Abstract
Berberine has been shown to regulate glucose and lipid metabolism in vitro and in vivo. This pilot study was to determine the efficacy and safety of berberine in the treatment of type 2 diabetic patients. In study A, 36 adults with newly diagnosed type 2 diabetes were randomly assigned to treatment with berberine or metformin (0.5 g t.i.d.) in a 3-month trial. The hypoglycemic effect of berberine was similar to that of metformin. Significant decreases in hemoglobin A1c (HbA1c; from 9.5% ± 0.5% to 7.5% ± 0.4%, P<0.01), fasting blood glucose (FBG; from 10.6 ± 0.9 mmol/L to 6.9 ± 0.5 mmol/L, P<0.01), postprandial blood glucose (PBG; from 19.8 ± 1.7 to 11.1 ± 0.9 mmol/L, P<0.01) and plasma triglycerides (from 1.13 ± 0.13 mmol/L to 0.89 ± 0.03 mmol/L, P<0.05) were observed in the berberine group. In study B, 48 adults with poorly controlled type 2 diabetes were treated supplemented with berberine in a 3-month trial. Berberine acted by lowering FBG and PBG from one week to the end of the trial. HbA1c decreased from 8.1% ± 0.2% to 7.3% ± 0.3% (P<0.001). Fasting plasma insulin and HOMA-IR were reduced by 28.1% and 44.7% (P<0.001), respectively. Total cholesterol and low-density lipoprotein cholesterol (LDL-C) were decreased significantly as well. During the trial, 20 (34.5%) patients suffered from transient gastrointestinal adverse effects. Functional liver or kidney damages were not observed for all patients. In conclusion, this pilot study indicates that berberine is a potent oral hypoglycemic agent with beneficial effects on lipid metabolism.
1. Introduction
Type 2 diabetes is a worldwide health threat and treatment of this disease is limited by availability of effective medications. All of the existing oral hypoglycemic agents have subsequent failure after long term administration. Thus, new oral medications are needed for long-term control of blood glucose in patients with type 2 diabetes. Certain botanical products from generally regarded as safe (GRAS) plants have been widely used in diabetes care because of their anti-oxidation, anti-inflammation, anti-obesity and anti-hyperglycemia properties.[1, 2]. However, the drawback of using GRAS plants is the difficulty in control their quality as most of these botanical products are mixtures of multiple compounds. Compared to other products from GRAS plants, berberine is a single purified compound, and has glucose-lowering effect in vitro and in vivo [3-6].
Berberine (molecular formula C20H19NO5 and molecular weight of 353.36) is the main active component of an ancient Chinese herb Coptis chinensis French, which has been used to treat diabetes for thousands of years. Berberine is an Over-the-Counter (OTC) drug, which is used to treat gastrointestinal infections in China. Berberine hydrochloride (B·HCl·nH2O) - the most popular form of berberine, is used in this pilot study. The chemical structure of Berberine and related isoquinoline alkaloids are quite different from the commonly used other hypoglycemic agents, such as sulphonylureas, biguanides, thiazolidinediones, or acarbose. Hence, if the efficacy and safety of berberine are confirmed, it can serve as a new class of anti-diabetic medication.
This pilot study was to assess the efficacy of berberine in human subjects with type 2 diabetes. Berberine was given to both newly diagnosed diabetic patients and poorly controlled diabetic patients alone or combination with other hypoglycemic agents for three months. HbA1c, blood glucose and HOMA index were used to determine the efficacy of berberine.
2. Subjects and methods
The subjects were recruited from diabetes outpatient department of Xinhua Hospital by advertising in the clinic. Ninety-seven Chinese volunteers were screened, and 13 subjects were excluded from the study due to failure to meet the recruitment criteria. Thus, 84 subjects (49 women and 35 men) with type 2 diabetes were included in the study. All participants received written and oral information regarding the natural and potential risks of the study and gave their informed consent. The experimental protocol was approved by the ethics committee of Xinhua Hospital. The monotherapy study was designed to compare berberine with metformin (study A, n = 36). The combination therapy was aimed at evaluating additive or synergistic effects of berberine on the classical anti-diabetic agents (study B, n = 48).
Major inclusion criteria were hemoglobin A1c (HbA1c) > 7.0% or fasting blood glucose (FBG) > 7.0 mmol/L, BMI > 22 kg/m2, age 25-75 years, and a negative pregnancy test for female patients. A total of 36 patients who were newly diagnosed for type 2 diabetes were assigned to study A. After a two-month phase during which the patients were treated with diet alone, they were randomly assigned to receive berberine or metformin. A total of 48 type 2 diabetic patients inadequately treated with diet plus sulfonylureas, metformin, acarbose or insulin therapy alone or with a combination were assigned to study B (Table 1). The dose of the medications was stable for at least 2 months before enrollment in the study and remained unchanged throughout the study. All participants were instructed to maintain their lifestyle habits during the course of the study.
Table 1.
Baseline characteristics of administration of hypoglycemic agents
| Subjects (n) | Diet | Sulfonylureas | Metformin | Acarbose | Insulin |
|---|---|---|---|---|---|
| 36 | + | ||||
| 7 | + | + | |||
| 3 | + | + | |||
| 1 | + | + | |||
| 8 | + | + | |||
| 12 | + | + | + | ||
| 9 | + | + | + | ||
| 4 | + | + | + | ||
| 1 | + | + | + | ||
| 1 | + | + | + |
Each study involved a 13-week treatment. For study A, 18 subjects took 500 mg berberine three times daily at the beginning of each major meal or 500 mg metformin three times daily after major meals. For study B, 500 mg berberine three times daily was added to their previous treatment for 3 months. If heavy gastrointestinal side-effects occurred, the dose of berberine was reduced to 300 mg three times daily.
Patients were evaluated weekly for the first 5 weeks of treatment and then every 4 weeks until the end of study. The primary efficacy end point was glycemic control as determined by HbA1c levels. Secondary efficacy parameters included changes in fasting blood glucose (FBG), postprandial blood glucose (PBG), plasma triglycerides, total cholesterol, high-density lipoprotein cholesterol (HDL-C) and low-density lipoprotein cholesterol (LDL-C) concentrations. Adverse events were recorded throughout the study by direct questioning.
2.1. Measurements
Blood glucose was determined by a glucose oxidase method (Roche, Basel, Switzerland). Serum insulin and C-peptide were determined by radioimmunoassay (Linco Research, St. Charles, MO). HbA1c was analyzed using the high-pressure liquid chromatography (BioRad, Hercules, CA). Plasma triglyceride, total cholesterol, HDL-C, LDL-C, alanine thansaminase (ALT), γ glutamyl transpeptidase (γ-GT) and creatinine concentrations were determined by enzymatic assays (Roche, Basel, Switzerland). The HOMA method was used to compare differences in the profiles for insulin resistance (HOMA-IR) and for β-cell dysfunction (HOMA-β cell) [7]. Ten insulin-treated subjects were excluded from the HOMA analysis.
HOMA-IR = fasting insulin (μU/ml)×fasting glucose (mmol/L)/22.5
HOMA-β cell = [20 − fasting insulin (μU/ml)] / [fasting glucose (mmol/L) - 3.5]
2.2. Statistical analysis
Descriptive statistics and analysis were performed in SPSS 12.0 for Windows. In study A, the significance of the differences between means of metformin and berberine groups was analyzed by Wilcoxon Rank Sum Test. The statistical differences between baseline and endpoint were calculated using Wilcoxon signed rank test. In study B, the significance of the differences among different time points was analyzed by repeated measure ANOVA. The α level was set at 0.05.
3. Results
In study A, 36 patients were included and randomly assigned to metformin or berberine treatment. Three patients of the berberine group and two patients of the metformin group withdrew from the study because of treatment failure. In study B, 48 patients were included, and 5 subjects were excluded from the study before week 13. Among the five subjects, three failed to complete the study in lack of efficacy, one failed in lack of participation time, and one was excluded due to lack of compliance (pill count < 80%). Thus, 74 participants were eligible for the final analysis.
3.1. Berberine verse metformin (study A)
In newly-diagnosed diabetic patients, berberine reduced blood glucose and lipids (Table 2). There were significant decreases in HbA1c (by 2%; P < 0.01), FBG (by 3.8 mmol/L; P < 0.01) and PBG (by 8.8 mmol/L; P < 0.01) in the berberine group. The FBG (or PBG) declined progressively during the berberine treatment, reaching a nadir that was 3.7 mmol/L (or 8.7 mmol/L) below baseline by week 5, and remained at this level until the end of the study (Fig 1A). Triglycerides and total cholesterol decreased by 0.24 mmol/L (P < 0.05) and 0.57 mmol/L (P < 0.05) with berberine treatment. It seemed there was a declining trend of HDL-C and LDL-C; however, no significant differences between week 1 and week 13 were observed in the berberine group. Compared with metformin, berberine exhibited an identical effect in the regulation of glucose metabolism, such as HbA1c, FBG, PBG, fasting insulin and postprandial insulin. In the regulation of lipid metabolism, berberine activity is better than metformin. By week 13, triglycerides and total cholesterol in the berberine group had decreased and were significantly lower than in the metformin group (P<0.05).
Table 2.
Monotherapeutic effects of metformin and berberine
| Metformin (n=16)
|
Berberine (n=15)
|
|||
|---|---|---|---|---|
| Baseline | End point | Baseline | End point | |
| HbA1c (%) | 9.15 ± 0.57 | 7.72 ± 0.43** | 9.47 ± 0.65 | 7.48 ± 0.40** |
| FBG (mmol/L) | 9.96 ± 0.64 | 7.16 ± 0.71** | 10.63 ± 0.88 | 6.85 ± 0.53** |
| PBG (mmol/L) | 20.53 ± 1.87 | 12.86 ± 0.77** | 19.83 ± 1.66 | 11.05 ± 0.92** |
| Fasting insulin (μU/ml) | 27.3 ± 4.4 | 22.9 ± 5.2 | 29.1 ± 5.3 | 24.0 ± 5.5 |
| Postprandial insulin (μU/ml) | 125.3 ± 29.8 | 110.5 ± 21.4 | 120.4 ± 31.4 | 116.0 ± 26.9 |
| Triglyceride (mmol/L) | 1.19 ± 0.12 | 1.17 ± 0.13 | 1.13 ± 0.13 | 0.89 ± 0.03* |
| Total cholesterol (mmol/L) | 4.31 ± 0.28 | 4.27 ± 0.15 | 4.40 ± 0.21 | 3.83 ± 0.09* |
| HDL-C (mmol/L) | 1.25 ± 0.06 | 1.31 ± 0.08 | 1.33 ± 0.10 | 1.22 ± 0.04 |
| LDL-C (mmol/L) | 2.55 ± 0.38 | 2.43 ± 0.11 | 2.47 ± 0.13 | 2.36 ± 0.06 |
Data are means ± SEM. Compared with baseline:
P<0.05,
P<0.01
Fig. 1.
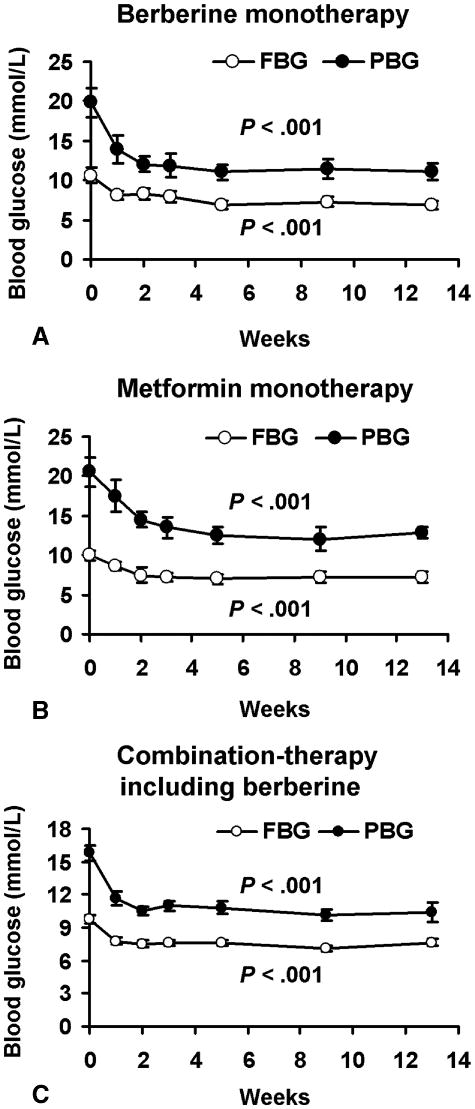
Both berberine and metformin decreased FBG and PBG of type 2 diabetic patients significantly from week 1 to week 13. A, means ± SEM of 15 patients treated with berberine alone. B, means ± SEM of 16 patients treated with metformin alone. C, means ± SEM of 43 patients with combination-therapy including berberine.
3.2. Combination-therapy of berberine (study B)
In the first 7 days of treatment, berberine led to a reduction in FBG from 9.6 ± 2.7 mmol/L to 7.8 ± 1.8 mmol/L (P<0.001; Fig 1C) and in PBG from 14.8 ± 4.1 mmol/L to 11.7 ± 3.6 mmol/L (P<0.001). During the second week, FBG and PBG declined further, reached a nadir that was 2.1 mmol/L (7.5 ± 2.1 mmol/L) and 3.3 mmol/L (10.5 ± 2.5 mmol/L) below the baseline, respectively, and remained at this level thereafter.
In the combination-therapy for 5 weeks, berberine led to a reduction in HbA1c from 8.1% to 7.3% (P < 0.001; Table 3). FBG and PBG declined remarkably, too (P < 0.001). Fasting insulin and HOMA-IR reduced by 29.0% (P < 0.01) and 46.7% (P < 0.001), respectively. Blood lipids including triglyceride, total cholesterol and LDL-C decreased and were significantly lower than baseline. In the absence of weight change, waist and waist/hip of the patients declined significantly. No significant changes in the criteria were observed between week 5 and week 13 except the increment of fasting C-peptide (P < 0.05) and postprandial C-peptide (P < 0.01). During the study, fasting C-peptide of the patients with insulin treatment went down then up and postprandial C-peptide increased by 70.5% (P<0.01) at 13 weeks.
Table 3.
Berberine in combination-therapy
| Week 0 | Week 5 | Week 13 | |
|---|---|---|---|
| BMI | 26.0 ± 0.6 | 26.1 ± 0.8 | 26.0 ± 0.8 |
| Waist (cm) | 89.0 ± 1.5 | 86.9 ± 1.8** | 87.0 ± 1.7** |
| Waist/hip | 0.89 ± 0.01 | 0.86 ± 0.01* | 0.86 ± 0.01 |
| HbA1c (%) | 8.1 ± 0.2 | 7.3 ± 0.2*** | 7.3 ± 0.3*** |
| FBG (mmol/L) | 9.6 ± 0.4 | 7.6 ± 0.3*** | 7.6 ± 0.3*** |
| PBG (mmol/L) | 14.8 ± 0.7 | 10.8 ± 0.6*** | 9.7 ± 0.9*** |
| Fasting insulin (μU/ml) | 35.2 ± 3.3 | 25.0 ± 2.6** | 25.3 ± 5.3** |
| Postprandial insulin (μU/ml) | 104.1 ± 9.5 | 88.0± 11.4 | 76.5 ± 15.6 |
| HOMA-IR | 15.2 ± 1.6 | 8.1 ± 1.0*** | 8.4 ± 1.8*** |
| HOMA-β cell | 128.6 ± 12.2 | 164.2 ± 27.3 | 151.7 ± 28.6 |
| Fasting C-peptide (ng/ml) | 0.96 ± 0.28 | 0.85 ± 0.24 | 1.12 ± 0.12*ˆ |
| Postprandial C-peptide (ng/ml) | 2.27 ± 0.72 | 2.28 ± 0.80 | 3.87 ± 0.14**ˆˆ |
| Triglyceride (mmol/L) | 1.73 ± 0.17 | 1.39 ± 0.18* | 1.49 ± 0.49 |
| Total cholesterol (mmol/L) | 4.97 ± 0.13 | 4.20 ± 0.13*** | 4.38 ± 0.40* |
| HDL-C (mmol/L) | 1.37 ± 0.04 | 1.31 ± 0.05 | 1.32 ± 0.05 |
| LDL-C (mmol/L) | 3.00 ± 0.10 | 2.50 ± 0.10*** | 2.59 ± 0.27** |
| ALT (U/L) | 31.5 ± 4.1 | 26.5 ± 2.6 | 25.2 ± 7.0 |
| γ-GT (U/L) | 41.8 ± 7.8 | 41.5 ± 8.4 | 41.4 ± 1.2 |
| Creatinine (mmol/L) | 88.5 ± 3.1 | 90.8 ± 3.7 | 90.8 ± 8.1 |
Data are means ± SEM of the patients with combination-therapy including berberine. Values of fasting insulin, postprandial insulin, HOMA-IR and HOMA-β cell were obtained from 33 patients treated only with oral hypoglycemic agents. Values of fasting c-peptide and postprandial c-peptide were obtained from the other 10 patients treated including insulin. The rest parameters were obtained from all the 43 patients with combination-therapy. Compared with Week 0:
P<0.05.
P<0.01.
P<0.001.
Compared with Week 5:
P<0.05,
P<0.01
3.3. Safety results
Incidence of gastrointestinal adverse events was 34.5% during the 13 weeks of berberine treatment including monotherapy and combination-therapy. These events included diarrhea (n: 6; percentage: 10.3%), constipation (4; 6.9%), flatulence (11; 19.0%) and abdominal pain (2; 3.4%). The side effects were observed only in the first four weeks in most patients. In 14 (24.1%) patients, berberine dosage decreased from 0.5 g t.i.d. to 0.3 g t.i.d. as a consequence of gastrointestinal adverse events. Of the 14 patients, ten were treated with metformin or acarbose in combination with berberine. The rest were treated with insulin combined with berberine. None of the patients suffered from severe gastrointestinal adverse events when berberine was used alone. In combination-therapy, the adverse events disappeared in one week after reduction in berberine dosage. The data suggest that berberine at dosage of 0.3 g t.i.d. is well tolerated in combination-therapy.
Liver and kidney functions were monitored in this study. No significant changes of plasma ALT, γ-GT and creatinine were observed during the 13 weeks of berberine treatment (Table 3). None of the patients were observed with pronounced (more than 50%) elevation in liver enzymes or creatinine.
4. Discussion
The hypoglycemic effect of berberine was reported in 1988 when it was used to treat diarrhea in diabetic patients in China [8]. Since then, berberine has often been used as an anti-hyperglycemic agent by many physicians in China. There are substantial numbers of clinical reports about the hypoglycemic action of berberine in Chinese literature. However, most of the previous studies were not well-controlled and experiments were not well-designed. Additionally, none of them used HbA1c as a parameter due to poor research conditions. Thus, the anti-diabetic effect of berberine needs to be carefully evaluated.
In this pilot study, berberine significantly decreased HbA1c levels in diabetic patients. The effect of decreasing HbA1c was comparable to that of metformin, a widely-used oral hypoglycemic agent [9, 10]. In monotherapy, berberine and metformin all improved glycemic parameters (HbA1c, FBG and PBG). But their effects on lipid metabolism were different. Berberine decreased serum triglyceride and total cholesterol significantly. HDL-C and LDL-C levels of patients treated with berberine were also reduced but the decreases did not reach statistic significance. Whether berberine has a lowering effect on HDL-C needs further investigation. Compared with berberine, metformin had little effects on these lipid parameters.
In combination with other agents, berberine exhibited consistent activities in improvement of glycemic and lipid parameters in diabetic patients. Insulin sensitivity was enhanced by berberine as the HOMA-IR value was reduced by nearly 50%. This effect may be related to fat distribution by berberine because waist and waist/hip of the patients were decreased significantly in the absence of weight change. Interestingly, both fasting and postprandial C-peptides increased significantly in patients when berberine was used together with insulin, which suggests that long-term berberine treatment may improve insulin secretion of the patients with consequent failure of oral hypoglycemic agents. The effects of berberine on islet function need further studies.
The mechanism of berberine on glucose metabolism is still under investigation. We and others have demonstrated that berberine has an insulin sensitizing effect in vivo and in vitro [3, 4, 5, 11, 12]. In diet-induced obese rats, berberine reduced insulin resistance, similar to metformin [13, 6]. In hepatocytes, adipocytes and myotubes, berberine increased glucose consumption and/or glucose uptake in the absence of insulin [3, 6, 14]. Berberine enhancing glucose metabolism may be due to stimulation of glycolysis, which is related to inhibition of oxidation in mitochondria [6]. Berberine may also act as an alpha-glucosidase inhibitor. It inhibited disaccharidases activities and decreased glucose transportation cross the intestinal epithelium [15, 16]. This may contribute to the adverse gastrointestinal effects of berberine in some patients. This side effect was often observed when berberine was used in combination with metformin or acarbose, which also have similar gastrointestinal side effects by themselves. Thus, when combined with these two agents, the dosage of berberine should be reduced to 0.3 g t.i.d. to avoid the severe flatulence or diarrhea.
Berberine is proposed to have potential as a therapeutic agent for lipid lowering. In this pilot study, berberine reduced serum cholesterol, triglycerides and LDL-C. This activity is similar to that reported elsewhere in vivo [17, 18]. However, further studies including outcome studies in humans are needed to confirm this activity and its benefit. The mechanism of berberine regulating lipid metabolism has been investigated by several groups. In hamsters with hyperlipidemia, berberine reduced serum cholesterol and LDL-C, and increased LDL receptor mRNA as well as protein in the liver [19]. These effects were partly due to stabilization of LDL receptor mRNA mediated by the ERK signaling pathway [20]. In addition to up-regulation of the LDL receptor, berberine was reported to inhibit lipid synthesis in human hepatocytes through activation of AMPK [21].
In summary, that berberine is a potent oral hypoglycemic agent with modest effect on lipid metabolism. It is safe and the cost of treatment by berberine is very low. It may serve as a new drug candidate in the treatment of type 2 diabetes. However, this is a pilot study. The efficacy of berberine needs to be tested in a much larger population and characterized as a function of the known duration of the diabetes. Further studies are needed to evaluate the action of berberine on type 2 diabetes in other ethnic groups.
Acknowledgments
Financial support for this study was provided by Xinhua Hospital. This study is partially supported by NIH grant (P50 AT02776-020002) to J Ye.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jayaprakasam B, Olson LK, Schutzki RE, et al. Amelioration of obesity and glucose intolerance in high-fat-fed C57BL/6 mice by anthocyanins and ursolic acid in Cornelian cherry (Cornus mas) J Agric Food Chem. 2006;54:243–8. doi: 10.1021/jf0520342. [DOI] [PubMed] [Google Scholar]
- 2.Matsui T, Ueda T, Oki T, et al. alpha-Glucosidase inhibitory action of natural acylated anthocyanins. 2. alpha-Glucosidase inhibition by isolated acylated anthocyanins. J Agric Food Chem. 2001;49:1952–6. doi: 10.1021/jf0012502. [DOI] [PubMed] [Google Scholar]
- 3.Yin J, Hu R, Chen M, et al. Effects of berberine on glucose metabolism in vitro. Metabolism. 2002;51:1439–43. doi: 10.1053/meta.2002.34715. [DOI] [PubMed] [Google Scholar]
- 4.Yin J, Chen M, Tang J, et al. Effects of berberine on glucose and lipid metabolism in animal experiment. Chinese Journal of Diabetes. 2004;12:215–8. [Google Scholar]
- 5.Lee YS, Kim WS, Kim KH, et al. Berberine, a natural plant product, activates AMP-activated protein kinase with beneficial metabolic effects in diabetic and insulin-resistant states. Diabetes. 2006;55:2256–64. doi: 10.2337/db06-0006. [DOI] [PubMed] [Google Scholar]
- 6.Yin J, Gao Z, Liu D, et al. Berberine improves glucose metabolism through induction of glycolysis. Am J Physiol Endocrinol Metab. doi: 10.1152/ajpendo.00211.2007. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matthews DR, Hosker JP, Rudenski AS, et al. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 8.Ni YX. Therapeutic effect of berberine on 60 patients with type II diabetes mellitus and experimental research. Zhong Xi Yi Jie He Za Zhi. 1988;8:711–3. 707. [PubMed] [Google Scholar]
- 9.DeFronzo R, Goodman A. Efficacy of metformin in patients with non-insulin-dependent diabetes mellitus. N Engl J Med. 1995;333:541–9. doi: 10.1056/NEJM199508313330902. [DOI] [PubMed] [Google Scholar]
- 10.Ceriello A, Johns D, Widel M, et al. Comparison of effect of pioglitazone with metformin or sulfonylurea (monotherapy and combination therapy) on postload glycemia and composite insulin sensitivity index during an oral glucose tolerance test in patients with type 2 diabetes. Diabetes Care. 2005;28:266–72. doi: 10.2337/diacare.28.2.266. [DOI] [PubMed] [Google Scholar]
- 11.Tang LQ, Wei W, Chen LM, et al. Effects of berberine on diabetes induced by alloxan and a high-fat/high-cholesterol diet in rats. J Ethnopharmacol. 2006;108:109–15. doi: 10.1016/j.jep.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 12.Zhou L, Yang Y, Wang X, et al. Berberine stimulates glucose transport through a mechanism distinct from insulin. Metabolism. 2007;56:405–12. doi: 10.1016/j.metabol.2006.10.025. [DOI] [PubMed] [Google Scholar]
- 13.Gao CR, Zhang JQ, Huang QL. Experimental study on berberin raised insulin sensitivity in insulin resistance rat models. Zhongguo Zhong Xi Yi Jie He Za Zhi. 1997;17:162–4. [PubMed] [Google Scholar]
- 14.Ko BS, Choi SB, Park SK, et al. Insulin sensitizing and insulinotropic action of berberine from Cortidis rhizoma. Biol Pharm Bull. 2005;28:1431–7. doi: 10.1248/bpb.28.1431. [DOI] [PubMed] [Google Scholar]
- 15.Pan GY, Wang GJ, Sun JG, et al. Inhibitory action of berberine on glucose absorption. Yao Xue Xue Bao. 2003;38:911–4. [PubMed] [Google Scholar]
- 16.Pan GY, Huang ZJ, Wang GJ, et al. The antihyperglycaemic activity of berberine arises from a decrease of glucose absorption. Planta Med. 2003;69:632–6. doi: 10.1055/s-2003-41121. [DOI] [PubMed] [Google Scholar]
- 17.Kong W, Wei J, Abidi P, et al. Berberine is a novel cholesterol-lowering drug working through a unique mechanism distinct from statins. Nat Med. 2004;10:1344–51. doi: 10.1038/nm1135. [DOI] [PubMed] [Google Scholar]
- 18.Leng SH, Lu FE, Xu LJ. Therapeutic effects of berberine in impaired glucose tolerance rats and its influence on insulin secretion. Acta Pharmacol Sin. 2004;25:496–502. [PubMed] [Google Scholar]
- 19.Doggrell SA. Berberine--a novel approach to cholesterol lowering. Expert Opin Investig Drugs. 2005;14:683–5. doi: 10.1517/13543784.14.5.683. [DOI] [PubMed] [Google Scholar]
- 20.Abidi P, Zhou Y, Jiang JD, et al. Extracellular signal-regulated kinase-dependent stabilization of hepatic low-density lipoprotein receptor mRNA by herbal medicine berberine. Arterioscler Thromb Vasc Biol. 2005;25:2170–6. doi: 10.1161/01.ATV.0000181761.16341.2b. [DOI] [PubMed] [Google Scholar]
- 21.Brusq JM, Ancellin N, Grondin P, et al. Inhibition of lipid synthesis through activation of AMP-kinase: An additional mechanism for the hypolipidemic effects of Berberine. J Lipid Res. 2006;47:1281–8. doi: 10.1194/jlr.M600020-JLR200. [DOI] [PubMed] [Google Scholar]


