Abstract
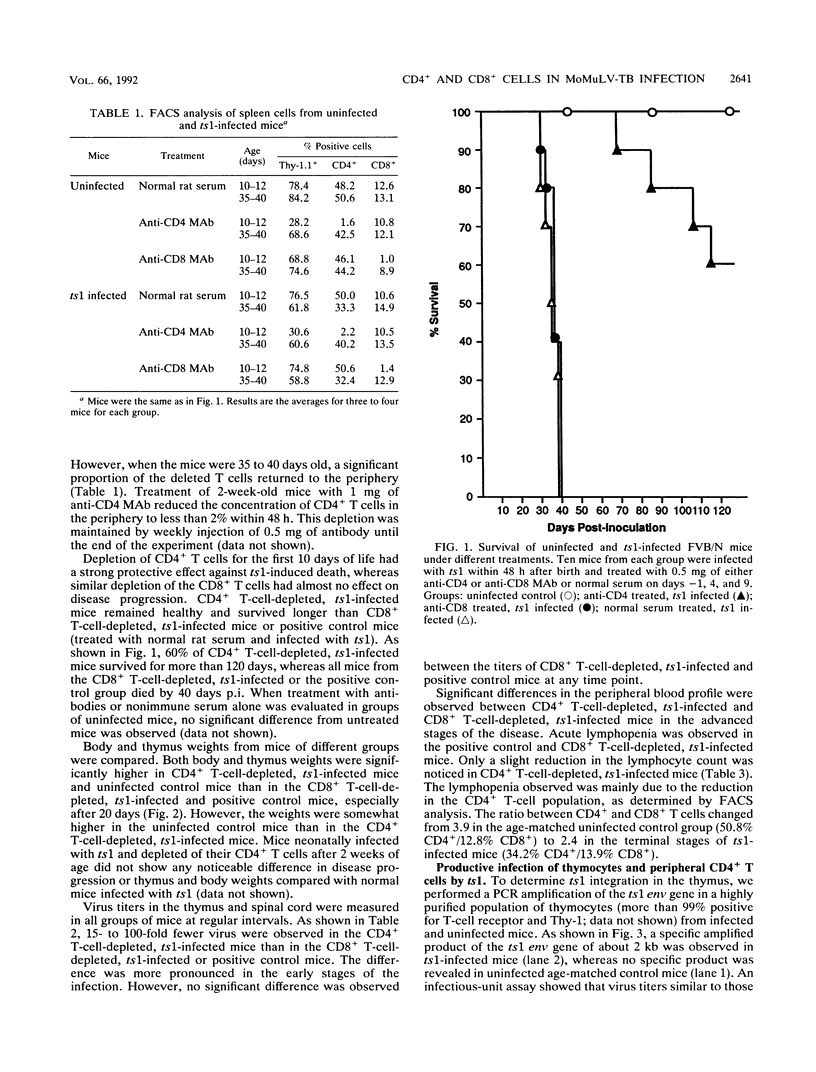
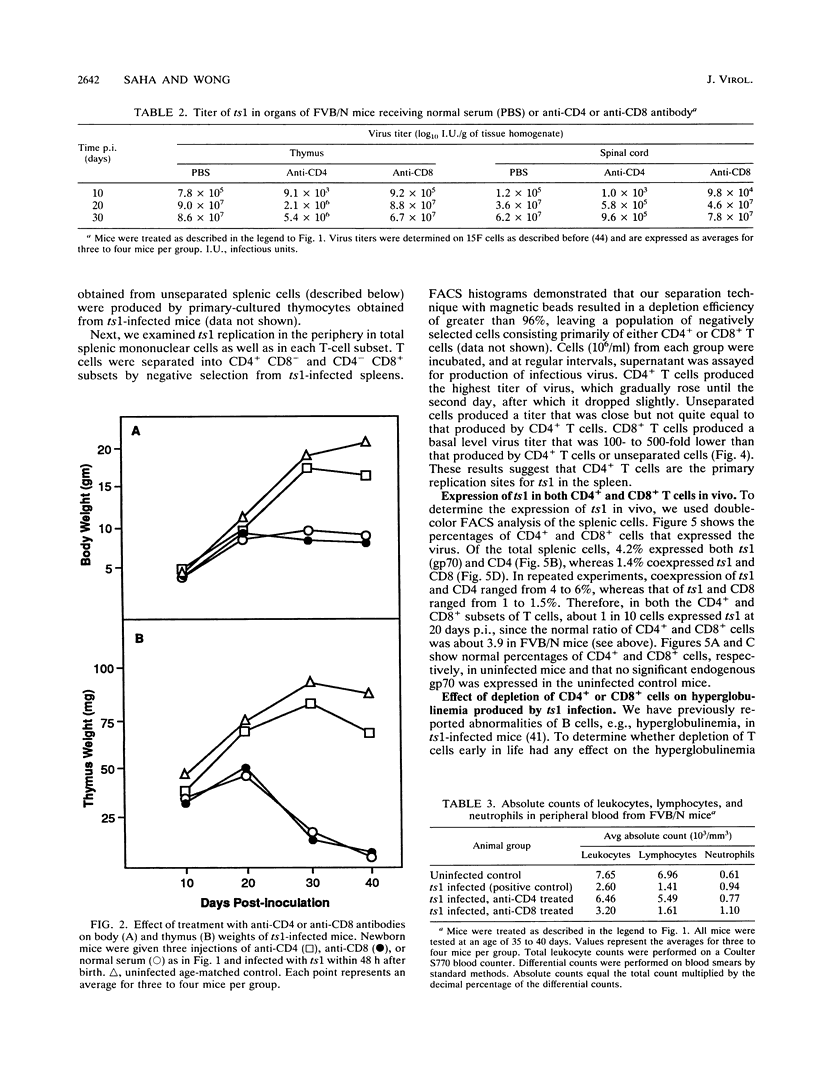
When neonatal FVB/N mice were inoculated with ts1, a temperature-sensitive mutant of Moloney murine leukemia virus TB, they developed a progressive bilateral hindlimb paralysis and immunodeficiency leading to death 4 to 6 weeks after inoculation. T lymphocytes have been shown to be primarily responsible for this ts1-induced syndrome. Here we compare the role played by each subset of T lymphocytes, i.e., CD4+ and CD8+ T cells, in disease development. Mice were depleted of a specific subset for the first 10 days of their lives by using either anti-CD4 or anti-CD8 monoclonal antibodies in vivo. Disease development in these mice was then monitored. Depletion of CD4+ T cells significantly attenuated the ts1-induced syndrome: virus replication was decreased, disease latency was extended, and death was prevented in 60% of the mice. Similar treatment with anti-CD8 antibody had almost no effect on disease progression. However, when depletion was begun 2 weeks after neonatal ts1 inoculation, CD4+ T cell depletion did not affect disease development. ts1 infected CD4+ and CD8+ T lymphocytes equally well in vivo, as shown by flow cytometric analysis, but virus replication was restricted primarily to the CD4+ subset of T cells, as found by in vitro assay. Hence, CD4+ T lymphocytes play an important role in the development of ts1-induced paralysis and immunodeficiency. The mechanism of this CD4+ T-cell-mediated disease production by ts1 is not clear; however, increased replication of ts1 in the CD4+ T cells, especially in the early stages of the disease, seems to play a crucial role.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed R., King C. C., Oldstone M. B. Virus-lymphocyte interaction: T cells of the helper subset are infected with lymphocytic choriomeningitis virus during persistent infection in vivo. J Virol. 1987 May;61(5):1571–1576. doi: 10.1128/jvi.61.5.1571-1576.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BALL J. K., HUH T. Y., MCCARTER J. A. ON THE STATISTICAL DISTRIBUTION OF EPIDERMAL PAPILLOMATA IN MICE. Br J Cancer. 1964 Mar;18:120–123. doi: 10.1038/bjc.1964.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blay R., Simpson K., Leslie K., Huber S. Coxsackievirus-induced disease. CD4+ cells initiate both myocarditis and pancreatitis in DBA/2 mice. Am J Pathol. 1989 Nov;135(5):899–907. [PMC free article] [PubMed] [Google Scholar]
- Bookman M. A., Swerdlow R., Matis L. A. Adoptive chemoimmunotherapy of murine leukemia with helper T lymphocyte clones. J Immunol. 1987 Nov 1;139(9):3166–3170. [PubMed] [Google Scholar]
- Borrow P., Tishon A., Oldstone M. B. Infection of lymphocytes by a virus that aborts cytotoxic T lymphocyte activity and establishes persistent infection. J Exp Med. 1991 Jul 1;174(1):203–212. doi: 10.1084/jem.174.1.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell A. E., Slater J. S., Futch W. S. Murine cytomegalovirus-induced suppression of antigen-specific cytotoxic T lymphocyte maturation. Virology. 1989 Nov;173(1):268–275. doi: 10.1016/0042-6822(89)90243-2. [DOI] [PubMed] [Google Scholar]
- Cantor H., Boyse E. A. Functional subclasses of T-lymphocytes bearing different Ly antigens. I. The generation of functionally distinct T-cell subclasses is a differentiative process independent of antigen. J Exp Med. 1975 Jun 1;141(6):1376–1389. doi: 10.1084/jem.141.6.1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christadoss P., Dauphinee M. J. Immunotherapy for myasthenia gravis: a murine model. J Immunol. 1986 Apr 1;136(7):2437–2440. [PubMed] [Google Scholar]
- Crocker P. R., Jefferies W. A., Clark S. J., Chung L. P., Gordon S. Species heterogeneity in macrophage expression of the CD4 antigen. J Exp Med. 1987 Aug 1;166(2):613–618. doi: 10.1084/jem.166.2.613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dialynas D. P., Wilde D. B., Marrack P., Pierres A., Wall K. A., Havran W., Otten G., Loken M. R., Pierres M., Kappler J. Characterization of the murine antigenic determinant, designated L3T4a, recognized by monoclonal antibody GK1.5: expression of L3T4a by functional T cell clones appears to correlate primarily with class II MHC antigen-reactivity. Immunol Rev. 1983;74:29–56. doi: 10.1111/j.1600-065x.1983.tb01083.x. [DOI] [PubMed] [Google Scholar]
- Harabuchi Y., Koizumi S., Osato T., Yamanaka N., Kataura A. Flow cytometric analysis of Epstein-Barr virus receptor among the different B-cell subpopulations using simultaneous two-color immunofluorescence. Virology. 1988 Jul;165(1):278–281. doi: 10.1016/0042-6822(88)90683-6. [DOI] [PubMed] [Google Scholar]
- Hom R. C., Finberg R. W., Mullaney S., Ruprecht R. M. Protective cellular retroviral immunity requires both CD4+ and CD8+ immune T cells. J Virol. 1991 Jan;65(1):220–224. doi: 10.1128/jvi.65.1.220-224.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferies W. A., Green J. R., Williams A. F. Authentic T helper CD4 (W3/25) antigen on rat peritoneal macrophages. J Exp Med. 1985 Jul 1;162(1):117–127. doi: 10.1084/jem.162.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolicoeur P. Murine acquired immunodeficiency syndrome (MAIDS): an animal model to study the AIDS pathogenesis. FASEB J. 1991 Jul;5(10):2398–2405. doi: 10.1096/fasebj.5.10.2065888. [DOI] [PubMed] [Google Scholar]
- Kamps C. A., Lin Y. C., Wong P. K. Oligomerization and transport of the envelope protein of Moloney murine leukemia virus-TB and of ts1, a neurovirulent temperature-sensitive mutant of MoMuLV-TB. Virology. 1991 Oct;184(2):687–694. doi: 10.1016/0042-6822(91)90438-h. [DOI] [PubMed] [Google Scholar]
- Koga Y., Sasaki M., Nakamura K., Kimura G., Nomoto K. Intracellular distribution of the envelope glycoprotein of human immunodeficiency virus and its role in the production of cytopathic effect in CD4+ and CD4- human cell lines. J Virol. 1990 Oct;64(10):4661–4671. doi: 10.1128/jvi.64.10.4661-4671.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korostoff J. M., Nakada M. T., Faas S. J., Blank K. J., Gaulton G. N. Neonatal exposure to thymotropic gross murine leukemia virus induces virus-specific immunologic nonresponsiveness. J Exp Med. 1990 Dec 1;172(6):1765–1775. doi: 10.1084/jem.172.6.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane H. C., Fauci A. S. Immunologic abnormalities in the acquired immunodeficiency syndrome. Annu Rev Immunol. 1985;3:477–500. doi: 10.1146/annurev.iy.03.040185.002401. [DOI] [PubMed] [Google Scholar]
- Leclerc J. C., Cantor H. T cell-mediated immunity to oncornavirus-induced tumors. II. Ability of different T cell sets to prevent tumor growth in vivo. J Immunol. 1980 Feb;124(2):851–854. [PubMed] [Google Scholar]
- Lukacher A. E., Braciale V. L., Braciale T. J. In vivo effector function of influenza virus-specific cytotoxic T lymphocyte clones is highly specific. J Exp Med. 1984 Sep 1;160(3):814–826. doi: 10.1084/jem.160.3.814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald H. R., Hengartner H., Pedrazzini T. Intrathymic deletion of self-reactive cells prevented by neonatal anti-CD4 antibody treatment. Nature. 1988 Sep 8;335(6186):174–176. doi: 10.1038/335174a0. [DOI] [PubMed] [Google Scholar]
- McChesney M. B., Fujinami R. S., Lampert P. W., Oldstone M. B. Viruses disrupt functions of human lymphocytes. II. Measles virus suppresses antibody production by acting on B lymphocytes. J Exp Med. 1986 May 1;163(5):1331–1336. [PMC free article] [PubMed] [Google Scholar]
- Mosier D. E., Yetter R. A., Morse H. C., 3rd Functional T lymphocytes are required for a murine retrovirus-induced immunodeficiency disease (MAIDS). J Exp Med. 1987 Jun 1;165(6):1737–1742. doi: 10.1084/jem.165.6.1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldstone M. B., Salvato M., Tishon A., Lewicki H. Virus-lymphocyte interactions. III. Biologic parameters of a virus variant that fails to generate CTL and establishes persistent infection in immunocompetent hosts. Virology. 1988 Jun;164(2):507–516. doi: 10.1016/0042-6822(88)90565-x. [DOI] [PubMed] [Google Scholar]
- Poss M. L., Quackenbush S. L., Mullins J. I., Hoover E. A. Characterization and significance of delayed processing of the feline leukemia virus FeLV-FAIDS envelope glycoprotein. J Virol. 1990 Sep;64(9):4338–4345. doi: 10.1128/jvi.64.9.4338-4345.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad G., Stoica G., Wong P. K. The role of the thymus in the pathogenesis of hind-limb paralysis induced by ts1, a mutant of Moloney murine leukemia virus-TB. Virology. 1989 Apr;169(2):332–340. doi: 10.1016/0042-6822(89)90158-x. [DOI] [PubMed] [Google Scholar]
- Ranges G. E., Sriram S., Cooper S. M. Prevention of type II collagen-induced arthritis by in vivo treatment with anti-L3T4. J Exp Med. 1985 Sep 1;162(3):1105–1110. doi: 10.1084/jem.162.3.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice G. P., Schrier R. D., Oldstone M. B. Cytomegalovirus infects human lymphocytes and monocytes: virus expression is restricted to immediate-early gene products. Proc Natl Acad Sci U S A. 1984 Oct;81(19):6134–6138. doi: 10.1073/pnas.81.19.6134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg Z. F., Fauci A. S. Immunopathogenesis of HIV infection. FASEB J. 1991 Jul;5(10):2382–2390. doi: 10.1096/fasebj.5.10.1676689. [DOI] [PubMed] [Google Scholar]
- Saha K., Wong P. K. T, not B, lymphocytes are required for immunodeficiency and paralysis induced by ts1, a mutant of Moloney murine leukemia virus-TB. Virology. 1991 Aug;183(2):815–820. doi: 10.1016/0042-6822(91)91017-b. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Salvato M., Borrow P., Shimomaye E., Oldstone M. B. Molecular basis of viral persistence: a single amino acid change in the glycoprotein of lymphocytic choriomeningitis virus is associated with suppression of the antiviral cytotoxic T-lymphocyte response and establishment of persistence. J Virol. 1991 Apr;65(4):1863–1869. doi: 10.1128/jvi.65.4.1863-1869.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart S. J., Fujimoto J., Levy R. Human T lymphocytes and monocytes bear the same Leu-3(T4) antigen. J Immunol. 1986 May 15;136(10):3773–3778. [PubMed] [Google Scholar]
- Szurek P. F., Floyd E., Yuen P. H., Wong P. K. Site-directed mutagenesis of the codon for Ile-25 in gPr80env alters the neurovirulence of ts1, a mutant of Moloney murine leukemia virus TB. J Virol. 1990 Nov;64(11):5241–5249. doi: 10.1128/jvi.64.11.5241-5249.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szurek P. F., Yuen P. H., Ball J. K., Wong P. K. A Val-25-to-Ile substitution in the envelope precursor polyprotein, gPr80env, is responsible for the temperature sensitivity, inefficient processing of gPr80env, and neurovirulence of ts1, a mutant of Moloney murine leukemia virus TB. J Virol. 1990 Feb;64(2):467–475. doi: 10.1128/jvi.64.2.467-475.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Topham D. J., Adesina A., Shenoy M., Craighead J. E., Sriram S. Indirect role of T cells in development of polioencephalitis and encephalomyelitis induced by encephalomyocarditis virus. J Virol. 1991 Jun;65(6):3238–3245. doi: 10.1128/jvi.65.6.3238-3245.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl S. M., Allen J. B., McCartney-Francis N., Morganti-Kossmann M. C., Kossmann T., Ellingsworth L., Mai U. E., Mergenhagen S. E., Orenstein J. M. Macrophage- and astrocyte-derived transforming growth factor beta as a mediator of central nervous system dysfunction in acquired immune deficiency syndrome. J Exp Med. 1991 Apr 1;173(4):981–991. doi: 10.1084/jem.173.4.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldor M. K., Sriram S., Hardy R., Herzenberg L. A., Herzenberg L. A., Lanier L., Lim M., Steinman L. Reversal of experimental allergic encephalomyelitis with monoclonal antibody to a T-cell subset marker. Science. 1985 Jan 25;227(4685):415–417. doi: 10.1126/science.3155574. [DOI] [PubMed] [Google Scholar]
- Wofsy D., Mayes D. C., Woodcock J., Seaman W. E. Inhibition of humoral immunity in vivo by monoclonal antibody to L3T4: studies with soluble antigens in intact mice. J Immunol. 1985 Sep;135(3):1698–1701. [PubMed] [Google Scholar]
- Wofsy D., Seaman W. E. Successful treatment of autoimmunity in NZB/NZW F1 mice with monoclonal antibody to L3T4. J Exp Med. 1985 Feb 1;161(2):378–391. doi: 10.1084/jem.161.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong P. K., Floyd E., Szurek P. F. High susceptibility of FVB/N mice to the paralytic disease induced by ts1, a mutant of Moloney murine leukemia virus TB. Virology. 1991 Jan;180(1):365–371. doi: 10.1016/0042-6822(91)90041-9. [DOI] [PubMed] [Google Scholar]
- Wong P. K. Moloney murine leukemia virus temperature-sensitive mutants: a model for retrovirus-induced neurologic disorders. Curr Top Microbiol Immunol. 1990;160:29–60. doi: 10.1007/978-3-642-75267-4_3. [DOI] [PubMed] [Google Scholar]
- Wong P. K., Prasad G., Hansen J., Yuen P. H. ts1, a mutant of Moloney murine leukemia virus-TB, causes both immunodeficiency and neurologic disorders in BALB/c mice. Virology. 1989 Jun;170(2):450–459. doi: 10.1016/0042-6822(89)90436-4. [DOI] [PubMed] [Google Scholar]
- Wong P. K., Soong M. M., Yuen P. H. Replication of murine leukemia virus in heterologous cells: interaction between ecotropic and xenotropic viruses. Virology. 1981 Mar;109(2):366–378. doi: 10.1016/0042-6822(81)90507-9. [DOI] [PubMed] [Google Scholar]
- Wong P. K., Szurek P. F., Floyd E., Saha K., Brooks B. R. Alteration from T- to B-cell tropism reduces thymic atrophy and cytocidal effects in thymocytes but not neurovirulence induced by ts1, a mutant of Moloney murine leukemia virus TB. Proc Natl Acad Sci U S A. 1991 Oct 15;88(20):8991–8995. doi: 10.1073/pnas.88.20.8991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L., Scollay R., Egerton M., Pearse M., Spangrude G. J., Shortman K. CD4 expressed on earliest T-lineage precursor cells in the adult murine thymus. Nature. 1991 Jan 3;349(6304):71–74. doi: 10.1038/349071a0. [DOI] [PubMed] [Google Scholar]
- Yetter R. A., Buller R. M., Lee J. S., Elkins K. L., Mosier D. E., Fredrickson T. N., Morse H. C., 3rd CD4+ T cells are required for development of a murine retrovirus-induced immunodeficiency syndrome (MAIDS). J Exp Med. 1988 Aug 1;168(2):623–635. doi: 10.1084/jem.168.2.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y., Kamps C. A., Yuen P. H., Wong P. K. Construction and characterization of expression systems for the env gene of ts1, a mutant of Moloney murine leukemia virus-TB. Virus Res. 1991 Mar;19(1):83–92. doi: 10.1016/0168-1702(91)90096-e. [DOI] [PubMed] [Google Scholar]
- Yuen P. H., Malehorn D., Nau C., Soong M. M., Wong P. K. Molecular cloning of two paralytogenic, temperature-sensitive mutants, ts1 and ts7, and the parental wild-type Moloney murine leukemia virus. J Virol. 1985 Apr;54(1):178–185. doi: 10.1128/jvi.54.1.178-185.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinkernagel R. M., Doherty P. C. MHC-restricted cytotoxic T cells: studies on the biological role of polymorphic major transplantation antigens determining T-cell restriction-specificity, function, and responsiveness. Adv Immunol. 1979;27:51–177. doi: 10.1016/s0065-2776(08)60262-x. [DOI] [PubMed] [Google Scholar]