM to 10 mM, concentrations in the 5 mM range were required for half-maximal blockade of KCl-induced chorda tympani (CT) nerve responses [24]. Moreover, 5 mM 4-AP, though effective at attenuating CT responses to up to 250 mM KCl, had no effect on CT responses to 500 mM KCl [24]. Had we presented 4-AP at 500 μM or higher, we may have observed an effect on licking for the KCl solutions. However, such a result would have been difficult to interpret, given that our pilot work indicated that 4-AP was aversive to rats at these concentrations. In any case, we are aware that our results with 4-AP could be explained either by ineffective action of the blocker, or by 4-AP-sensitive channels being unimportant to the ingestive behavior of K+-deprived rats, and further work will be needed to resolve this issue.
In Experiment 2, we found that rats licked 500 mM NaCl+ 200 μM amiloride solution less than 500 mM NaCl alone. In contrast, the addition of the VR-1 channel blocker ruthenium red did not affect licking for NaCl in either group. One explanation for this is that the amiloride-sensitive portion of NaCl’s taste response is more palatable than its RR-sensitive portion, with the lack of a group difference suggesting that this is true to about the same degree regardless of K+ status.
The amiloride-insensitive component of NaCl taste has been reported to be mediated by an ion channel derived from the VR-1 gene [32], but no investigations have been made of the role of the VR-1 channel in the expression of sodium appetite. Lyall et al. [32] successfully blocked CT responses to NaCl with the specific VR-1 antagonist SB-366791, but we and others (S.J. St. John, personal communication) have been unable to dissolve SB-366791 in water. Although water soluble, ruthenium red has not been used in behavioral studies of taste, and we are unsure whether the 100 μM dose (the strongest concentration that was not behaviorally aversive) used in this study is sufficient to block amiloride-insensitive Na+ channels. Although RR has been reported to effectively block VR-1 channels at concentrations ranging from 1 μM in some in vitro studies [35,51] to 3–30 μM in some in situ preparations [33,34,51], the efficacy of ruthenium red at blocking the VR-1 variant responsible for the amiloride-insensitive component of NaCl taste has never been evaluated. Thus, there are two likely interpretations of our finding that RR did not alter licking to NaCl in either group. One is that the compound was not effective at blocking passage of sodium through VR-1 channels in this experiment. The other is that it effectively enhanced the proportion of the sodium taste response derived from ENaC-related transduction, possibly making NaCl taste more “salty”, but such “salty” taste was not especially appetitive for either group of rats.
4.3. Other observations and perspectives
The lower water licking scores for K+-deprived relative to replete rats in Experiment 1 were unexpected, especially as there are prior reports of polydipsia induced by K+ deprivation [32]. This discrepancy may relate to specifics of the procedures, such as brief access versus daily testing or restricted versus free access to water. The reduced water licking in the K+-deprived group was apparent in training sessions prior to exposure to the taste solutions, and thus it was not due to some factor related to the testing procedure. Moreover, rats did not appear to be satiated over the course of the testing session, which makes a reduction in water licking due to hydration unlikely. A more probable explanation is provided by the observation that the K+-deprived rats failed to grow as quickly as the replete rats, leading to large differences in weights between the two groups; perhaps K+-deprived rats consumed less water due to their smaller size. Consistent with this hypothesis, the group differences in training trials and brief access testing were reduced when consumption was expressed as ratios of licks to body weight. In addition, differences in water licking were not observed in Experiment 2, which used older and larger rats in which group body weights diverged less markedly. It is also possible that K+ deprivation reduced thirst by an unknown physiological mechanism and that this effect was not apparent in Experiment 2 because it was counteracted by an increase in thirst caused by the K+-deprived rats’ consumption of the more concentrated salts that were used in Experiment 1. However, this explanation seems unlikely, given that no group differences in water licking during training trials were apparent in Experiment 2, despite the fact that K+-deprived rats were clearly K+-deficient by the end of these trials.
The rats in this study ingested drugs, such as amiloride, that are known to have physiological effects, such as increased sodium excretion. However, the volumes of solutions ingested would have resulted in negligible effects of this sort. Given a maximal lick rate of approximately 120 licks/20 s [39], two 20-s presentations of 200 μM amiloride (as in experiment 2) daily, and a lick volume of 6 μL, each rat could have maximally consumed 0.22 μg/kg per day, a dose at which diuretic action is not expected [52]. The possibility that K+-deprived rats were repleted by ingestion of K+ salts during brief access testing is also ruled out by the K+ deficiencies of the rats at the conclusion of brief access testing (see Figs. 1A and 4A).
K+-deprived rats exhibited an unlearned appetite for cations other than K+ even though these cations did not relieve their deficiency. This result is consistent with several studies of mineral appetites in rats, which have been reported to generalize to stimuli that do not provide long-term repletion of the mineral needed by the animals, even in tests lasting 24 h or longer, though the pattern of generalization has not been as easy to characterize as was the case here. For example, calcium-deprived rats showed appetites for some chloride-containing stimuli, such as CaCl2, NaCl, NH4Cl, FeCl2, MgCl2, KCl, and ZnCl2, but not for CsCl, and only within a narrow concentration range for some of the chloride salts listed above, while they also showed elevated intake of calcium gluconate [2]. Magnesium-deprived rats showed appetites for MgCl2 and CaCl2, but not NaCl [3]. Sodium-deprived rats displayed elevated consumption of a variety of sodium-containing compounds [20,43] and LiCl [20], but reports vary as to whether they also show an appetite for KCl [20,30,31,53]. Like this other work, the current experiment reinforces the danger of making conclusions about appetitive behavior based on tests with a small number of taste stimuli (e.g., 2 bottle tests). Thus, while our data do support the prior claim that K+-deprived rats develop an appetite for KCl, our results also reveal that this result is due to a generalized appetite for several minerals. Furthermore, our data are useful in showing that unlearned factors, such as the sense of taste, are sufficient to guide this appetite.
Acknowledgments
Supported by Amherst College (J.P.B.), NIH DK-46791 (M.G.T.), a Howard Hughes Medical Institute research fellowship (C.J.G.), and an Amherst College Dean of the Faculty student research award to C.J.G.
References
- 1.Richter CP. Increased salt appetite in adrenalectomized rats. Am J Physiol. 1936;115:115–61. [Google Scholar]
- 2.Coldwell SE, Tordoff MG. Acceptance of minerals and other compounds by calcium-deprived rats: 24-h tests. Am J Physiol. 1996;271(1 Pt 2):R1–R10. doi: 10.1152/ajpregu.1996.271.1.R1. [DOI] [PubMed] [Google Scholar]
- 3.McCaughey SA, Tordoff MG. Magnesium appetite in the rat. Appetite. 2002;38(1):29–38. doi: 10.1006/appe.2001.0443. [DOI] [PubMed] [Google Scholar]
- 4.Czarnogorski M, Woda CB, Schulkin J, Mulroney SE. Induction of a phosphate appetite in adult male and female rats. Exp Biol Med. 2004;229(9):914–9. doi: 10.1177/153537020422900907. (Maywood) [DOI] [PubMed] [Google Scholar]
- 5.Sweeny JM, Seibert HE, Woda C, Schulkin J, Haramati A, Mulroney SE. Evidence for induction of a phosphate appetite in juvenile rats. Am J Physiol. 1998;275(4 Pt 2):R1358–65. doi: 10.1152/ajpregu.1998.275.4.R1358. [DOI] [PubMed] [Google Scholar]
- 6.Woods SC, Vasselli JR, Milam KM. Iron appetite and latent learning in rats. Physiol Behav. 1977;19(5):623–6. doi: 10.1016/0031-9384(77)90036-1. [DOI] [PubMed] [Google Scholar]
- 7.Chesters JK, Quarterman J. Effects of zinc deficiency on food intake and feeding patterns of rats. Br J Nutr. 1970;24(4):1061–9. doi: 10.1079/bjn19700109. [DOI] [PubMed] [Google Scholar]
- 8.Rutkoski NJ, Levenson CW. Self-selection of copper-containing diets by copper-deficient and overloaded rats. Physiol Behav. 2000;71(1–2):117–21. doi: 10.1016/s0031-9384(00)00324-3. [DOI] [PubMed] [Google Scholar]
- 9.Zuberbuehler CA, Messikommer RE, Wenk C. Choice feeding of selenium-deficient laying hens affects diet selection, selenium intake and body weight. J Nutr. 2002;132(11):3411–7. doi: 10.1093/jn/132.11.3411. [DOI] [PubMed] [Google Scholar]
- 10.Blake WD, Jurf AN. Increased voluntary Na intake in K deprived rats. Commun Behav Biol. 1968;1:1–7. [Google Scholar]
- 11.Adam WR, Dawborn JK. Effect of potassium depletion on mineral appetite in the rat. J Comp Physiol Psychol. 1972;78(1):51–8. doi: 10.1037/h0032183. [DOI] [PubMed] [Google Scholar]
- 12.Ugolev AR, Roshchina GM. Variations in appetite for water, glucose, sodium and potassium in rats when the sodium-pump is inhibited by the administration of strophanthin. K Dokl Akad Nauk SSSR. 1965;165(5):1211–4. [PubMed] [Google Scholar]
- 13.Tordoff MG. Salt intake of rats fed diets deficient in calcium, iron, magnesium, phosphorus, potassium, or all minerals. Appetite. 1992;18(1):29–41. doi: 10.1016/0195-6663(92)90208-n. [DOI] [PubMed] [Google Scholar]
- 14.Berl T, Linas SL, Aisenbrey GA, Anderson RJ. On the mechanism of polyuria in potassium depletion. The role of polydipsia. J Clin Invest. 1977;60(3):620–5. doi: 10.1172/JCI108813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brokaw A. Renal hypertrophy and polydipsia in potassium-deficient rats. Am J Physiol. 1953;172(2):333–46. doi: 10.1152/ajplegacy.1953.172.2.333. [DOI] [PubMed] [Google Scholar]
- 16.McKay AJ, Poirier CD, Peterson LN. Converting-enzyme inhibition abolishes polydipsia induced by dietary NaCl and K depletion. Am J Physiol. 1990;258(5 Pt 2):F1164–72. doi: 10.1152/ajprenal.1990.258.5.F1164. [DOI] [PubMed] [Google Scholar]
- 17.Markison S, Gietzen DW, Spector AC. Essential amino acid deficiency enhances long-term intake but not short-term licking of the required nutrient. J Nutr. 1999;129(8):1604–12. doi: 10.1093/jn/129.8.1604. [DOI] [PubMed] [Google Scholar]
- 18.Tordoff MG. Intragastric calcium infusions support flavor preference learning by calcium-deprived rats. Physiol Behav. 2002;76:521–9. doi: 10.1016/s0031-9384(02)00723-0. [DOI] [PubMed] [Google Scholar]
- 19.Epstein AN, Stellar E. The control of salt preference in the adrenalectomized rat. J Comp Physiol Psychol. 1955;48(3):167–72. doi: 10.1037/h0045626. [DOI] [PubMed] [Google Scholar]
- 20.Nachman M. Taste preferences for sodium salts by adrenalectomized rats. J Comp Physiol Psychol. 1962;55:1124–9. doi: 10.1037/h0041348. [DOI] [PubMed] [Google Scholar]
- 21.Coldwell SE, Tordoff MG. Immediate acceptance of minerals and HCl by calcium-deprived rats: brief exposure tests. Am J Physiol. 1996;271(1 Pt 2):R11–7. doi: 10.1152/ajpregu.1996.271.1.R11. [DOI] [PubMed] [Google Scholar]
- 22.Milner P, Zucker I. Specific hunger for potassium in the rat. Psychon Sci. 1965;2:17–8. [Google Scholar]
- 23.Zucker I. Short-term salt preference of potassium-deprived rats. Am J Physiol. 1965;208:1071–4. doi: 10.1152/ajplegacy.1965.208.6.1071. [DOI] [PubMed] [Google Scholar]
- 24.Kim M, Mistretta CM. 4-aminopyridine reduces chorda tympani nerve taste responses to potassium and alkali salts in rat. Brain Res. 1993;612(1–2):96–103. doi: 10.1016/0006-8993(93)91648-c. [DOI] [PubMed] [Google Scholar]
- 25.Dahl M, Erickson RP, Simon SA. Neural responses to bitter compounds in rats. Brain Res. 1997;756(1–2):22–34. doi: 10.1016/s0006-8993(97)00131-5. [DOI] [PubMed] [Google Scholar]
- 26.Lemon CH, Smith DV. Neural representation of bitter taste in the nucleus of the solitary tract. J Neurophysiol. 2005;94(6):3719–29. doi: 10.1152/jn.00700.2005. [DOI] [PubMed] [Google Scholar]
- 27.Spector AC, Kopka SL. Rats fail to discriminate quinine from denatonium: implications for the neural coding of bitter-tasting compounds. J Neurosci. 2002;22(5):1937–41. doi: 10.1523/JNEUROSCI.22-05-01937.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.St John SJ, Spector AC. Behavioral discrimination between quinine and KCl is dependent on input from the seventh cranial nerve: implications for the functional roles of the gustatory nerves in rats. J Neurosci. 1998;18(11):4353–62. doi: 10.1523/JNEUROSCI.18-11-04353.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.St John SJ, Hallagan LD. Psychophysical investigations of cetylpyridinium chloride in rats: its inherent taste and modifying effects on salt taste. Behav Neurosci. 2005;119(1):265–79. doi: 10.1037/0735-7044.119.1.265. [DOI] [PubMed] [Google Scholar]
- 30.Morrison GR. In: Taste psychophysics in animals, in Olfaction and Taste III. Pfaffmann C, editor. New York: Rockefeller University Press; 1969. pp. 512–6. [Google Scholar]
- 31.Morrison GR. Behavioural response patterns to salt stimuli in the rat. Can J Psychol. 1967;21(2):141–52. [Google Scholar]
- 32.Lyall V, Heck GL, Vannikova AK, Ghosh S, Phan TH, Alam RI, et al. The mammalian amiloride-insensitive non-specific salt taste receptor is a vanilloid receptor-1 variant. J Physiol. 2004;558(Pt 1):147–59. doi: 10.1113/jphysiol.2004.065656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maggi CA, Bevan S, Walpole CS, Rang HP, Giuliani S. A comparison of capsazepine and ruthenium red as capsaicin antagonists in the rat isolated urinary bladder and vas deferens. Br J Pharmacol. 1993;108(3):801–5. doi: 10.1111/j.1476-5381.1993.tb12881.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Amann R, Donnerer J, Lembeck F. Ruthenium red selectively inhibits capsaicin-induced release of calcitonin gene-related peptide from the isolated perfused guinea pig lung. Neurosci Lett. 1989;101(3):311–5. doi: 10.1016/0304-3940(89)90551-x. [DOI] [PubMed] [Google Scholar]
- 35.McIntyre P, McLatchie LM, Chambers A, Phillips E, Clarke M, Savidge J, et al. Pharmacological differences between the human and rat vanilloid receptor 1 (VR1) Br J Pharmacol. 2001;132(5):1084–94. doi: 10.1038/sj.bjp.0703918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Spector AC, Guagliardo NA, St John SJ. Amiloride disrupts NaCl versus KCl discrimination performance: implications for salt taste coding in rats. J Neurosci. 1996;16(24):8115–22. doi: 10.1523/JNEUROSCI.16-24-08115.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.St John SJ, Smith DV. Neural representation of salts in the rat solitary nucleus: brain stem correlates of taste discrimination. J Neurophysiol. 2000;84(2):628–38. doi: 10.1152/jn.2000.84.2.628. [DOI] [PubMed] [Google Scholar]
- 38.Smith JC. The history of the “vis Rig”. Appetite. 2001;36(1):93–8. doi: 10.1006/appe.2000.0372. [DOI] [PubMed] [Google Scholar]
- 39.Stellar E, Hill JH. The rats rate of drinking as a function of water deprivation. J Comp Physiol Psychol. 1952;45(1):96–102. doi: 10.1037/h0062150. [DOI] [PubMed] [Google Scholar]
- 40.Nakanishi T, Takamitsu Y, Nakahama H, Sugita M. Impairment of renal medullary osmolyte accumulation in potassium-depleted rats. Am J Physiol. 1994;267(1 Pt 2):F139–45. doi: 10.1152/ajprenal.1994.267.1.F139. [DOI] [PubMed] [Google Scholar]
- 41.Boudreau J, Hoang NK, Oravec J, Do LT. Neurophysiological taste responses to salt solutions. Chem Senses. 1983;8(2):131–50. [Google Scholar]
- 42.Doetsch GS, Erickson RP. Synaptic processing of taste-quality information in the nucleus tractus solitarius of the rate. J Neurophysiol. 1970;33(4):490–507. doi: 10.1152/jn.1970.33.4.490. [DOI] [PubMed] [Google Scholar]
- 43.Morrison GR, Young JC. Taste control over sodium intake in sodium deficient rats. Physiol Behav. 1972;8(1):29–32. doi: 10.1016/0031-9384(72)90125-4. [DOI] [PubMed] [Google Scholar]
- 44.Bernstein IL, Hennessy CJ. Amiloride-sensitive sodium channels and expression of sodium appetite in rats. Am J Physiol. 1987;253(2 Pt 2):R371–4. doi: 10.1152/ajpregu.1987.253.2.R371. [DOI] [PubMed] [Google Scholar]
- 45.Brot MD, Watson CH, Bernstein IL. Amiloride-sensitive signals and NaCl preference and appetite: a lick-rate analysis. Am J Physiol Regul Integr Comp Physiol. 2000;279(4):R1403–11. doi: 10.1152/ajpregu.2000.279.4.R1403. [DOI] [PubMed] [Google Scholar]
- 46.McCutcheon NB. Sodium deficient rats are unmotivated by sodium chloride solutions mixed with the sodium channel blocker amiloride. Behav Neurosci. 1991;105(5):764–6. doi: 10.1037//0735-7044.105.5.764. [DOI] [PubMed] [Google Scholar]
- 47.Schiffman SS, McElroy AE, Erickson RP. The range of taste quality of sodium salts. Physiol Behav. 1980;24(2):217–24. doi: 10.1016/0031-9384(80)90077-3. [DOI] [PubMed] [Google Scholar]
- 48.McCaughey SA, Giza BK, Tordoff MG. Taste and acceptance of pyrophosphates by rats and mice. Am J Physiol Regul Integr Comp Physiol. 2007;292(6):R2159–67. doi: 10.1152/ajpregu.00886.2006. [DOI] [PubMed] [Google Scholar]
- 49.Miyamoto T, Miyazaki T, Fujiyama R, Okada Y, Sato T. Differential transduction mechanisms underlying NaCl- and KCl-induced responses in mouse taste cells. Chem Senses. 2001;26(1):67–77. doi: 10.1093/chemse/26.1.67. [DOI] [PubMed] [Google Scholar]
- 50.Ye Q, Heck GL, DeSimone JA. The anion paradox in sodium taste reception: resolution by voltage-clamp studies. Science. 1991;254(5032):724–6. doi: 10.1126/science.1948054. [DOI] [PubMed] [Google Scholar]
- 51.Liu L, Simon SA. A rapid capsaicin-activated current in rat trigeminal ganglion neurons. Proc Natl Acad Sci U S A. 1994;91(2):738–41. doi: 10.1073/pnas.91.2.738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Baer JE, Jones CB, Spitzer SA, Russo HF. The potassium-sparing and natriuretic activity of N-amidino-3,5-diamino-6-chloropyrazinecarboxamide hydrochloride dihydrate (amiloride hydrochloride) J Pharmacol Exp Ther. 1967;157(2):472–85. [PubMed] [Google Scholar]
- 53.Jalowiec JE, Crapanzano JE, Stricker EM. Specificity of salt appetite elicited by hypovolemia. Psychon Sci. 1966;6:331–2. [Google Scholar]


