Abstract
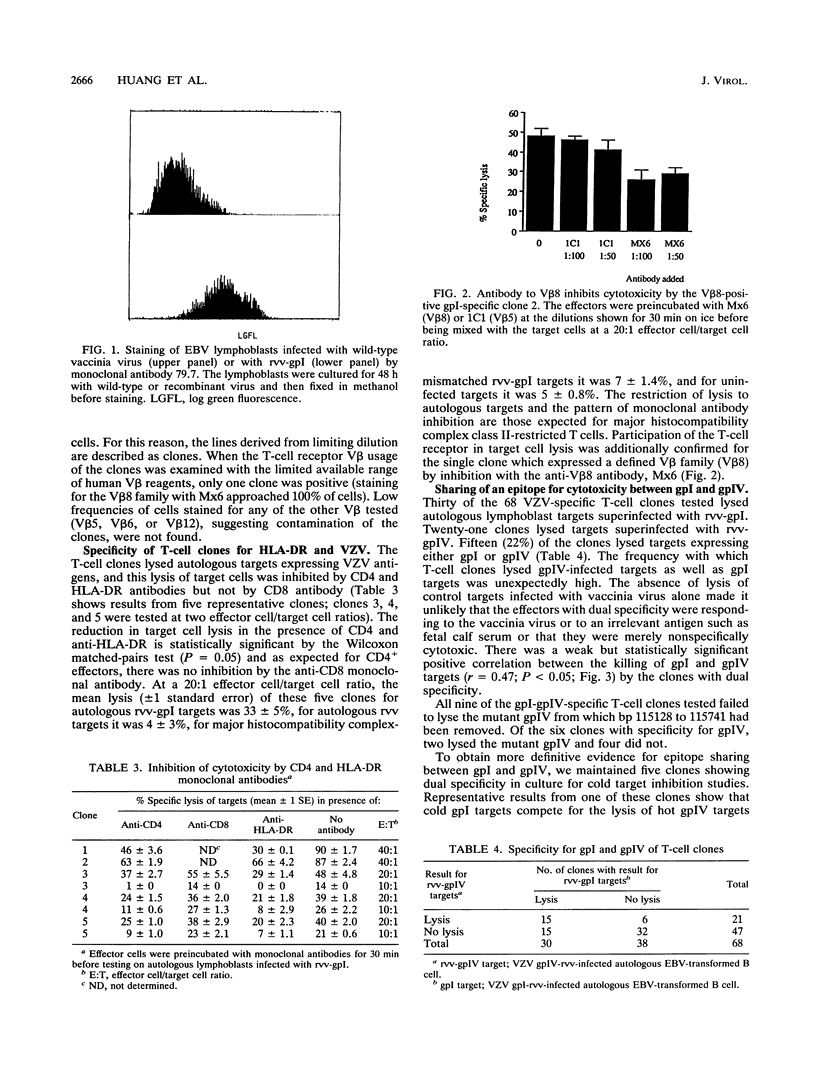
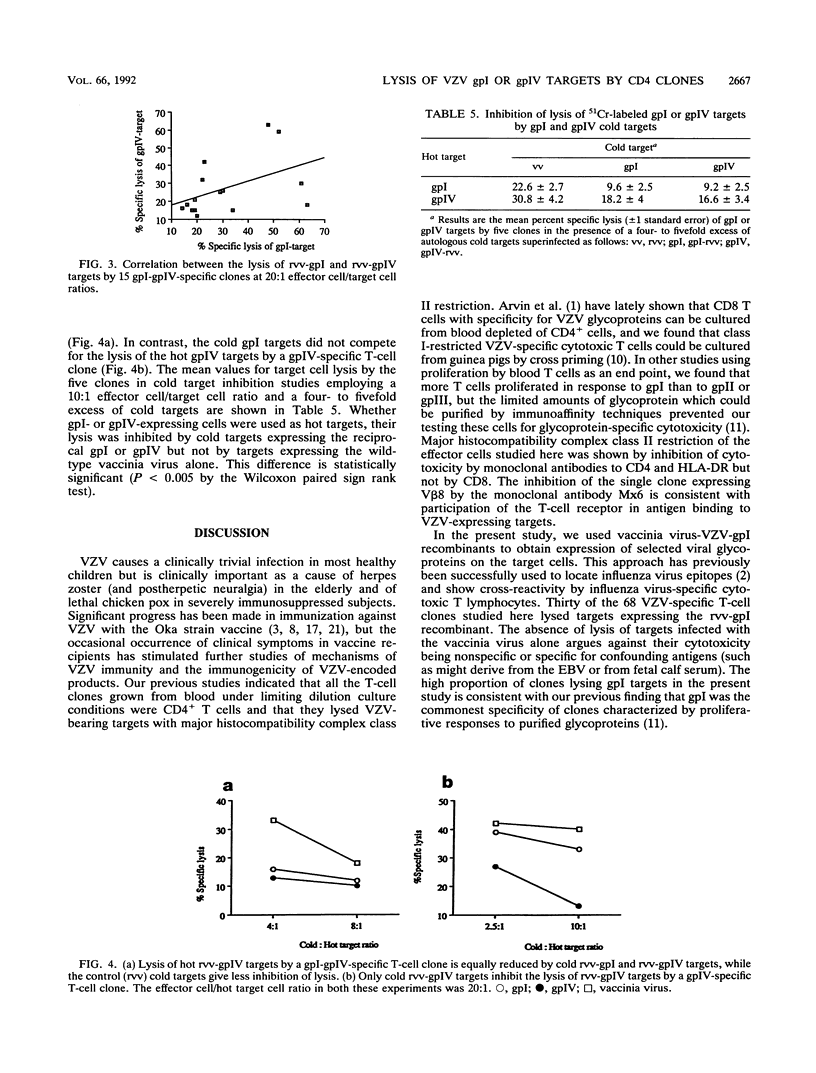
Varicella-zoster virus (VZV)-specific CD4-positive T cells are known to lyse targets expressing VZV antigen, but little is known of the glycoprotein specificity or phenotype of these cells. To test the ability of T cells to distinguish between gpI and gpIV (which share an antibody-defined epitope), we prepared clones from blood from four healthy individuals by limiting dilution. Among 68 T-cell clones from four donors which were VZV specific in tests of proliferation, 30 lysed autologous Epstein-Barr virus-transformed lymphoblasts which had been superinfected with a recombinant vaccinia virus which included the whole VZV gpI sequence. These clones were characterized as major histocompatibility complex class II restricted by inhibition of their cytotoxicity with HLA-DR and CD4 monoclonal antibodies. Twenty-one clones lysed targets expressing gpIV. Fifteen of these clones lysed targets expressing gpI and gpIV. Four clones with gpI-gpIV specificity were examined in detail, and their dual specificity was confirmed by cold target inhibition. These four clones failed to kill target cells infected with a mutant gpIV recombinant vaccinia virus from which amino acid residues 212 to 354 had been deleted. This region includes one of the two gpIV decapeptides which have 50% homology with amino acids 111 to 121 of gpI. Our data confirm that T-cell-receptor-associated structures are required for specific lysis of VZV targets and indicate that (i) gpI-specific CD4 cytotoxic T cells outnumber gpIV-specific T cells in blood and (ii) 50% of gpI-specific T-cell clones also lyse gpIV-expressing targets.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arvin A. M., Sharp M., Smith S., Koropchak C. M., Diaz P. S., Kinchington P., Ruyechan W., Hay J. Equivalent recognition of a varicella-zoster virus immediate early protein (IE62) and glycoprotein I by cytotoxic T lymphocytes of either CD4+ or CD8+ phenotype. J Immunol. 1991 Jan 1;146(1):257–264. [PubMed] [Google Scholar]
- Bennink J. R., Yewdell J. W., Smith G. L., Moss B. Recognition of cloned influenza virus hemagglutinin gene products by cytotoxic T lymphocytes. J Virol. 1986 Mar;57(3):786–791. doi: 10.1128/jvi.57.3.786-791.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergen R. E., Diaz P. S., Arvin A. M. The immunogenicity of the Oka/Merck varicella vaccine in relation to infectious varicella-zoster virus and relative viral antigen content. J Infect Dis. 1990 Nov;162(5):1049–1054. doi: 10.1093/infdis/162.5.1049. [DOI] [PubMed] [Google Scholar]
- Cabirac G., Gilden D., Wellish M., Vafai A. Expression of varicella-zoster virus glycoprotein I in cells infected with a vaccinia virus recombinant. Virus Res. 1988 May;10(2-3):205–213. doi: 10.1016/0168-1702(88)90016-0. [DOI] [PubMed] [Google Scholar]
- Chilmonczyk B. A., Levin M. J., McDuffy R., Hayward A. R. Characterization of the human newborn response to herpesvirus antigen. J Immunol. 1985 Jun;134(6):4184–4188. [PubMed] [Google Scholar]
- Cooper E. C., Vujcic L. K., Quinnan G. V., Jr Varicella-zoster virus-specific HLA-restricted cytotoxicity of normal immune adult lymphocytes after in vitro stimulation. J Infect Dis. 1988 Oct;158(4):780–788. doi: 10.1093/infdis/158.4.780. [DOI] [PubMed] [Google Scholar]
- Davison A. J., Waters D. J., Edson C. M. Identification of the products of a varicella-zoster virus glycoprotein gene. J Gen Virol. 1985 Oct;66(Pt 10):2237–2242. doi: 10.1099/0022-1317-66-10-2237. [DOI] [PubMed] [Google Scholar]
- Diaz P. S., Smith S., Hunter E., Arvin A. M. T lymphocyte cytotoxicity with natural varicella-zoster virus infection and after immunization with live attenuated varicella vaccine. J Immunol. 1989 Jan 15;142(2):636–641. [PubMed] [Google Scholar]
- Hayward A. R., Burger R., Scheper R., Arvin A. M. Major histocompatibility complex restriction of T-cell responses to varicella-zoster virus in guinea pigs. J Virol. 1991 Mar;65(3):1491–1495. doi: 10.1128/jvi.65.3.1491-1495.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward A. R., Herberger M. Lymphocyte responses to varicella zoster virus in the elderly. J Clin Immunol. 1987 Mar;7(2):174–178. doi: 10.1007/BF00916011. [DOI] [PubMed] [Google Scholar]
- Hayward A. R., Pontesilli O., Herberger M., Laszlo M., Levin M. Specific lysis of varicella zoster virus-infected B lymphoblasts by human T cells. J Virol. 1986 Apr;58(1):179–184. doi: 10.1128/jvi.58.1.179-184.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward A. R. T-cell responses to predicted amphipathic peptides of varicella-zoster virus glycoproteins II and IV. J Virol. 1990 Feb;64(2):651–655. doi: 10.1128/jvi.64.2.651-655.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward A., Giller R., Levin M. Phenotype, cytotoxic, and helper functions of T cells from varicella zoster virus stimulated cultures of human lymphocytes. Viral Immunol. 1989 Fall;2(3):175–184. doi: 10.1089/vim.1989.2.175. [DOI] [PubMed] [Google Scholar]
- Keller P. M., Lonergan K., Neff B. J., Morton D. A., Ellis R. W. Purification of individual varicella-zoster virus (VZV) glycoproteins gpI, gpII, and gpIII and their use in ELISA for detection of VZV glycoprotein-specific antibodies. J Virol Methods. 1986 Sep;14(2):177–188. doi: 10.1016/0166-0934(86)90048-0. [DOI] [PubMed] [Google Scholar]
- Slørdahl S. H., Wiger D., Strømøy T., Degre M., Thørsby E., Lie S. O. Vaccination of children with malignant disease against varicella. Postgrad Med J. 1985;61 (Suppl 4):85–92. [PubMed] [Google Scholar]
- Vafai A., Jensen K., Kubo R. Existence of similar antigenic-sites on varicella-zoster virus gpI and gpIV. Virus Res. 1989 Aug;13(4):319–336. doi: 10.1016/0168-1702(89)90077-4. [DOI] [PubMed] [Google Scholar]
- Vafai A., Wroblewska Z., Mahalingam R., Cabirac G., Wellish M., Cisco M., Gilden D. Recognition of similar epitopes on varicella-zoster virus gpI and gpIV by monoclonal antibodies. J Virol. 1988 Aug;62(8):2544–2551. doi: 10.1128/jvi.62.8.2544-2551.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vafai A., Yang W. N. Neutralizing antibodies induced by recombinant vaccinia virus expressing varicella-zoster virus gpIV. J Virol. 1991 Oct;65(10):5593–5596. doi: 10.1128/jvi.65.10.5593-5596.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson B., Keller P. M., Ellis R. W., Starr S. E. Cell-mediated immune responses after immunization of healthy seronegative children with varicella vaccine: kinetics and specificity. J Infect Dis. 1990 Oct;162(4):794–799. doi: 10.1093/infdis/162.4.794. [DOI] [PubMed] [Google Scholar]
- Zaia J. A., Leary P. L., Levin M. J. Specificity of the blastogenic response of human mononuclear cells to herpesvirus antigens. Infect Immun. 1978 Jun;20(3):646–651. doi: 10.1128/iai.20.3.646-651.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]


