Abstract
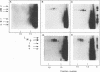
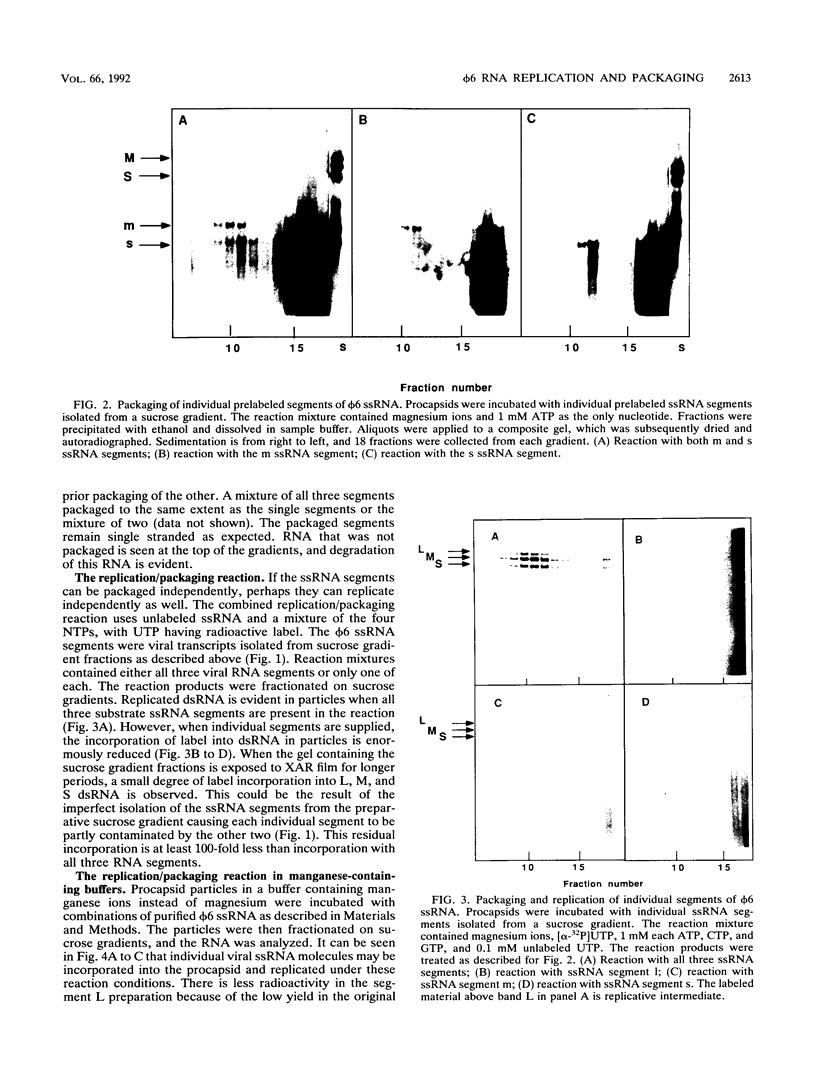
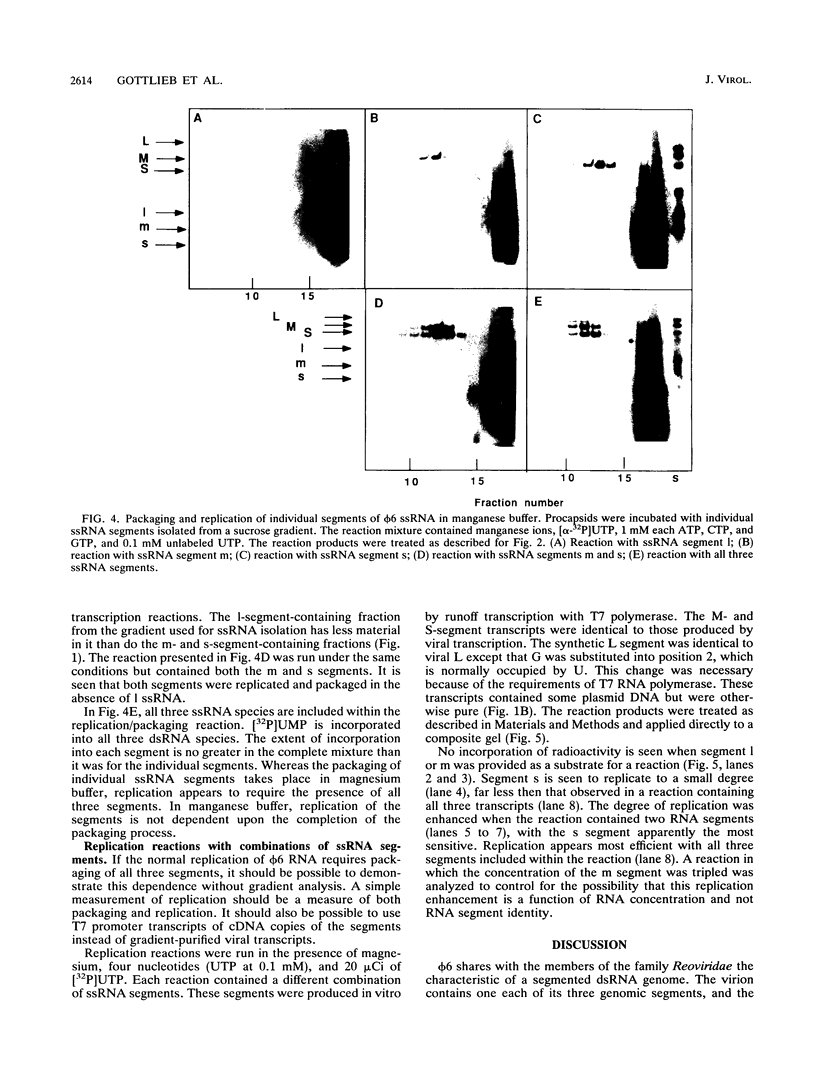
The genome of bacteriophage phi 6 contains three segments of double-stranded RNA. Procapsid structures whose formation was directed by cDNA copies of the large genomic segment are capable of packaging the three viral message sense RNAs in the presence of ATP. Addition of UTP, CTP, and GTP results in the synthesis of minus strands to form double-stranded RNA. In this report, we show that procapsids are capable of taking up any of the three plus-strand single-stranded RNA segments independently of the others. In manganese-containing buffers, synthesis of the corresponding minus strand takes place. In magnesium-containing buffers, individual message sense viral RNA segments were packaged, but minus-strand replication did not take place unless all three viral single-stranded RNA segments were packaged. Since the conditions of packaging in magnesium buffer more closely resemble those in vivo, these results indicated that there is no specific order or dependence in packaging and that replication is regulated so that it does not begin until all segments are in place.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Day L. A., Mindich L. The molecular weight of bacteriophage phi 6 and its nucleocapsid. Virology. 1980 Jun;103(2):376–385. doi: 10.1016/0042-6822(80)90196-8. [DOI] [PubMed] [Google Scholar]
- Emori Y., Iba H., Okada Y. Transcriptional regulation of three double-stranded RNA segments of bacteriophage phi 6 in vitro. J Virol. 1983 Apr;46(1):196–203. doi: 10.1128/jvi.46.1.196-203.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewen M. E., Revel H. R. In vitro replication and transcription of the segmented double-stranded RNA bacteriophage phi 6. Virology. 1988 Aug;165(2):489–498. doi: 10.1016/0042-6822(88)90593-4. [DOI] [PubMed] [Google Scholar]
- Gottlieb P., Strassman J., Bamford D. H., Mindich L. Production of a polyhedral particle in Escherichia coli from a cDNA copy of the large genomic segment of bacteriophage phi 6. J Virol. 1988 Jan;62(1):181–187. doi: 10.1128/jvi.62.1.181-187.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb P., Strassman J., Frucht A., Qiao X. Y., Mindich L. In vitro packaging of the bacteriophage phi 6 ssRNA genomic precursors. Virology. 1991 Apr;181(2):589–594. doi: 10.1016/0042-6822(91)90892-f. [DOI] [PubMed] [Google Scholar]
- Gottlieb P., Strassman J., Qiao X. Y., Frucht A., Mindich L. In vitro replication, packaging, and transcription of the segmented double-stranded RNA genome of bacteriophage phi 6: studies with procapsids assembled from plasmid-encoded proteins. J Bacteriol. 1990 Oct;172(10):5774–5782. doi: 10.1128/jb.172.10.5774-5782.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo P. X., Erickson S., Xu W., Olson N., Baker T. S., Anderson D. Regulation of the phage phi 29 prohead shape and size by the portal vertex. Virology. 1991 Jul;183(1):366–373. doi: 10.1016/0042-6822(91)90149-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Icho T., Wickner R. B. The double-stranded RNA genome of yeast virus L-A encodes its own putative RNA polymerase by fusing two open reading frames. J Biol Chem. 1989 Apr 25;264(12):6716–6723. [PubMed] [Google Scholar]
- Koonin E. V., Gorbalenya A. E., Chumakov K. M. Tentative identification of RNA-dependent RNA polymerases of dsRNA viruses and their relationship to positive strand RNA viral polymerases. FEBS Lett. 1989 Jul 31;252(1-2):42–46. doi: 10.1016/0014-5793(89)80886-5. [DOI] [PubMed] [Google Scholar]
- Mindich L. Bacteriophage phi 6: a unique virus having a lipid-containing membrane and a genome composed of three dsRNA segments. Adv Virus Res. 1988;35:137–176. doi: 10.1016/s0065-3527(08)60710-1. [DOI] [PubMed] [Google Scholar]
- Mindich L., Davidoff-Abelson R. The characterization of a 120 S particle formed during phi 6 infection. Virology. 1980 Jun;103(2):386–391. doi: 10.1016/0042-6822(80)90197-x. [DOI] [PubMed] [Google Scholar]
- Mindich L., MacKenzie G., Strassman J., McGraw T., Metzger S., Romantschuk M., Bamford D. cDNA cloning of portions of the bacteriophage phi 6 genome. J Bacteriol. 1985 Jun;162(3):992–999. doi: 10.1128/jb.162.3.992-999.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mindich L., Qiao X., Onodera S., Gottlieb P., Strassman J. Heterologous recombination in the double-stranded RNA bacteriophage phi 6. J Virol. 1992 May;66(5):2605–2610. doi: 10.1128/jvi.66.5.2605-2610.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olkkonen V. M., Gottlieb P., Strassman J., Qiao X. Y., Bamford D. H., Mindich L. In vitro assembly of infectious nucleocapsids of bacteriophage phi 6: formation of a recombinant double-stranded RNA virus. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9173–9177. doi: 10.1073/pnas.87.23.9173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton J. T. Evidence for equimolar synthesis of double-strand RNA and minus-strand RNA in rotavirus-infected cells. Virus Res. 1990 Nov;17(3):199–208. doi: 10.1016/0168-1702(90)90065-j. [DOI] [PubMed] [Google Scholar]
- Semancik J. S., Vidaver A. K., Van Etten J. L. Characterization of segmented double-helical RNA from bacteriophage phi6. J Mol Biol. 1973 Aug 25;78(4):617–625. doi: 10.1016/0022-2836(73)90283-0. [DOI] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Usala S. J., Brownstein B. H., Haselkorn R. Displacement of parental RNA strands during in vitro transcription by bacteriophage phi 6 nucleocapsids. Cell. 1980 Apr;19(4):855–862. doi: 10.1016/0092-8674(80)90076-8. [DOI] [PubMed] [Google Scholar]
- Van Etten J. L., Burbank D. E., Cuppels D. A., Lane L. C., Vidaver A. K. Semiconservative synthesis of single-stranded RNA by bacteriophage phi 6 RNA polymerase. J Virol. 1980 Feb;33(2):769–773. doi: 10.1128/jvi.33.2.769-773.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidaver A. K., Koski R. K., Van Etten J. L. Bacteriophage phi6: a Lipid-Containing Virus of Pseudomonas phaseolicola. J Virol. 1973 May;11(5):799–805. doi: 10.1128/jvi.11.5.799-805.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]