Calcium signals were recorded from glial cells in acutely isolated rat retina to determine whether Ca2+ waves occur in glial cells of intact central nervous system tissue. Chemical (adenosine triphosphate), electrical, and mechanical stimulation of astrocytes initiated increases in the intracellular concentration of Ca2+ that propagated at ~23 micrometers per second through astrocytes and Müller cells as intercellular waves. The Ca2+ waves persisted in the absence of extracellular Ca2+ but were largely abolished by thapsigargin and intracellular heparin, indicating that Ca2+ was released from intracellular stores. The waves did not evoke changes in cell membrane potential but traveled synchronously in astrocytes and Müller cells, suggesting a functional linkage between these two types of glial cells. Such glial Ca2+ waves may constitute an extraneuronal signaling pathway in the central nervous system.
Glial cells, long considered to be passive elements in the central nervous system (CNS), are now known to generate active responses (1), including intracellular Ca2+ signals (2). Stimulation of astrocytes triggers increases in the intracellular Ca2+ concentration ([Ca2+]i) that can propagate as waves between cells coupled by gap junctions (3, 4). These glial Ca2+ waves have been observed only in dissociated cell (3–7) and organotypic (8) culture preparations, which differ from cells in situ in several respects (9). Because these waves may represent a form of intercellular signaling in the CNS (5) and can potentially modulate neuronal activity (10, 11), we tested whether Ca2+ waves occur in situ in glial cells of acutely isolated rat retina.
The rat retina contains two types of macroglial cells: astrocytes, which form a two-dimensional syncytium at the vitreal surface of the retina, and Müller cells, which are radial glial cells whose end feet terminate at the vitreal surface and whose trunks project downward into the retina (12). We detected [Ca2+]i in these cells with the fluorescent Ca2+ indicator dye Calcium Green-1 (13). The vitreal surfaces of flat-mounted retinas were imaged with video-rate confocal microscopy (14). Both astrocytes and Müller cells incorporated the dye and were identified by their morphology (Fig. 1A).
Fig. 1.
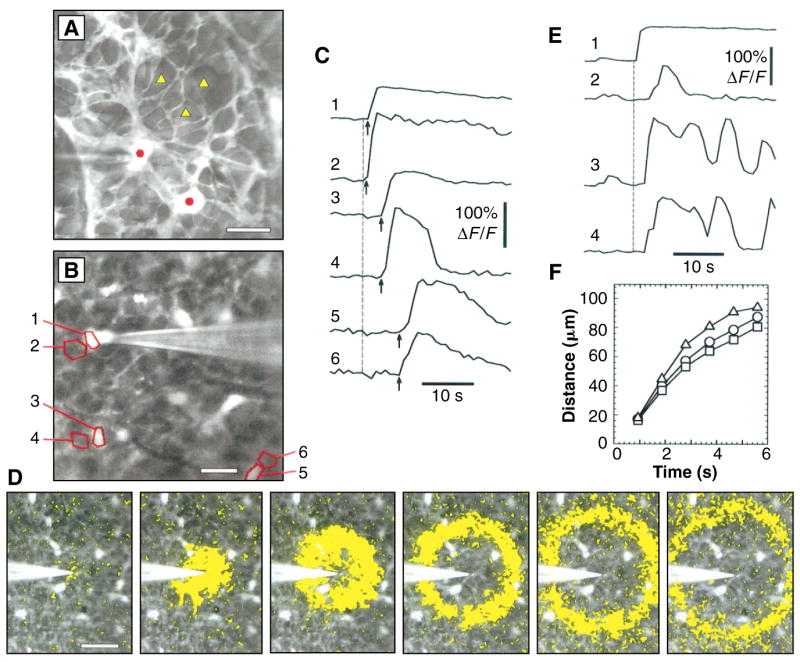
Propagation of Ca2+ waves in glial cells. (A) Astrocytes (circles) and end feet of Müller cells (triangles) in a confocal fluorescence image of the vitreal surface of the rat retina labeled with Calcium Green-1. (B) The retinal surface with six measurement regions outlined. The stimulating pipette is touching region 1. Regions 1, 3, and 5 outline astrocytes; regions 2, 4, and 6 outline adjacent Müller cell endfeet. (C) Change in fluorescence, normalized to baseline fluorescence (ΔF/F ), for the six regions in (B). Onset of the mechanical stimulus is indicated by vertical dashed line; arrival of the Ca2+ wave is marked by arrows. (D) Spread of a Ca2+ wave initiated by a mechanical stimulus. The fluorescence image is shown in black and white. Superimposed yellow rings mark the leading edge of the Ca2+ wave (where the change in fluorescence between successive panels exceeded a threshold value). Interval between panels, 0.93 s. (E) Increases of Ca2+ within one astrocyte (trace 1) and three Müller cells (traces 2 through 4), initiated by a mechanical stimulus. (F) Propagation velocity of Ca2+ waves initiated by mechanical (circles), electrical (squares), and chemical (ATP, triangles) stimuli. Mean distance to the outer edge of the Ca2+ wave rings is plotted. Scale bars: (A), 20 μm; (B), 25 μm; and (D), 50 μm.
Stimulation of a single astrocyte evoked increases in [Ca2+]i in the simulated cell and in neighboring astrocytes and Müller cells. This Ca2+ response propagated outward from the site of stimulation as a wave across the retinal surface (Fig. 1, B to D). Chemical, electrical, and mechanical stimuli were all effective in initiating Ca2+ waves. Pressure ejection of adenosine triphosphate (ATP) (200 μM), carbachol (1 mM), or phenylephrine (100 μM) from micropipettes onto astrocyte somata initiated Ca2+ waves. In contrast to findings in cultured cells (2, 5, 15), local ejection of glutamate (2 mM) or its application in the bath (0.3 to 1 mM) was ineffective at evoking [Ca2+]i increases, but bath application (10 to 100 μM) did potentiate the Ca2+ responses initiated by other stimuli. Both electrical stimulation (−2 μA, 40 to 200 ms, delivered through a micropipette) of astrocyte end feet or somata and mechanical stimulation (5- to 10-μm movement of a micropipette tip pressed against an astrocyte cell body) also initiated Ca2+ waves.
The Ca2+ waves spread outward radially from the point of stimulation and propagated synchronously in astrocytes and Müller cells. Within the temporal resolution of our measurements (~1 s), Ca2+ waves arrived at astrocytes and adjacent Müller cells simultaneously (Fig. 1C). All astrocyte regions, including somata, processes, and end feet, exhibited increased [Ca2+]i upon arrival of a Ca2+ wave, and the propagation of waves was often observed within processes of individual cells. In Müller cells, increases in [Ca2+]i were observed in cell end feet at the retinal surface and in primary cell processes within the ganglion cell and inner plexiform layers. There was no increase in [Ca2+]i in cell somata (in the inner nuclear layer), however, indicating that Ca2+ waves initiated at the proximal end of Müller cells did not propagate into the distal half of the cell. Movies of glial Ca2+ waves can be viewed at http://enlil.med.umn.edu/www/phsl/work/caw.htm.
The time course of the [Ca2+]i increases observed in astrocytes differed from that in Müller cells (Fig. 1E). In astrocytes, [Ca2+]i remained elevated for ~10 to 100 s after the arrival of a wave. In Müller cells, in contrast, the arrival of the Ca2+ wave often triggered a more transient increase in [Ca2+]i or initiated a series of [Ca2+]i oscillations.
The propagation velocities of the Ca2+ waves were nearly identical regardless of the stimulus used to initiate them, suggesting that once initiated, the waves were all propagated by the same mechanism. The velocity [measured from wavefront images (Fig. 1D) 1.5 s after stimulation] averaged 25.3 ± 5.4 (mean ± SD, n = 10), 21.9 ± 7.6 (n = 13), and 23.1 ± 6.7 (n = 17) μm/s, respectively, for chemical (ATP), electrical, and mechanical stimuli (Fig. 1F). The propagation velocity slowed as the wave spread outward from the point of stimulation. Calcium increases in both astrocytes and Müller cells were observed at distances as large as 180 μm from the point of stimulation and with latencies as long as 10.6 s.
The increases in [Ca2+]i observed in astrocytes and Müller cells distant from the site of stimulation might not result from propagated Ca2+ waves but perhaps arise from the direct excitation of these cells by the stimulus. The latter explanation is highly unlikely for several reasons. (i) The onset of Ca2+ responses in cells distant from the stimulus was usually abrupt and could have latencies as long as 10.6 s. (ii) The propagation velocity of Ca2+ waves was nearly identical for chemically, electrically, and mechanically initiated waves. (iii) The Ca2+ waves evoked by ejection of ATP did not spread preferentially in the direction of ejection or bath perfusion but rather spread uniformly in all directions.
Additional experiments confirmed that stimuli did not directly trigger Ca2+ responses in distant cells. After initiation of a Ca2+ wave by mechanical or electrical stimulation, the stimulated astrocyte was refractory for several minutes (Fig. 2). During this refractory period, stimulation at the same site initiated a weak, local response or no response at all. However, stimulation of another astrocyte close to the refractory cell initiated a large Ca2+ response in the surrounding glial cells (in all 13 trials conducted), demonstrating that these cells were capable of conducting a propagated Ca2+ wave.
Fig. 2.
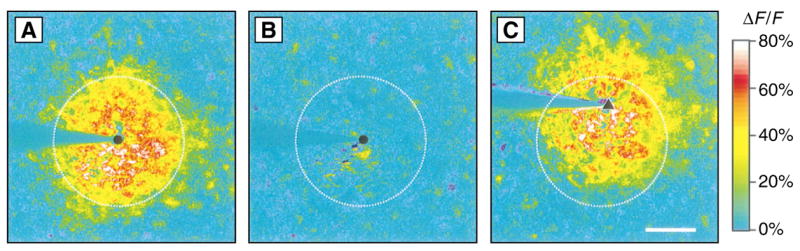
Normalized fluorescence-difference images of Ca2+ waves evoked by repeated stimulation. The pseudocolor images represent a comparison of the mean fluorescence of 15 consecutive images after stimulation and the mean fluorescence of 20 images before stimulation. (A) Ca2+ wave evoked by a mechanical stimulus. (B) Stimulation at the same site (circle) 180 s later elicited no response. (C) Ca2+ wave evoked by stimulation at a second site (triangle) after an additional 90 s. Dashed circles indicate the response region in (A). Scale bar, 50 μm.
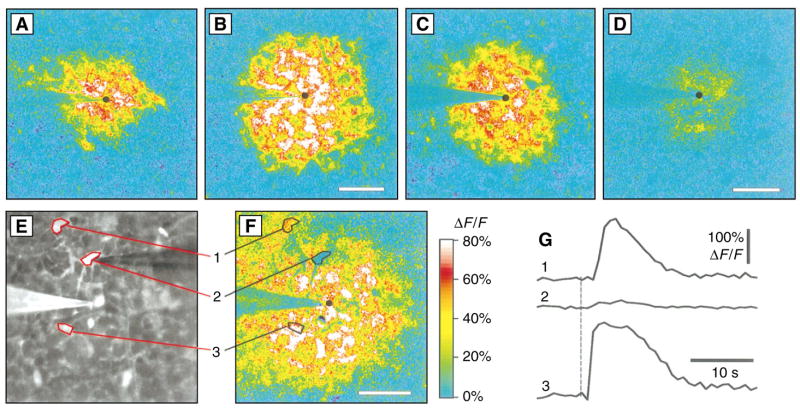
The propagated increases in [Ca2+]i in astrocytes and Müller cells apparently arise largely from the release of Ca2+ from internal stores, mediated by an inositol 1,4,5-trisphosphate (IP3) receptor, rather than from an influx of extracellular Ca2+. Normal Ca2+ waves were observed when no external Ca2+ was present (Fig. 3, A and B). In contrast, bath application of thapsigargin (1.5 μM, for 10 to 16 min), which depletes internal Ca2+ stores (16), reduced the Ca2+ response (Fig. 3, C and D). The Ca2+ response was also reduced in cells in which IP3 receptors were blocked by heparin (17) introduced into the cells through a patch pipette (18) (Fig. 3, E to G). Astrocytes and Müller cells near heparin-filled cells showed normal Ca2+ responses as Ca2+ waves swept past. In contrast, the Ca2+ response of heparin-treated cells (after 15 min of dialysis) was reduced to 18.7 ± 7.8% (n = 12) of untreated neighboring cells, whereas the Ca2+ response of control cells, dialyzed with patch pipette solution without heparin (for an average of 20 min), was 80.3 ± 27.2% (n = 6) of untreated neighbors. The reduction caused by heparin was significantly greater than that caused by dialysis without heparin (P < 0.0001, Student’s unpaired t test).
Fig. 3.
Generation of Ca2+ waves by the release of Ca2+ from internal stores. (A) Ca2+ wave evoked by an electrical stimulus. (B) Different region of the retina shown in (A), stimulated after 31 min in 0 mM Ca2+ and 0.5 mM EGTA. (C) Ca2+ wave evoked by an electrical stimulus under control conditions. (D) Different region of the retina shown in (C), stimulated 16 min after the addition of 1.5 μM thapsigargin. (E) Fluorescence image showing retina, stimulating pipette [left, made visible by coating with DiI (1,1′-diocta-decyl-3,3,3′,3′-tetramethylindocarbo-cyanine perchlorate)], and patch pipette (right, used to introduce heparin and Calcium Green-1 into the cell). A heparin-containing astrocyte (cell 2) and two untreated astrocytes (cells 1 and 3) are outlined. (F) Ca2+ wave evoked by ejection of ATP in same retinal area as shown in (E). (G) Change in fluorescence of regions shown in (E) and (F ). Large [Ca2+]i increases occurred in untreated cells but not in the heparin-filled cell. Panels (A) through (D) and (F ) are normalized fluorescence-difference images. Black circles indicate stimulation sites. Scale bars for all panels, 50 μm.
The electrophysiological consequences of propagated Ca2+ waves were examined. Waves evoked little change in cell membrane potential or input resistance in glial cells, which were monitored in whole cell, current-clamp recordings. Ca2+ waves elicited by ejection of ATP were associated with weak depolarizations: 0.15 ± 0.20 mV (n = 19; P < 0.005, one-population t test) in astrocytes and 0.16 ± 0.13 mV (n = 20; P < 0.001) in Müller cells. Electrical and mechanical stimuli did evoke depolarizations as large as 37 and 8 mV, recorded from astrocytes and Müller cells, respectively. These responses probably represented depolarizations conducted electrotonically from the stimulated cell because (i) the depolarization often occurred before the arrival of the Ca2+ wave, (ii) the depolarization was sometimes observed even when the Ca2+ wave failed to reach the cell, and (iii) the amplitude of the depolarization declined rapidly as the distance between the stimulation site and the cell increased.
Our results demonstrate that glial cells in freshly excised CNS tissue support the propagation of Ca2+ waves. The Ca2+ waves we observed resemble those in cultured cells in some respects. The waves have similar propagation velocities (2, 5, 7, 8) and both are generated by the release of Ca2+ from internal stores (2, 4, 7), mediated, at least in part, by IP3 receptors (19). In contrast to cultured cells (2, 5), however, astrocytes in the retina were unresponsive to glutamate and did not exhibit oscillations in [Ca2+]i when stimulated.
Our results further suggest that the propagation of Ca2+ waves through astrocytes and Müller cells is linked. Such linkage is consistent with the findings of chemical tracer studies (20), which show that astrocytes and Müller cells are coupled. The synchronous spread of Ca2+ waves in these cells suggests that, as waves spread outward through the astrocyte syncytium, they may secondarily propagate into Müller cells directly coupled to the astrocytes.
The propagation of calcium waves through a glial syncytium represents an extraneuronal signaling pathway that may influence neuronal activity (10, 11). The absence of membrane potential changes suggests that Ca2+ waves do not lead to the voltage activation of ion channels in glial cells, which have been postulated to influence potassium buffering and neurotransmitter uptake (1). But an increase in [Ca2+]i within glial cells could lead to the release of neuroactive substances (10, 21) and to the modulation of neuronal activity.
REFERENCES AND NOTES
- 1.Kettenmann H, Ransom BR. Neuroglia. Oxford Univ. Press; Oxford: 1995. [Google Scholar]
- 2.Finkbeiner SM. Glia. 1993;9:83. doi: 10.1002/glia.440090202. [DOI] [PubMed] [Google Scholar]
- 3.Finkbeiner SM. Neuron. 1992;8:1101. doi: 10.1016/0896-6273(92)90131-v. [DOI] [PubMed] [Google Scholar]; Charles AC, et al. J Cell Biol. 1992;118:195. doi: 10.1083/jcb.118.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Enkvist MOK, McCarthy KD. J Neurochem. 1992;59:519. doi: 10.1111/j.1471-4159.1992.tb09401.x. [DOI] [PubMed] [Google Scholar]
- 5.Cornell-Bell AH, Finkbeiner SM, Cooper MS, Smith SJ. Science. 1990;247:470. doi: 10.1126/science.1967852. [DOI] [PubMed] [Google Scholar]
- 6.Cornell-Bell AH, Finkbeiner SM. Cell Calcium. 1991;12:185. doi: 10.1016/0143-4160(91)90020-f. [DOI] [PubMed] [Google Scholar]
- 7.Charles AC, Merril JE, Dirksen ER, Sanderson MJ. Neuron. 1991;6:983. doi: 10.1016/0896-6273(91)90238-u. [DOI] [PubMed] [Google Scholar]
- 8.Dani JW, Chernjavsky A, Smith SJ. ibid. 1992;8:429. doi: 10.1016/0896-6273(92)90271-e. [DOI] [PubMed] [Google Scholar]
- 9.Barres BA, Koroshetz WJ, Chun LLY, Corey DP. ibid. 1990;5:527. doi: 10.1016/0896-6273(90)90091-s. [DOI] [PubMed] [Google Scholar]; Porter JT, McCarthy KD. J Neurochem. 1995;65:1515. doi: 10.1046/j.1471-4159.1995.65041515.x. [DOI] [PubMed] [Google Scholar]; Duffy S, MacVicar BA. J Neurosci. 1995;15:5535. doi: 10.1523/JNEUROSCI.15-08-05535.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]; Porter JT, McCarthy KD. Glia. 1995;13:101. doi: 10.1002/glia.440130204. [DOI] [PubMed] [Google Scholar]
- 10.Parpura V, et al. Nature. 1994;369:744. doi: 10.1038/369744a0. [DOI] [PubMed] [Google Scholar]
- 11.Nedergaard M. Science. 1994;263:1768. doi: 10.1126/science.8134839. [DOI] [PubMed] [Google Scholar]; Hassinger TD, et al. J Neurobiol. 1995;28:159. doi: 10.1002/neu.480280204. [DOI] [PubMed] [Google Scholar]
- 12.Newman EA, Reichenbach A. Trends Neurosci. 1996;19:307. doi: 10.1016/0166-2236(96)10040-0. [DOI] [PubMed] [Google Scholar]
- 13.Retinas from Long-Evans rats were incubated in collagenase/dispase (2 mg/ml) and deoxyribonuclease (DNase) (0.1 mg/ml) for 12 to 20 min before removal of the vitreous humor, then incubated in Calcium Green-1 AM (33.3 mg/ml; Molecular Probes) and pluronic acid (4.7 mg/ml) for 1 hour at room temperature, mounted onto nitrocellulose filters, and secured in a chamber perfused at ~3 ml/min with HCO3−-buffered Ringer’s (24°C) [117 mM NaCl, 3 mM KCl, 2 mM CaCl2, 1 mM MgSO4, 0.5 mM NaH2PO4, 15 mM dextrose, 26 mM NaHCO3, equilibrated with 5% CO2 in O2 (pH ~ 7.4)]. In most experiments, 2 μM ATP and 10 or 100 μM glutamate were added to the perfusate to potentiate the Ca2+ waves. Ca2+ responses were also seen without ATP and glutamate added. Glutamate concentration at the surface of the retina in vivo ranges between 10 and 400 μM; Heinamaki AA, Muhonen ASH, Piha RS. Neurochem Res. 1986;11:535. doi: 10.1007/BF00965323. [DOI] [PubMed] [Google Scholar]; Gunnarson G, Jakobbson AK, Hamberger A, Sjostrand J. Exp Eye Res. 1987;44:235. doi: 10.1016/s0014-4835(87)80008-8. [DOI] [PubMed] [Google Scholar]
- 14.Labeled glial cells were imaged with a Noran Odyssey confocal scanner and a BX60 Olympus microscope with 20× [0.5 numerical aperture (NA)] and 40× (0.8 NA) water immersion objectives. Calcium Green-1 fluorescence was monitored with 488-nm argon excitation and a 515-nm-long pass barrier filter. Images, averages of 16 video frames, were acquired every 0.93 s with MetaMorph software (Universal Imaging). Measurements were corrected for baseline drift resulting from bleaching.
- 15.Kim WT, Rioult MG, Cornell-Bell AH. Glia. 1994;11:173. doi: 10.1002/glia.440110211. [DOI] [PubMed] [Google Scholar]
- 16.Thastrup O, Cullen PJ, Drobak BK, Hanley MR, Dawson AP. Proc Natl Acad Sci USA. 1990;87:2466. doi: 10.1073/pnas.87.7.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ghosh TK, Eis PS, Mullaney JM, Ebert CL, Gill DL. J Biol Chem. 1988;263:11075. [PubMed] [Google Scholar]
- 18.The patch pipette solution contained 5 mM NaCl, 120 mM KCl, 1 mM CaCl2, 7 mM MgCl2, 5 mM M Calcium EGTA, 5 mM Na2ATP, 5 mM Hepes, 2 μg/ml) (6 kD), and Green-1 K6 salt, and heparin (100 μ the solution was adjusted to pH 7.2 with KOH. Cells were judged to be successfully dialyzed with the pipette solution if Calcium Green-1 fluorescence in the cells increased at least 30% after achieving whole cell recording.
- 19.Shao Y, McCarthy KD. Cell Calcium. 1995;17:187. doi: 10.1016/0143-4160(95)90033-0. [DOI] [PubMed] [Google Scholar]; Charles AC, Dirksen ER, Merrill JE, Sanderson MJ. Glia. 1993;7:134. doi: 10.1002/glia.440070203. [DOI] [PubMed] [Google Scholar]
- 20.Robinson SR, Hampson ECGM, Munro MN, Vaney DI. Science. 1993;262:1072. doi: 10.1126/science.8093125. [DOI] [PubMed] [Google Scholar]
- 21.Parpura V, Liu F, Jeftinija KV, Haydon PG, Jeftinija SD. J Neurosci. 1995;15:5831. doi: 10.1523/JNEUROSCI.15-08-05831.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.We thank P. Ceelen for technical assistance, M. H. Newman for suggestions concerning data analysis, and J. I. Gepner for helpful comments on the manuscript. Supported by NIH grants EY04077 and EY10383.