Abstract
Limb-sparing surgeries have been performed more frequently than amputation based on the belief that limb-sparing surgeries provide improved function and quality-of-life (QOL). However, this has not been extensively studied in the paediatric population, which has unique characteristics that have implications for function and QOL. Using the Childhood Cancer Survivor Study, 528 adult long-term survivors of pediatric lower extremity bone tumours, diagnosed between 1970 and 1986, were contacted and completed questionnaries assessing function and QOL. Survivors were an average of 21 years from diagnosis with an average age of 35 years. Overall they reported excellent function and QOL. Compared to those who had a limb-sparing procedure, amputees were not more likely to have lower function and QOL scores and self-perception of disability included general health status, lower educational attainment, older age and female gender. Findings from this study suggest that, over time, amputees do as well as those who underwent limb-sparing surgeries between 1970 and 1986. However, female gender, lower educational attainment and older current age appear to influence function, QOL and disability.
Keywords: osteosarcoma, Ewing's sarcoma, quality of life, function, lower extremity, bone tumour
Malignant bone tumours, predominantly osteosarcoma and Ewing's sarcoma, account for approximately 6% of all cancer diagnosed under the age of 20 years (Ries et al, 1999) in the United States. Nearly two-thirds of these tumours have a primary site in the lower extremity of pelvis. Historically, treatment of extremity bone tumours with amputation resulted in poor survival rates (Wang and Schulz, 1953; Dahlin et al, 1961; Friedman and Carter, 1972). Beginning in the 1970s, treatment strategies changed to include the use of multiagent chemotherapy (Rosen et al, 1979; Link et al, 1986; Nesbit et al, 1990). This led to significant improvements in the prognosis of children and young adults with osteosarcoma and Ewing's sarcoma and increased the overall survival rate for patients with nonmetastatic disease from 10 to 20% (Wang and Schulz, 1953; Dahlin et al, 1961; Friedman and Carter, 1972) to approximately 60% (Burgert et al, 1990; Bacci et al, 1998; Nesbit et al, 1990; Provisor et al, 1997). Enabled by improved radiographic and surgical techniques, the surgical management of patients with lower extremity lesions was increasingly characterized by an expanded use of limb-sparing surgeries (Springfield, 1991).
While there is general agreement that limb-sparing techniques are the preferred approach for patients with upper extremity tumours (Cheng and Gebhardt, 1991; Aboulafia and Malawer, 1993), debate continues regarding the long term outcomes of amputation compared to limb-sparing surgery for lower extremity primaries (Nagarajan et al, 2002). The current investigation assessed self-reported function and quality of life (QOL) among a large cohort of long-term survivors of childhood lower extremity bone tumours.
MATERIALS AND METHODS
Subject selection and eligibility
The childhood Cancer Survivor Study (CCSS) is a cohort of individuals with a confirmed diagonosis of cancer who participated in the Long-Term Follow-Up Study, a multi-institutional study of individuals who survived for 5 or more years after treatment for cancer, leukaemia, tumours or similar illnesses diagnosed during childhood or adolescence. The methods and cohort characteristics of the CCSS have been previously presented in detail (Robison et al, 2002). Briefly, as of 1 November 2000, 20 276 participants met the cohort inclusion criteria of: (1) diagnosis of one of the following cancers: brain tumour, leukaemia, Hodgkin's disease, non-Hodgkin's lymphoma, kidney cancer, neuroblastoma, soft-tissue sarcoma, or cancer of the bone; (2) initial treatment of one of the 25 collaborating CCSS institution – see Appendix; (3) diagnosis date between 1 January 1970 and 31 December 1986; (4) age less than 21 years at diagnosis; and (5) survival 5 years from diagnosis. A total of 1042 of the 1596 5-year survivors of a bone tumour directly participated by completing the CCSS baseline data collection questionnaire. Excluded were 328 survivors who declined participation, 221 considered lost to follow-up after extensive tracing, and five who were pending. The present report is restricted to the subset of 629 participants who fulfilled the following additional criteria: (1) diagnosis of osteosarcoma or Ewing's sarcoma: (2) tumour located in the lower extremity or the pelvis: (3) 18 years of age or older at the time of the present evaluation: (4) availability of complete medical records: (5) alive and able to complete a self-reported (not by proxy) function and QOL assessment.
Overall, 84% (528/629) of the subjects contacted for the current study participated by completing the function and QOL questionnaire. Of the 101 nonparticipants, two were lost to follow-up, 75 declined participation and 24 were pending participation at the time of analysis. Nonparticipants were slightly more likely than participants to be younger at diagnosis (12.7 years vs 13.5 years) and to have an extremity lesion (97 vs 91%), but were less likely to have graduated from college (27 vs 48%).
The CCSS protocol and contact documents were reviewed and approved by the Human Subjects Committee at each participating institution. All contact documents including the baseline questionnaire and the function and QOL assessments can be viewed at www.cancer.umn.edu/ccss.
Assessment of function and QOL
Subjects completed a self-administered questionnaire that included the Toronto Extremity Salvage Score (TESS) (Davis et al, 1996, 1999) and the Quality of Life for Cancer Survivors (QOL-CS) instrument (Ferrel et al, 1995a, 1995b) The TESS (30 questions) measures physical disability based on the patient's report of their function and was developed and validated specifically for individuals who have undergone surgery for lower extremity musculoskeletal tumours. The QOL-CS questionnaire (41 questions), developed and validated specifically for cancer survivors, measures four domains of QOL (physical, psychological, social, and spiritual well-being).
Cancer treatment information
Information on the characteristics of the original cancer diagnosis was obtained on all consented eligible cases from the treating institution. Surgical local control procedures were grouped into amputation and nonamputation by using the abstracted ICD-9 codes and participants' questionnaire responses. Subjects (n=24) with an amputation 3 or more years after diagnosis were classified by their initial surgical treatment.
Site codes (long bones of the lower extremity or pelvis) from the CCSS medical records abstraction forms were the primary source for classifying tumour site. More specific site information (e.g. distal femur or proximal tibia) was obtained using a skeletal diagram given to the bone tumour survivors, who were asked to indicate the site of their tumour.
Data analysis
A priori participants were categorized to one of four groups defined by age at diagnosis of the bone tumour (⩽12 years vs ⩾12 years) and by the type of surgery (amputation vs limb-sparing surgery) resulting in four age/surgery groups (⩽12/Amp and ⩾12/Amp: under and over the age of 12 years with an amputation; ⩽12/LS and ⩾12/LS: under and over the age of 12 years with a limb-sparing surgery). For comparison purposes, the ⩾12/LS age-surgery group was used as the reference group. The age cutoff of 12 years old was chosen because it represents an approximate marker for emotional maturity (preadolescence and early adolescence) (Brindis et al, 1992) and pubertal development, and is the approximate age at which significant bone growth starts (Kreipe, 1992). Amputees were further subdivided into amputation only and amputation with radiation therapy. Nonamputees (limb-salvage) were categorised into: (1) radiation treatment only, (2) arthrodesis, (3) arthrodesis with radiation treatment, (4) endoprosthetic reconstruction, (5) endoprosthetic reconstruction with radiation treatment, (6) surgery not otherwise specified and (7) surgery not otherwise specified with radiation treatment. There were no rotationplasties noted. General health status was assessed from questions from the initial baseline survey of CCSS participants that asked about self-perceived general health. Responses were dichotomised into good/very good/excellent health vs fair/poor health.
TESS and QOL-CS questionnaires were scored according to instructions of the authors of the respective scales. TESS and QOL-CS scores were dichotomised for analyses by using the 25th percentile of the scores. Comparisons of TESS and QOL-CS scores across the age/surgery groups were performed using general linear models, adjusting for perceived general health, gender, educational attainment and age at the completion of the CCSS baseline questionnaire. The TESS scale incorporated a self-rating scale of disability, which was collapsed into a dichotomous variable: not disabled (not at all disabled and mildly disabled) and disabled (moderately and completely/severely disabled). The associations of the age/surgery variable with the dichotomous disability variable and with scoring below the 25th percentile on TESS or QOL-CS were assessed using logistic regression models, adjusting for general health, gender, educational attainment, and a categorical age at questionnaire completion variable. In order to assure that adjustment for general health status did not influence the relationship between the age/surgery groups and the outcomes, multivariate analyses were conducted with and without inclusion of the general health status variable, which provided comparable results. Given that the primary comparisons are based upon testing of a priori hypotheses, no correction was made for multiple comparisons.
RESULTS
Characteristics of study participants
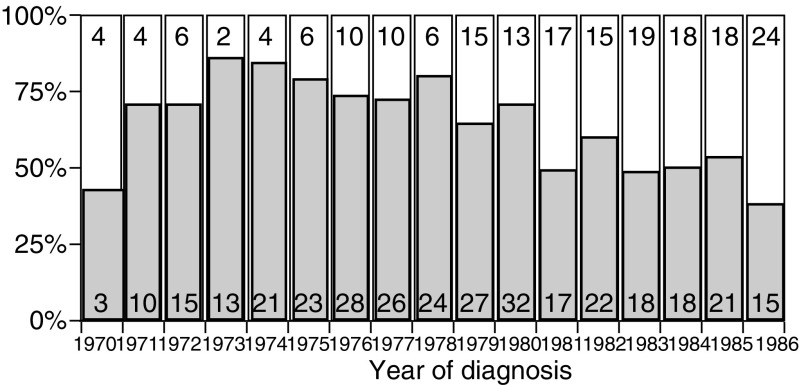
In total, 80% of the participants had a previous diagnosis of osteosarcoma. The most frequent site of tumour was the distal femur (42%), followed by the proximal tibia (18%) (see Table 1). The majority of participants had undergone amputation (63) and was over the age of 12 years at diagnosis (65%). Figure 1 graphically represents the number of amputations performed by year that appears to decrease with time. The median time between diagnosis and evaluation was 21 years (range 12–31 years). The majority of survivors reported an educational level beyond high school and considered themselves to be in good health.
Table 1. Characteristics of study population.
| Characteristic | Lower extremity survivors (n=528) |
|---|---|
| Gender | |
| Female | 270 (51.1%) |
| Male | 258 (48.9%) |
| General health | |
| Poor | 49 (9.7%) |
| Good | 457 (90.3%) |
| Education | |
| No high school | 21 (4.2%) |
| High school graduate | 235 (47.5%) |
| College graduate | 239 (48.3%) |
| Specific site | |
| Distal femur | 226 (42.8%) |
| Proximal tibia | 98 (18.6%) |
| Pelvis | 46 (8.7%) |
| Other femoral sites | 63 (11.9%) |
| Other tibial sites | 46 (8.7%) |
| Fibular sites | 39 (7.4%) |
| Nonspecific, nonpelvic site | 10 (1.9%) |
| Tumor type | |
| Osteosarcoma | 422 (79.9%) |
| Ewing's sarcoma | 106 (20.1%) |
| Surgery type | |
| Amputation | |
| Amputation only | 317 (60.0%) |
| Amputation/XRT | 19 (3.6%) |
| Limb-sparing | |
| XRT only | 32 (6.1%) |
| Arthrodesis/XRT | 1 (0.2%) |
| Endoprosthesis/XRT | 7 (1.3%) |
| NOS/XRT | 40 (7.6%) |
| Arthrodesis | 10 (1.9%) |
| Endoprosthesis | 49 (9.3%) |
| Nonamputation, nonspecific | 53 (10.0%) |
| Age at diagnosis | |
| Mean (range) | 13.5 (1–20) |
| Median | 14.0 |
| Years from diagnosis to questionnaire completion | |
| Mean (range) | 20.8 (13–31) |
| Median | 21.0 |
| Age at questionnaire completion (years) | |
| Mean (range) | 34.8 (19.49) |
| Median | 35.0 |
| ⩽30 | 132 (25.0%) |
| 31–35 | 145 (27.5%) |
| 36–39 | 128 (24.2%) |
| ⩾40 | 123 (23.3%) |
Figure 1.
Frequency of amputation over time. Shaded bars represent the number of amputations and open bars represent the number of limb sparing procedures.
QOL, function and self-perception of disability (Tables 2, 3 and 4)
Table 2. Raw scores of function (TESS), quality of life (QOL-CS), quality of life subscales and percent self-reported as disabled.
|
Age/surgery subgroups |
|||||
|---|---|---|---|---|---|
| Outcome | All participants (n=528) | ⩽12/Amp (n=121) | ⩽12/LS (n=63) | >12/Amp (n=215) | >12/LS (n=129) |
| TESS | |||||
| Mean (SD) | 85.5 (14.3) | 87.6 (12.4) | 88.6 (15.7) | 83.8 (13.1) | 84.5 (16.8) |
| Range | 17.2–100 | 64.2–100 | 23.3–100 | 33.7–100 | 17.2–100 |
| QOL-CS | |||||
| Overall | |||||
| Mean (SD) | 6.9 (1.4) | 7.0 (1.4) | 7.0 (1.2) | 6.8 (1.3) | 6.8 (1.4) |
| Range | 1.4–9.7 | 6.3–9.4 | 3.4–9.0 | 3.2–9.7 | 2.4–8.9 |
| Spirituality | |||||
| Mean (SD) | 6.0 (2.0) | 5.6 (19) | 6.3 (1.8) | 6.0 (2.0) | 6.1 (1.9) |
| Range | 0.1–10 | 0.1–10 | 2.3–10 | 0.7–10 | 1.3–9.7 |
| Social | |||||
| Mean (SD) | 7.4 (1.9) | 7.5 (1.9) | 7.6 (2.0) | 7.3 (1.9) | 7.4 (2.0) |
| Range | 0.9–10 | 0.9–10 | 1.0–10 | 1.4–10 | 1.0–10.0 |
| Psychosocial | |||||
| Mean (SD) | 6.5 (1.6) | 6.8 (1.6) | 6.5 (1.5) | 6.4 (1.6) | 6.4 (1.6) |
| Range | 0.9–9.9 | 0.9–9.9 | 2.7–9.2 | 2.2–9.8 | 2.0–9.3 |
| Physical | |||||
| Mean (SD) | 7.9 (1.6) | 8.2 (1.6) | 8.2 (1.5) | 7.9 (1.7) | 7.7 (1.6) |
| Range | 1.0–10 | 1.9–10 | 4.0–10 | 1.0–10 | 1.6–10.0 |
| Self-rating disability (expressed as subjects (%)) | |||||
| Severely/completely | 24 (4.6%) | 2 (1.7%) | 3 (4.8%) | 11 (5.1%) | 8 (6.3%) |
| Moderately | 110 (21.0%) | 27 (22.5%) | 8 (12.7%) | 54 (25.4%) | 21 (16.4%) |
| Mildly | 241 (46.0%) | 60 (50.0%) | 22 (34.9%) | 112 (52.6%) | 47 (36.7%) |
| Not disabled | 149 (28.4) | 31 (25.8%) | 30 (47.6%) | 36 (16.9%) | 52 (40.6%) |
Table 3. Characteristics of survivors reporting disability and TESS and QOL-CS scores below the 25th.
| Characteristic | No. | Self-rating disabled (%) | TESS score below 25th percentile (%) | QOL-CS score below 25th percentile (%) |
|---|---|---|---|---|
| Age/surgery group | ||||
| ⩽12/Amp | 121 | 24.2 | 15.8 | 20.0 |
| ⩽12/LS | 63 | 17.5 | 17.5 | 22.2 |
| >12/Amp | 215 | 30.5 | 28.6 | 29.1 |
| >12/LS | 129 | 22.7 | 28.1 | 25.8 |
| Amputation status | ||||
| Amputation | 336 | 28.2 | 24.0 | 25.8 |
| Limb-sparing surgery | 192 | 20.9 | 24.6 | 24.6 |
| General health | ||||
| Good/excellent health | 457 | 27.7 | 21.9 | 21.4 |
| Fair/poor health | 49 | 61.2 | 53.1 | 69.4 |
| Gender | ||||
| Female | 270 | 26.2 | 29.2 | 28.8 |
| Male | 258 | 24.9 | 19.1 | 21.8 |
| Education | ||||
| No H.S. graduation | 21 | 57.1 | 52.4 | 47.6 |
| H.S. graduation | 235 | 27.4 | 23.9 | 23.5 |
| College graduation | 239 | 21.1 | 21.1 | 25.3 |
| Age at questionnaire completion | ||||
| ⩽30 years old | 132 | 16.8 | 15.3 | 17.6 |
| 31–35 years old | 145 | 22.9 | 22.8 | 22.9 |
| 36–39 years old | 128 | 26.0 | 26.8 | 30.7 |
| ⩾40 years old | 123 | 27.7 | 32.8 | 31.2 |
Table 4. Odds ratio for considering themselves disabled (moderately, severely or completely disabled) or having a TESS or QOL-CS score below the 25th percentile.
|
Univariate |
Multivariate |
|||||
|---|---|---|---|---|---|---|
| Subgroups | Self-rating disable | TESS score | QOL-CS score | Self-rating disable | TESS score | QOL-CS score |
| ⩽12/Amp | 1.1 (0.60–1.96) | 0.5 (0.26–0.90)* | 0.7 (0.40–1.31) | 1.5 (0.77–3.10) | 0.4 (0.19–0.92)* | 1.1 (0.54–2.37) |
| ⩽12/LS | 0.7 (0.33–1.56) | 0.5 (0.25–1.15) | 0.8 (0.40–1.68) | 0.9 (0.37–2.19) | 0.6 (0.25–1.45) | 0.9 (0.37–2.06) |
| >12/Amp | 1.5 (0.90–2.49) | 1.0 (0.63–1.67) | 1.2 (0.72–1.94) | 1.1 (0.74–1.78) | 0.9 (0.55–1.59) | 1.2 (0.69–2.25) |
| >12/LS | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) |
| Poor health | 5.4 (2.90–9.93)** | 4.1 (2.27–7.59)** | 8.3 (4.35–15.89)** | 4.9 (2.49–9.76)** | 2.8 (1.41–5.47)** | 7.5 (3.69–15.24)** |
| Good health | 1.0 (ref) | 1.0 (ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) |
| Female | 1.1 (0.72–1.59) | 1.7 (1.17–2.63)* | 1.5 (0.98–2.16) | 1.1 (0.74–1.78) | 1.7 (1.08–2.68)* | 1.6 (1.05–2.59)* |
| Male | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) |
| Current age (years) | ||||||
| ⩽30 | 0.3 (0.19–0.60)** | 0.4 (0.20–0.68)* | 0.5 (0.26–0.85)* | 0.3 (0.13–0.62)** | 0.5 (0.22–1.06) | 0.5 (0.22–1.03) |
| 31–35 | 0.5 (0.29–0.84)* | 0.6 (0.35–1.00) | 0.7 (0.38–1.13) | 0.5 (0.26–0.86)* | 0.6 (0.33–1.12) | 0.7 (0.38–1.34) |
| 36–39 | 0.6 (0.34–1.0)* | 0.7 (0.44–1.29) | 1.0 (0.57–1.68) | 0.5 (0.28–0.95)* | 0.8 (0.42–1.39) | 1.0 (0.54–1.79) |
| ⩾40 | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) |
| No high school | 5.0 (1.99–2.50)** | 4.1 (12.65–10.24)** | 2.7 (1.09–6.63)* | 4.3 (1.52–12.24)** | 4.2 (1.52–12.24)** | 1.5 (0.51–4.36) |
| High school graduate | 1.4 (0.92–2.15) | 1.2 (0.76–1.81) | 0.9 (0.60–1.38) | 1.4 (0.88–2.19) | 1.2 (0.77–1.96) | 0.6 (0.53–1.36) |
| College graduate | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) |
| Ewing's sarcoma | 0.7 (0.43–1.20) | 0.9 (0.54–1.49) | 0.8 (0.47–1.29) | — | — | — |
| Osteosarcoma | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | — | — | — |
| Pelvic site | 1.3 (0.67–2.52) | 1.6 (0.82–3.03) | 2.0 (1.08–3.80)* | — | — | 0.9 (0.25–3.56) |
| Limb site | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | — | — | 1.0 (Ref) |
| Pelvic irradiation | 1.3 (0.60–2.61) | 1.6 (0.76–3.18) | 2.4 (1.22–4.76)* | — | — | 2.6 (0.63–10.87) |
| No pelvic irradiation | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | — | — | 1.0 (Ref) |
| Limb irradiation | 1.1 (0.58–1.94) | 0.7 (0.42–1.33) | 1.6 (0.82–3.10) | — | — | — |
| No limb irradiation | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | — | — | — |
| Ampution | 1.5 (0.97–2.27) | 1.0 (0.64–1.47) | 1.1 (0.71–1.61) | — | — | — |
| Limb sparing surgery | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | — | — | — |
| ⩽12 years old at surgery | 0.7 (0.48–1.2) | 0.5 (0.31–0.78)** | 0.7 (0.44–1.04) | — | — | — |
| >12 years old at surgery | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | — | — | — |
P<0.05,
P<0.01.
Subjects in all four age/surgery groups scored relatively high with mean function scores (scale of 1–100) between 83 and 88 and mean quality-of-life scores (scale of 1–10) between 6.8. and 7.0 (Table 2) with no statistical difference in scores across groups. TESS and QOL-CS scores correlated with self-report assessments of disability, having Pearson's, correlation coefficients of 0.62 and 0.45, respectively. Mean TESS scores for self-ratings of completely/severely disabled, moderately disabled, mildly disabled, and not at all disabled were 61.2, 74.4, 86.9, and 95.4, respectively. Mean QOL-CS scores for these same groups were 5.0, 6.2, 7.0, and 7.6. When the self-rating scores were collapsed into those who considered themselves disabled or not disabled, significant differences were found between the two disability groups in mean TESS (72.1 vs 90.1, P⩽0.001) and QOL-CS scores (6.0 vs 7.2, P⩽0.001).
Characteristics of the survivors classified as disabled or scoring below the 25th percentile of TESS and QOL-CS are provided in Table 3. In pairwise comparisons of the age/surgery groups, no significant differences in mean TESS or QOL-CS scores, self-perception of disability, or scoring below the 25th percentile on QOL-CS or TESS scores were found after adjusting for general health, gender, educational status, and age at questionnaire completion (data not shown).
Poor general health status, older current age, and failure to graduate from high school predicted self-perception of disability and lower TESS and QOL-CS scores and were all statistically significant in univariate analyses (Table 4). Those in the ⩽12/Amp group were significantly less likely to score below the 25th percentile of TESS scores compared to subjects in other age/surgery groups, while females were more likely to score below the 25th percentile of TESS scores compared to the males. Those with a pelvic lesion and those receiving pelvic irradiation were more likely to score below the 25th percentile of QOL-CS scores (Table 4).
In multivariable analysis (Table 4) of self-perceived disability, age/surgery group was not predictive; however, poor health status, older current age (>40 years), and not graduating high school predicted an increased likelihood of perceived disability. Those in the ⩽12/Amp group were significantly less likely to score below the 25th percentile in TESS scores. Poor health status, female gender, and not graduating from high school were predictive of TESS scores below the 25th percentile. When examining QOL-CS scores, only female gender and poor health status were predictive of scoring below the 25th percentile.
Tumour location/surgical procedure
Primary tumour site was not predictive of QOL-CS or TESS scores below the 25th percentile; however, those with a proximal tibia lesion appeared less likely to rate themselves as disabled (see Table 5). There was a slightly lower than expected proportion of cases with pelvic tumours (46/528) which typically would account for 25% of Ewing's sarcoma and 10% of osteosarcoma presentations (Arndt and Crist, 1999). This is likely due to the poorer prognosis of those with pelvic tumours at the time this cohort was diagnosed. Analysis of the two surgical groups (amputation vs nonamputation) revealed no differences in self-perception of disability or in scoring below the 25th percentile on TESS or QOL-CS after adjusting for general health status, gender, education, and age at questionnaire completion. In the site and surgery group analyses, poor general health and older age at questionnaire completion predicted worse outcomes (data not shown). Female gender and less than a high school education were less consistent in predicting poorer outcomes in the surgical group analysis.
Table 5. Odds ratio of disability, TESS score (below 25th percentile), or QOL-CS score (below 25th percentile).
| Self-rating disabled OR (CI) | TESS score OR (CI) | QOL-CS score OR (CI) | |
|---|---|---|---|
| Site | |||
| Pelvis | 0.9 (0.36–2.15) | 1.3 (0.56–3.16) | 1.3 (0.55–3.14) |
| Proximal tibia | 0.5 (0.25–0.96)* | 0.7 (0.39–1.42) | 0.7 (0.38–1.44) |
| Distal femur | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) |
| Surgery | |||
| Amputation | 1.3 (0.82–2.08) | 0.8 (0.54–1.34) | 1.0 (0.66–1.64) |
| Limb-sparing | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) |
Comparisons by site adjusted for general health status, amputation, education, gender and age. Comparisons by surgical procedure adjusted for general health status, gender, education and age.
P<0.05.
DISCUSSION
As cure rates for childhood cancers continue to rise, increasing numbers of childhood cancer survivors will be entering adulthood and will require close follow-up for late effects of their therapy. One particular group warranting close evaluation are survivors of bone tumours because of the nature of treatment, which often includes intensive chemotherapy, surgery and, at times, radiation therapy. As reviewed by Langeveld, several studies have already highlighted problems encountered by adult survivors of paediatric bone tumours (Langeveld et al, 2002). Late effects relating to chemotherapy and irradiation (e.g. infertility, cardiomyopathy) are varied and have been the subject of extensive research (Bhatia et al, 2003). Late effects relating to surgical local control of paediatric lower extremity bone tumours have been less thoroughly explored (Nagarajan et al, 2002). Local control procedures include amputation and limb-sparing procedures (e.g. the use of radiation, arthrodesis, endoprosthesis, allograft bone, etc.). The decision to perform a limb-sparing surgery is based on the ability to achieve an equivalent oncologic result and a comparable or better functional result compared to amputation (Yaw, 1999).
Amputation was standard treatment prior to the development of radiological and surgical techniques that have made limb-sparing surgery the more frequent treatment choice (Provisor et al, 1997; Bacci et al, 1998; Bielack et al, 2002). The preference for limb-sparing surgery to treat paediatric lower extremity bone tumours had been the subject of debate and warranted careful evaluation in the past. The issues surrounding the debate have been succinctly described in four questions (Simon, 1991): (1) will survival be the same? (2) how do the immediate and delayed morbidities (complications) compare? (3) how does function compare? and (4) does limb-sparing surgery impart improved psychosocial/quality of life outcomes? Regarding the first question, survival and local recurrence rates between amputation and limb-sparing surgery have not been found to be significantly different when adequate margins are achieved and adjuvant chemotherapy is used (Simon et al, 1986; Sluga et al, 1999; Bacci et al, 2000; Bielack et al, 2002). When considering short- and long-term complications, several studies have shown that there are more complications following limb-sparing surgery (Ruggieri et al, 1993; Rougraff et al, 1994; Lindner et al, 1999; Nagarajan et al, 2002). Of note, with new techniques and materials being developed for limb-sparig surgeries, long-term outcomes need to be continually evaluated.
In contrast, the last two questions have not been extensively studied, which is the basis for the current investigations. Overall, a trend toward an improvement of function has been reported for limb-sparing approaches. No differences in quality of life have thus far been shown between amputation and limb-sparing operative procedures. However, it is important to note that despite a number of authors addressing these outcomes, the overall conclusions have limitations because of the differing methodologic approaches and assessment tools used, small sample sizes, short follow-up, and limited study of adult survivors of childhood cancer (Nagarajan et al, 2002). The most common instrument that has been used for functional assessment has been the Musculoskeletal Tumour Society survey, which relies on the subjective ratings given by the administering clinician with no other objective measure of function. This has been questioned as to whether this is an accurate reflection of function (Marchese et al, 2004) and whether other more global functional assessments with QOL measures (TESS and QOL-CS) are needed to better represent the clinical status.
Children with lower extremely bone tumours have unique characteristics with implications for functional and quality-of-life outcomes. The emotional maturity of patients at the time of diagnosis can influence their ability to accept the loss of limb due to cancer (Kagan, 1976; Ettinger and Heiney, 1993; Felder-Puig et al, 1998). Skeletal maturity is another important determinant of functional and QOL outcomes in children with lower extremity bone sarcomas because of its importance in determining the type of local surgical control (amputation/rotationplasty/expanding prosthesis) and the associated risk of complications. Those who are skeletally immature and have substantial growth potential at diagnosis are often treated with limb-sparing surgery, which requires removal of a skeletal growth plate. Such patients often need multiple subsequent surgeries to accommodate growth of the unaffected limb. Moreover, children have a substantial lifespan ahead of them following successful treatment of their malignancy and this may increase the potential risk of further reconstructive procedures and complications.
With the establishment of the CCSS to facilitate the investigation of late effects among long-term survivors of childhood cancer, we were able to examine some of these key issues. The current study represents the largest series of adult survivors of paediatric lower extremity bone tumours thus far evaluated for function and QOL. However, some limitations must be considered in the interpretation of the results of this study. These include the inability to further classify the procedures that nonamputees (e.g. allograft vs. endoprosthesis) and amputees (above the knee vs below the knee amputation) received and the fact that this study provides information regarding treatments performed between 1970 and 1986, which are likely quite different from today's surgical treatments. However, this study does provide an excellent assessment of the outcomes of amputees, which would be difficult to examine today given the infrequency with which amputations are performed for paediatric lower extremity bone tumours. An additional shortcoming of the study relates to the length of time from diagnosis (median 21 years) to current assessment, which precludes the examination of outcomes within 10 years of diagnosis. It is in this time frame that differences between groups may be substantial and may be masked or lost by longer follow-up. Other issues involved the assessment of subsequent surgeries or complications. Since the assessment of surgeries was restricted to the initial treating institution and complications were not specifically noted, the accurate enumeration of subsequent surgeries and complications is not possible. Further limitation of the study includes ascertainment bias since the cohort includes only those who have agreed to participate in the CCSS. This may underestimate deficits by excluding those who are having more difficulty adjusting and are unable or unwilling to participate.
We found no major differences in function and quality of life between those who had an amputation and those treated with limb-sparing surgery. Additionally, our assessment of the large population of patients who underwent amputation provides clear indication that amputees do well long-term. Thus, when an amputation is clinically indicated, patients, families and clincians can assume that long-term amputees have no different function and QOL compared to those without an amputation. One may attribute the lack of reported differences between amputees and nonamputees to ‘adjustment and accommodation’ to their current condition, rather than having comparable physical ability such as range of motion and strength. To determine actual physical ability, via clinical examination, in a cohort as large as this and as far from diagnosis would be very difficult to accomplish. Additionally, one may argue that ‘actual’ physical ability is only a component of overall perceived physical function, which is influenced by other individualised factors including self-image, motivation and social support (Neugebauer, 2000).
Females reported significantly lower function and QOL, but did not report more disability. Survivors with a lower educational attainment also appeared to have lower function and QOL scores and a significantly increased likelihood of self-reported disability. In the author's prior study of psychosocial outcomes (Nagarajan et al, 2003) in this group of survivors, female survivors were less likely to be employed and those with a higher educational attainment were more likely to ever been employed or married or ever have insurance. Female gender-related deficiencies may be related to differences in coping styles (Znajda et al, 1999) and other gender-specific issues and the benefit of higher educational attainment may suggest more available opportunities or better social support. In the current study, those who were younger in age at completion of the questionnaire were less likely to have lower TESS and QOL-CS scores and significantly less likely to consider themselves disabled. This is most likely due to issues related to normal aging.
Further follow-up of this cohort, including reassessment to observe any changes in scores and perceived disability over time, is clearly warranted. It will also be important to prospectively investigate post-1986 populations of paediatric lower extremity bone tumour survivors in order to see how current surgical techniques affect function and quality of life. These prospective studies must encompass long-term assessments and integrate uniform methods of recording complications associated with the surgical procedures, as well as uniform collection of function and QOL data. Such an approach will aid paediatric oncologists and orthopaedic surgeons by providing insight into disease control, functional outcomes, and quality of life. We hope that this research study will help provide patients and families with needed information on anticipated long-term outcomes and on how to maximise function and quality of life as these paediatric patients grow into adulthood.
Acknowledgments
This work was supported by National Institutes of Health Grants U24-CA55727 and T32-CA09607, Bethesda, MD, USA; Children's Cancer Research Fund, Minneapolis, MN, USA; National Childhood Cancer Foundation Research Fellowship, Arcadia, CA, USA and American Society of Clinical Oncology Young Investigator Award, Alexandria, VA, USA.
Appendix
CCSS Institutions and Investigators
Table A1.
| University of California, San Francisco, CA | Arthur Ablin, MD* |
| University of Alabama, Birmingham, AL | Roger Berkow, MD* |
| International Epidemiology Institute, Rockville, MD | John Boice, ScD‡ |
| University of Washington, Seattle, WA | Norman Breslow, PhD‡ |
| UT-Southwestern Medican Center at Dallas, TX | George R, Buchanan, MD*, Kevin Oeffinger. MD‡ |
| Cincinnati Children's Hospital Medical Center | Stella Davies, MD, PhD‡ |
| Dana-Farber Cancer Institute, Boston, MA | Lisa Diller, MD*, Holcombe Grier, MD†, Frederick Li MD‡ |
| Texas Children's Center, Houston, TX | Zoann Dreyer, MD* |
| Children's Hospital and Medical Center, Seattle, WA | Debra Friedman, MD, MPH*, Thomas Pendergrass, MD† |
| Reswell Park Cancer Institute, Buffalo, NY | Daniel M Green, MD*,‡ |
| Hospital for Sick Children, Toronto, NY | Mark Greenberg, MB ChB* |
| St. Louis Children ‘s Hospital, MO | Robert Hayashi, MD*, Teresa Vietti, MD† |
| St. Jude Children's Research Hospital, Memphis, TN | Melissa Hudson MD*,‡ |
| University of Michigan, Ann Arbor, MI | Raymond Hutchinson MD* |
| Stanford University School of Medicine, Stanford, CA | Michael P Link, MD*, Sarah S Donaldson, MD‡ |
| Emory University, Atlanta, GA | Lillian Meacham, MD* |
| Children's Hospital of Philadelphia PA | Anna Meadows, MD*,‡ |
| Children's Hospital, Oklahoma City, OK | John Mulvihill MD‡ |
| Children's Hospital, Denver, CO | Brain Greffe*, Lorrie Odom, MD† |
| Children's Health Care-Minneapolis, MN | Maura O'Leary, MD* |
| Columbus Children's Hospital. OH | Amanda Termuhlen MD*, Frederick Ruymann, MD†, Stephen Qualman, MD‡ |
| Children's National Medical Center. Washington, DC | Gregory Reaman, MD*, Roger Packer, MD‡ |
| Children's Hospital of Pittsburgh, PA | A Kim Ritchey, MD*, Julie Blatt MD† |
| University of Minnesota. Minneapolis. MN | Leslie L. Robison PhD*,‡, Ann Mertens, PhD‡, Joseph Neglia, MD, MPH‡, Mark Nesbit, MD‡, |
| Children's Hospital Los Angeles, CA | Kathy Ruccione, RN, MPH* |
| Memorial Sloan-Kettering Cancer Center New York | Charles Sklar, MD*,‡ |
| National Cancer Institute, Bethesda, MD | Malcolm Smith, MD‡, Peter Inskip, ScD‡ |
| Mayo Clinic, Rochester, MN | W. Anthony Smithson, MD*, Gerald Gilchrist, MD† |
| UTMD Anderson Cancer Center, Houston, TX | Louise Strong, MD*,‡, Marilyn Stovall, PhD‡ |
| Riley Hospital for Children, Indianapolis IN | Terry A, Vik, MD*,‡, Robert Weetman, MD‡ |
| Fred Hutchinson Cancer Center, Seattle. WA | Yutaka Yasui, PhD*,‡, John Potter, MD., PhD*,‡ |
| University of California-Los Angeles. CA | Lonnie Zeltzer, MD*,‡ |
Institutional Principal Investigator
Former Institutional Principal Investigator
Member CCSS Steering Committee
References
- Aboulafia A, Malawer M (1993) Surgical management of pelvic and extremity osteosarcoma. Cancer 71(Suppl): 3358–3366 [DOI] [PubMed] [Google Scholar]
- Arndt CA, Crist WM (1999) Common musculoskeletal tumors of childhood and adolescence. N Engl J Med 341: 342–352 [DOI] [PubMed] [Google Scholar]
- Bacci G, Ferrari S, Bertoni F, Rimondini S, Longhi A, Bacchini P, Farni C, Manfrini M, Donati D, Picci P (2000) Prognostic factors in non-metastatic Ewing's sarcoma of bone treated with adjuvant chemotherapy: analysis of 359 patients at the Instituto Orthopedico Rizzoli. J Clin Oncol 18: 4–11 [DOI] [PubMed] [Google Scholar]
- Bacci G, Ferrai S, Mercuri M, Longhi A, Capanna R, Tienghi A, Brach del Prever A, Comandone A, Cesari M, Bernini G, Picci P (1998) Neoadjuvant chemotherapy for extremity osteosarcoma – preliminary results of the Rizzoli's 4th study. Acta Oncol 37: 41–48 [DOI] [PubMed] [Google Scholar]
- Bhatia S, Landier W, Robison L (2003) Late effects of childhood cancer therapy. In Progress in Oncology 2002 De Vita V, Hellman S, Rosenberg S (eds) pp 171–201. Sudbury, MA: Jone and Barlett Publications [Google Scholar]
- Bielack SS, Kempf-Bielack B, Delling G, Exner GU, Flege S, Helmke K, Kotz R, Salzner-Kuntschik M, Werner M, Winkelmann W, Zoubek A, Jurgens H, Winkler K (2002) Prognostic factors in high-grade osteosarcoma of the extremities or trunk: an analysis of 1702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol 20: 776–790 [DOI] [PubMed] [Google Scholar]
- Brindis C, Irwin C, Millstein S (1992) United States profile. In Textbook of Adolescent Medicine McAarnaey E, Kreipe R, Orr D, Comerci G (eds) pp 12–27. Philadelphia, PA: W.B. Saunders [Google Scholar]
- Burgert EO, Nesbit ME, Garnsey LA, Gehan EA, Herrmann J, Vietti TJ, Cangir A, Tefft M, Evans R, Thomas P, Askin FB, Kissane JM, Pritchard DJ, Neff J, Markley JT, Gilula L (1990) Multimodal therapy for the management of nonpelvic, localized Ewing's sarcoma of bone: intergroup study IESS-II. J Clin Oncol 8: 1514–1524 [DOI] [PubMed] [Google Scholar]
- Cheng E, Gebhardt M (1991) Allograft reconstruction of the shoulder after bone tumor resection. Orthop Clin North Am 22: 37–48 [PubMed] [Google Scholar]
- Dahlin DC, Coventry MH, Scalon P (1961) Ewing's sarcoma. J Bone Joint Surg Am 43: 185–192 [PubMed] [Google Scholar]
- Davis AM, Devlin M, Griffin AM, Wunder JS, Bell RS (1999) Functional outcome in amputation versus limb sparing of patients with lower extremity sarcoma: a matched case–control study. Arch, Phys Med Rehabil 80: 615–618 [DOI] [PubMed] [Google Scholar]
- Davis AM, Wright JG, Williams JI, Bombardier C, Griffin A, Bell RS (1996) Development of a measure of physical function for patients with bone and soft tissue sarcoma. Qual Life Res 5: 508–516 [DOI] [PubMed] [Google Scholar]
- Ettinger R, Heiney S (1993) Cancer in adolescents and young adults. Cancer 71(Suppl): 3276–3280 [DOI] [PubMed] [Google Scholar]
- Felder-Puig R, Formann AK, Mildner A, Bretschneider W, Bucher B, Windhager R, Zoubek A, Puig S, Topf R (1998) Quality of life and psychosocial adjustment of young patients after treatment of bone cancer. Cancer 83: 69–75 [DOI] [PubMed] [Google Scholar]
- Ferrel BR, Hassay Dow K, Grant M (1995a) Measurements of the quality of life in cancer survivors. Qual Life Res 4: 523–531 [DOI] [PubMed] [Google Scholar]
- Ferrel BR, Hassay Dow K, Leigh S, Ly, J. Gulasekaram P (1995b) Quality of life in long-term cancer survivors. Oncol Nurs Forum 22: 915–922 [PubMed] [Google Scholar]
- Friedman MA, Carter SK (1972) The therapy of osteogenic sarcoma: current status and thoughts for the future. J Surg Oncol 4: 482–510 [DOI] [PubMed] [Google Scholar]
- Kagan L (1976) Use of denial in adolescents with bone cancer. Health Soc Work 1: 70–87 [DOI] [PubMed] [Google Scholar]
- Kreipe R (1992) Normal somatic adolescent growth and development. In Textbook of Adolescent Medicine McAarnaey E, Kreipe R, Orr D, Comerci G (eds) pp 44–67. Philadelphia, PA: W.B. Saunders [Google Scholar]
- Langeveld NE, Stam H, Grootenhuis MA, Last BF (2002) Quality of life in young adult survivors of childhood cancer. Support Care Cancer 10: 579–600 [DOI] [PubMed] [Google Scholar]
- Lindner NJ, Ramm O, Hillmann A, Roedl R, Gosheger G, Brinkschmidt C, Juergens H, Winkelmann W (1999) Limb salvage and outcome of osteosarcoma. The University of Muenster experience. Clin Orthop 358: 83–89 [DOI] [PubMed] [Google Scholar]
- Link MP, Goorin AL, Miser AW, Green AA, Pratt CB, Belasco JB, Pritchard J, Malpas JS, Baker AR, Kirkpatrick JA, Ayala AG, Shuster JJ, Abelson HT, Simone JV, Vietti TJ (1986) The effect of adjuvant chemotherapy on relapse-free survival in patients with osteosracoma of the extremity. N Engl J Med 314: 1600–1606 [DOI] [PubMed] [Google Scholar]
- Marchese V, Ogle S, Womer R, Dormans J, Ginsberg JP (2004) An examination of outcome measures to assess functional mobility in childhood survivors of osteosarcoma. Pediatr Blood Cancer 42: 41–45 [DOI] [PubMed] [Google Scholar]
- Nagarajan R, Neglia JP, Clohisy DR, Robison LL (2002) Limb salvage and amputation in survivors of pediatric lower-extremity bone tumors: what are the long-term implications? J Clin Oncol 20: 4493–4501 [DOI] [PubMed] [Google Scholar]
- Nagarajan R, Neglia JP, Clohisy DR, Yasui Y, Greenberg M, Hudson M, Zevon MA, Tersak JM, Ablin A, Robison LL (2003) Education, employment, insurance and marital status among 694 survivors of pediatric lower extremity bone tumors. Cancer 97: 2554–2564 [DOI] [PubMed] [Google Scholar]
- Nesbit ME, Gehan EA, Burgert EO, Vietti TJ, Cangir A, Tefft M, Evans R, Thomas P, Askin FB, Kissane JM, Pritchard DJ, Herrmann J, Neff J, Makley JT, Gilula L (1990) Multimodal therapy for the management of primary, nonmetastaitic Ewing's sarcoma of bone: a long term follow up of the first intergroup study. J Clin Oncol 8: 1664–1674 [DOI] [PubMed] [Google Scholar]
- Neugebauer A (2000) The experience of chronic illness and physical disability: consequences for quality of life and social relationships. In Psychology pp 146. Berkeley CA: University of California [Google Scholar]
- Provisor AJ, Ettinger LJ, Nachman JB, Krailo MD, Makley JT, Yunis EJ, Huvos AG, Betcher DL, Baum ES, Kisker CT, Miser JS (1997) Treatment of nonmetastatic osteosarcoma of the extremity with preoperative and postoperative chemotherapy: a report from the Children's Cancer Group. J Clin Oncol 15: 76–84 [DOI] [PubMed] [Google Scholar]
- Ries L, Smith M, Gurney J, Linet M, Tamra T, Young J, Bunin G (1999) Cancer incidence and survival among children and adolescents: United States SEER Program 1975–1995. In National Cancer Institute, SEER Program. Vol. NIH Publication No.99–4649. Bethesda, MD: NIH [Google Scholar]
- Robison L, Mertens A, Boice JD, Breslow NE, Donaldson SS, Green D, Li FP, Meadows AT, Mulvihill JJ, Neglia JP, Nesbit ME, Packer RJ, Potter JD, Sklar C, Malcolm AS, Stovall M, Strong CS, Yasui Y, Zeltzer LK (2002) Study design and cohort characteristics of the childhood cancer survivor study: a multi institutional collaborative project. Med Pediatr Oncol 38: 229–239 [DOI] [PubMed] [Google Scholar]
- Rosen G, Marcove RC, Caparros B, Nirenberg A, Kosloff C, Huvos AG (1979) Primary osteogenic sarcoma: the rationale for preoperative chemotherapy and delayed surgery. Cancer 43: 2163–2177 [DOI] [PubMed] [Google Scholar]
- Rougraff BT, Simon MA, Kneisl JS, Greenberg DB, Mankin HJ (1994) Limb salvage compared with amputation for osteosarcoma of the distal end of the femur. A long-term oncological, functional, and quality-of-life study. J Bone Joint Surg Am 76: 649–656 [DOI] [PubMed] [Google Scholar]
- Ruggieri P, De Cristofaro R, Picci P, Bacci G, Biagini R, Casadei R, Ferraro A, Ferruzzi A, Fabbri N, Cazzola A, Campanacci M (1993) Complications and surgical indications in 144 cases of nonmetastatic osteosarcoma of the extremities treated with neoadjuvant chemotherapy. Clin Orthop 295: 226–238 [PubMed] [Google Scholar]
- Simon MA (1991) Limb salvage for osteosarcoma in the 1980s. Clin Orthop 270: 264–270 [PubMed] [Google Scholar]
- Simon MA, Aschliman MA, Thomas N, Mankin HJ (1986) Limb-salvage treatment versus amputation for osteosarcoma of the distal end of the femur. J Bone Joint Surg Am 68: 1331–1337 [PubMed] [Google Scholar]
- Sluga M, Windhager R, Lang S, Heinzl H, Bielack S, Kotz R (1999) Local and systemic control after ablative and limb sparing surgery in patients with osteosarcoma. Clin Orthop 358: 120–127 [PubMed] [Google Scholar]
- Springfield DS (1991) Introduction to limb-salvage surgery for sarcomas. Orthop Clin North Am 22: 1–5 [PubMed] [Google Scholar]
- Wang CC, Schulz MD (1953) A study of fifty cases treated at Massachusetts general hospital 1930–1952 inclusive. N Engl J Med 248: 571–576 [DOI] [PubMed] [Google Scholar]
- Yaw KM (1999) Pediatric bone tumors. Semin Surg Oncol 16: 173–183 [DOI] [PubMed] [Google Scholar]
- Znajda T, Wunder J Bell R, Davis A (1999) Gender issues in patients with extremity soft-sarcoma: a pilot study. Cancer Nurs 22: 111–118 [DOI] [PubMed] [Google Scholar]