SUMMARY
Transgenic mice expressing hepatocyte growth factor (HGF) develop cutaneous melanocytic tumors following neonatal UV exposure. On the albino FVB background, tumors arise from the epidermal-dermal junction and exhibit radial growth phase pattern reminiscent of human melanoma. Here, we examined the histologic spectrum of UV-induced melanocytic tumors in HGF mice on a pigmented (C57BL/6 x C3H/HeN)F1 background. Neonatally-irradiated (4000 J/m2) mice were monitored for 43 weeks, and 31/34 (91%) animals developed a total of 163 melanocytic tumors. Of 54 primary tumors analyzed, most (49/54, 91%) demonstrated exclusively dermal collections of epithelioid cells with voluminous densely pigmented cytoplasm. Seven of these also demonstrated a population of spindled cells with mitoses. Several (3/54, 6%) tumors exhibited a junctional component with melanocytes present in the epidermis. Staining with PEP8 confirmed the presence of interfollicular melanocytes at the epidermal junction in neonatal skin. Thus in contrast to HGF animals on the FVB background, HGF animals on the pigmented (C57BL/6 x C3H/HeN)F1 background do not develop classic radial growth phase melanoma but rather predominantly develop dermal melanocytomas resembling the epithelioid blue nevi and pigmented epithelioid melanocytomas occasionally seen in humans. These results demonstrate the influence of genetic background on histologic pattern of UV-induced melanomas in mice.
INTRODUCTION
Approximately 62,000 cases of invasive malignant melanoma in the United States will be diagnosed in 2006, with 7,900 deaths attributable to this disease (Jemal et al., 2006). Environmental influences, particularly blistering sunburns in childhood, is a known risk factor for cutaneous melanoma, along with other constitutional factors (red hair, blue eyes), family history of melanoma, and the number of cutaneous melanocytic nevi (Tsao et al., 2004). In human skin, melanocytes are situated within the epidermis along the dermoepidermal junction, as well as within hair follicles. Melanocytic nevi and most melanomas arise from melanocytes situated within the epidermis. In the early stages, most melanomas are confined to the dermoepidermal junction and exhibit a radial growth phase pattern.
Mice are naturally resistant to development of melanoma, due in part to a lack of melanocytes within the interfollicular epidermis (Walker and Hayward, 2002). Nevertheless, various regimens including combinations of 7,12-dimethylbenz(a)anthracene (DMBA) and UV light (Epstein et al., 1967), DMBA and croton oil (Berkelhammer et al., 1982), DMBA/croton oil and UV (Romerdahl et al., 1989), and aloe emodin and UV (Strickland et al., 2000) result in the formation of pigmented cutaneous tumors. Most of these tumors, however, arose within the dermis and lacked the intraepithelial (radial) growth pattern commonly seen in human melanoma. Similar lesions occur in more recently described genetically engineered mice either expressing various oncogenes or deficient in tumor suppressor genes that confer increased susceptibility to melanoma. Examples include transgenic mice with melanocyte-specific expression of SV40 T antigen (Bradl et al., 1991; Penna et al., 1998), Ret receptor tyrosine kinase (Schmidt et al., 1999), and activated Hras (G12V) (Kramer et al., 1998) that spontaneously developed ocular (but not cutaneous) melanoma. In some of these, neonatal UV (Kelsall and Mintz, 1998) or DMBA treatment (Broome Powell et al., 1999) resulted in a low rate of cutaneous melanoma that metastasized. While mice deficient in both Ink4a and Arf do not develop melanomas (Serrano et al., 1996), knockins of Hras (G12V) (Chin et al., 1997) or Arf (Krimpenfort et al., 2001) on this genetic background did develop spontaneous melanoma. In contrast to the melanomas observed in these mouse models, those induced by neonatal UV exposure in albino FVB mice with transgenic expression of hepatocyte growth factor (HGF) were metastatic and displayed radial growth pattern, with large atypical epithelioid intraepidermal melanocytes with Pagetoid scatter of cells (Noonan et al., 2001) strikingly reminiscent of final common pathway human melanoma (Reed, 1985).
HGF signaling through the c-Met transmembrane receptor tyrosine kinase confers broad effects on cell motility, morphology, proliferation, and survival (Gentile and Comoglio, 2004). Mutational activation of c-Met via has been associated with several human carcinomas and acquisition of a metastatic phenotype (Park et al., 1999; Di Renzo et al., 2000), and autocrine loops involving HGF and c-Met have been reported in human sarcomas (Ferracini et al., 1995). Overexpression of HGF in normal human melanocytes results in hyperproliferation, but not malignant transformation (Hamoen et al., 2001). In human melanocytic lesions, c-Met expression has been detected in both benign and malignant melanocytic processes (Natali et al., 1993; Saitoh et al., 1994). Overexpression of c-Met is associated with melanoma progression and metastatic spread (Cruz et al., 2003), and thus may be an important prognostic indicator.
The highly pigmented dermal melanomas produced in most of the mouse models described above, with the exception of the HGF model, resemble the tumors found in equine melanotic disease (Levene, 1971) and have been denoted collectively by the term “animal-type melanoma” (Crowson et al., 1999). Histologically, these animal-type melanomas resemble two rare but distinct entities encountered in humans: epithelioid blue nevi (EBN) and pigmented epithelioid melanocytomas (PEM). While EBN were originally described in the setting of familial lentiginosis, cardiac myxoma, schwannomas and endocrine dysfunction (Carney syndrome) (Carney and Ferreiro, 1996), they may also occur sporadically. Although PEM are commonly (approximately 50% of cases) associated with ulceration and metastasis to localized lymph nodes – they generally have an indolent course and are rarely fatal (Zembowicz et al., 2004). With the exception of ulceration in PEM, these two entities are indistinguishable histologically; both are characterized by exclusively dermal collections of heavily pigmented epithelioid or spindled melanocytes with minimal mitotic activity that may penetrate along adnexal structures into the deep dermis and subcutaneous tissue (Zembowicz et al., 2004).
In this study, we examined the histologic spectrum of UV-induced melanocytic tumors in HGF mice on a pigmented (C57BL/6 x C3H)F1 background. We were surprised to find that unlike the tumors reported in HGF/FVB mice, these tumors were predominantly dermal melanocytomas resembling animal-type melanoma and EBN/PEM with limited metastatic potential. These findings illustrate a role for genetic background in determining the histologic patterns of UV-induced melanoma in mice.
MATERIALS AND METHODS
HGF mice
HGF transgenic (MH19) mice (Takayama et al., 1996) were obtained from Glenn Merlino (National Cancer Institute) and propagated as heterozygotes on a C57BL/6 background. The HGF mice (usually males) were mated with C3H/HeN animals obtained from Charles River Laboratories (Wilmington, MA). The resulting litters, on a (C57BL/6 x C3H/HeN)F1 background, were used for tumor induction experiments. Treatment of mice was carried out according to established guidelines and all procedures were approved by the IACUC of the University of Utah (#11002). Genotype was apparent by visual inspection, as HGF animals displayed darkly pigmented ears and tails compared to non-transgenic littermates that was evident by 1 week of age.
UV-induced tumors
Neonatal mice (1–2 days old) were UV-irradiated unrestrained in open cages with a single dose of 4,000 J/m2 (4 J/m2/s) using a bank of four fan-cooled unfiltered sun lamps (FS20T12-UVB, National Biological Corporation, Twinsburg, OH). The bulbs emit wavelengths between 250 and 420 nm (72.6% UVB, 27.4% UVA, 0.01% UVC), with peak emission at 313 nm, according to the manufacturer. Dosimetry was determined with a calibrated UVB-500C meter (National Biological Corporation). This level of UV exposure resulted in mild erythema and scaling, but not ulceration, of the skin. After irradiation, mice were returned to their cages and weaned at three weeks of age, then monitored for 40 weeks. Mice developing ulcerated tumors, or tumors ≥1 cm in diameter prior to 40 weeks were euthanized. All cutaneous lesions >2 mm in diameter at experimental endpoints were excised for pathologic analysis. A subset of animals with large tumors was subjected to necropsy and samples were taken of lung, liver, spleen, and regional lymph nodes.
Histologic and immunohistochemical examination
Tissues were fixed in formalin, embedded in paraffin, and 4-μm thick sections were applied to glass microscopic slides and stained with hematoxylin and eosin. In many cases, sections were bleached to remove melanin prior to H&E staining. For melanin bleaching, sections were deparaffinized, hydrated, and incubated in 0.25% potassium permanganate (Sigma) for 30 min. After washing in water, sections were treated with 5% oxalic acid (Sigma) for 2–5 min until sections appeared clear, then washed again with water.
Immunoperoxidase staining for melanocytes in neonatal skin using the PEP8 antibody was performed as described previously (Recio et al., 2002). For Fontana-Masson staining for melanin, sections were deparaffinized and hydrated in water, placed into a 2.5% silver nitrate solution, then subjected to microwaving on high power for one minute. After rinsing in water, slides were placed in 1% gold chloride for one minute, rinsed in water, then in 5% sodium thiosulfate for one minute. After rinsing with water, sections were counterstained with Nuclear Fast Red.
RESULTS
UV-induced melanocytic tumors
Melanocytes can be easily seen in the skin of adult mice carrying the HGF transgene on the pigmented (C57BL/6 x C3H/HeN)F1 background. Heavily pigmented cells populate the entire dermis including the dermal-epidermal junction (Figure 1A). A similar pattern is seen in mice that received a single UV exposure as neonates (Figure 1B). These cells are not seen in non-transgenic mice that underwent similar neonatal UV exposure (Figure 1C). Thirty-one of 34 (91%) HGF mice developed at least one melanocytic tumor following neonatal UV exposure. Of 29 control non-transgenic animals receiving similar UV treatment, none developed tumors. These tumors generally appeared to arise from within the dermis, as very early tumors demonstrated collections of pigmented cells clustered exclusively within the dermis (Figure 1D). We examined a total of 54 tumors >2 mm in diameter at experimental endpoints. Based on histologic features, the tumors could be segregated into four types: (1) dermal melanocytosis; (2) dermal melanocytosis with spindle cell component; (3) melanoma with junctional involvement; and (4) amelanotic tumor consistent with sarcoma (Table I).
Figure 1.
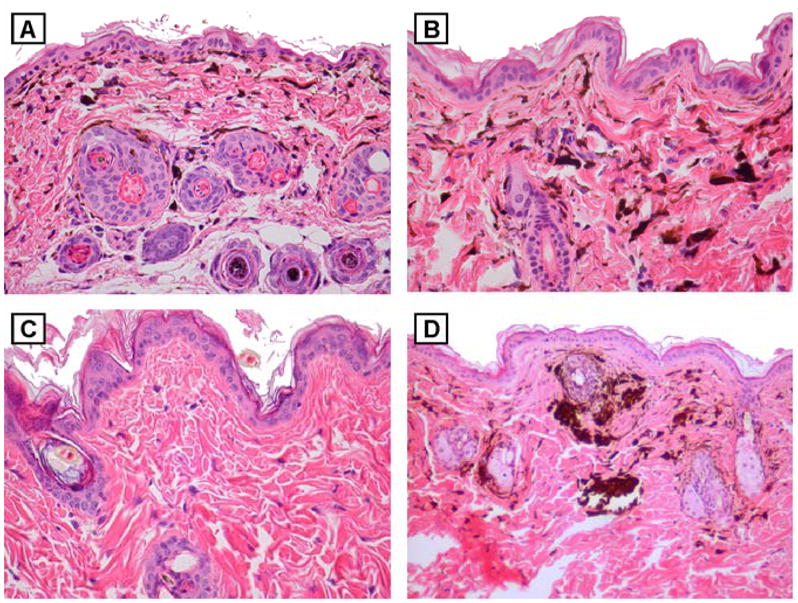
Dermal melanocytosis in skin and early UV-induced tumor from adult HGF (C57BL/6xC3H)F1 mice. H&E-stained sections of skin from (A) untreated and (B) UV-irradiated (neonatally) HGF (C57BL/6xC3H)F1 mouse, both showing dermal infiltration of pigmented cells. Original magnification, x200. (C) H&E-stained section of skin from UV-irradiated (neonatally) non-transgenic (C57BL/6xC3H)F1 mouse, lacking dermal pigmented cells. Original magnification, x200. (D) H&E-stained sections of an early developing tumor in UV-irradiated (neonatally) HGF (C57BL/6xC3H)F1 mouse, showing dermal aggregates of pigmented cells. Original magnification, x100.
Table I.
Summary of UV-induced tumors in HGF (C57BL/6xC3H/HeN)F1 mice
| Mice developing tumors | 31/34 (91%) |
| Total tumors | 163 |
| Tumors analyzed (>2 mm) | 54 |
| Dermal melanocytosis | 49/54 (91%) |
| Dermal melanocytosis with spindle-cells | 7/49 (13%) |
| Melanomas with junctional component | 3/54 (6%) |
| Sarcomas | 2/54 (4%) |
Dermal melanocytosis
Consistent with the dermal aggregates of pigmented cells seen in early tumors (Figure 1D), the vast majority (49/54, 91%) of pigmented cutaneous lesions examined demonstrated striking dermal melanocytosis (Figure 2A). These lesions were characterized by deposition of large epithelioid cells with abundant, densely pigmented cytoplasm that obscured an eccentrically placed nucleus (Figure 2C). These cells filled and expanded the dermis and often extended through the subcutis into the underlying skeletal muscle. The cells abutted the overlying epidermis and were generally separated from the epidermis by a narrow zone of collagen, though most of the tumors showed focal areas of pigment transfer to keratinocytes. Sections in which the melanin was bleached revealed large, histiocytic-appearing cells with uniform, bland nuclei with small and single eosinophilic nucleoli, and voluminous granular cytoplasm (Figure 2E). In seven of these tumors, rare, single heavily pigmented cells were observed in the epidermis (Figure 3A). Bleached sections from two of these seven tumors, however, failed to demonstrate cells with melanocyte morphology in the epidermis in the area seen by routine histology (not shown). The histologic pattern of these tumors was strikingly similar to a case of EBN (Figure 2B, D, F).
Figure 2.
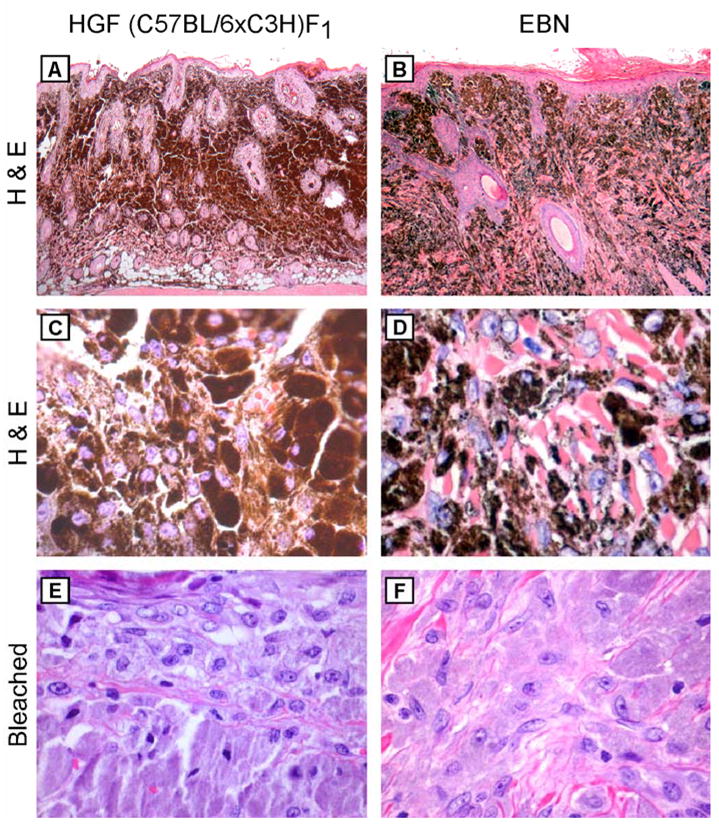
Striking similarity of UV-induced tumors in HGF (C57BL/6xC3H)F1 mice and EBN. Histology of a UV-induced dermal melanocytoma arising in a HGF (C57BL/6xC3H)F1 mouse (left panel) and an EBN excised from a 8 year-old child (right panel). (A, B) H&E-stained sections, showing dermal infiltration of pigmented cells. Original magnification, x40. (C, D) H&E-stained sections, showing highly pigmented tumor cells. Original magnification, x400. (E, F) Melanin-bleached sections, revealing large bland histiocytic-like cells. Original magnification, x400.
Figure 3.
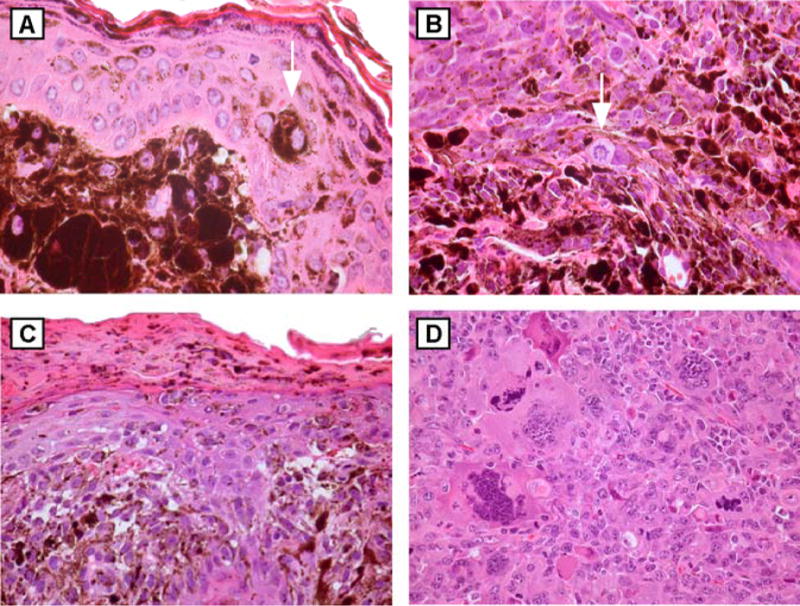
Histology of UV-induced tumors in HGF (C57BL/6xC3H)F1 mice. H&E-stained sections of tumors demonstrating (A) dermal melanocytosis, original magnification x400, with isolated epidermal melanocyte overlying tumor (arrow); (B) spindled tumor composed of epithelioid and pigmented cells, original magnification x400, with mitotic figures (arrow); (C) pigmented tumor with junctional involvement, original magnification x200. (D) amelanotic tumor consistent with sarcoma, original magnification x200.
Dermal melanocytosis with spindle cell component
Seven tumors were centered mainly within the deep dermis and subcutis, but tumor cells also expanded the papillary dermis and abutted the overlying epidermis. Rare, large pigmented cells were seen in the epidermis and variable pigment transfer to keratinocytes was seen, though none of these tumors demonstrated a radial growth phase component within the epidermis. The tumors were composed of an admixture of two distinct cell types: (1) large, epithelioid cells with abundant heavily pigmented cytoplasm identical to those described above; and (2) fascicles of spindled cells with vesicular nuclei and condensed chromatin with several small eosinophilic nucleoli and variable amounts of lightly pigmented cytoplasm (Figure 3B). Scattered mitotic figures were also identified. All of these tumors were seen in association with areas of dermal melanocytosis as described above. An epidermal melanocytic proliferation was not seen in these tumors.
Melanoma with junctional involvement
Three pigmented lesions demonstrated clear epidermal involvement. These lesions were similar to the pigmented malignant spindle cell tumors in that two cell types were present, as described above. These tumors showed clusters of spindled and lightly pigmented melanocytes within the overlying epidermis (Figure 3C). Two of these three lesions demonstrated epidermal ulceration. These tumors occupied the full thickness of the dermis and extended through the subcutis into the underlying muscle. Though an epidermal melanocytic population was present, these lesions did not resemble typical human malignant melanoma with a radial growth phase component (Reed, 1985), nor the UV-induced melanomas described (Noonan et al., 2001) in the HGF mouse on the FVB background.
Sarcoma
Finally, two ulcerated lesions demonstrated sheets of undifferentiated, amelanotic, cytologically malignant cells within the dermis and subcutis (Figure 3D). Areas of necrosis were evident. These tumors are consistent with the sarcomas that have been reported to arise occasionally in HGF transgenic mice (Takayama et al., 1996).
Metastasis
Necropsies were performed on 10 animals bearing large tumors. Lymph nodes from the draining basins of biopsied pigmented skin lesions were grossly pigmented and histologically demonstrated small clusters (< 20 cells) of pigmented epithelioid cells within the lymph node parenchyma and subcapsular sinuses. These cells resembled those seen in the tumors showing dermal melanosis. This pattern of lymphoid pigmentation was comparable to what has been reported in lymph node of untreated adult HGF (C57BL/6 x FVB)F1 mice (Takayama et al., 1996), and definitive metastatic foci were not appreciated. Visceral organs, including lung, liver, and spleen were grossly normal in appearance. In 9 of 10 animals, sections of lung tissue revealed focal perivascular collections of pigmented epithelioid cells similar to what was seen in the draining lymph nodes. Lung tissue from one animal demonstrated a metastatic focus composed of pigmented spindled cells. The remainder of the organs sampled was histologically normal. Thus these UV-induced tumors demonstrated limited capacity for metastatic spread.
Melanocyte distribution and density
A possible explanation for the predominant development of dermal melanocytomas in HGF (C57BL/6 x C3H)F1 mice could be altered neonatal melanocyte distribution that might preclude epidermal involvement of UV-induced tumors. We examined melanocytes in 2-day-old neonatal skin (corresponding to the time of UV irradiation) of these mice and non-transgenic littermates. The minimal pigmentation in neonatal skin made it difficult to discern melanocytes (Figure 4A, B), but melanocytes were easily visualized in sections stained with Fontana-Masson (Figure 4C, D) and PEP8 (Figure 4E, F). Skin of HGF (C57BL/6 x C3H)F1 mice demonstrated prominent staining in cells located in the epidermis and upper dermis as well as hair follicles (Figure 4C, E), similar to what has been reported previously in (C57BL/6 x FVB)F1 mice (Takayama et al., 1996), while that of non-transgenic mice revealed melanocytes only in the hair follicles (Figure 4D, F). Thus the predominance of dermal melanocytic tumors elicited in this model was not a result of ectopic neonatal melanocyte distribution or altered melanocyte density on the (C57BL/6 x C3H)F1 background.
Figure 4.
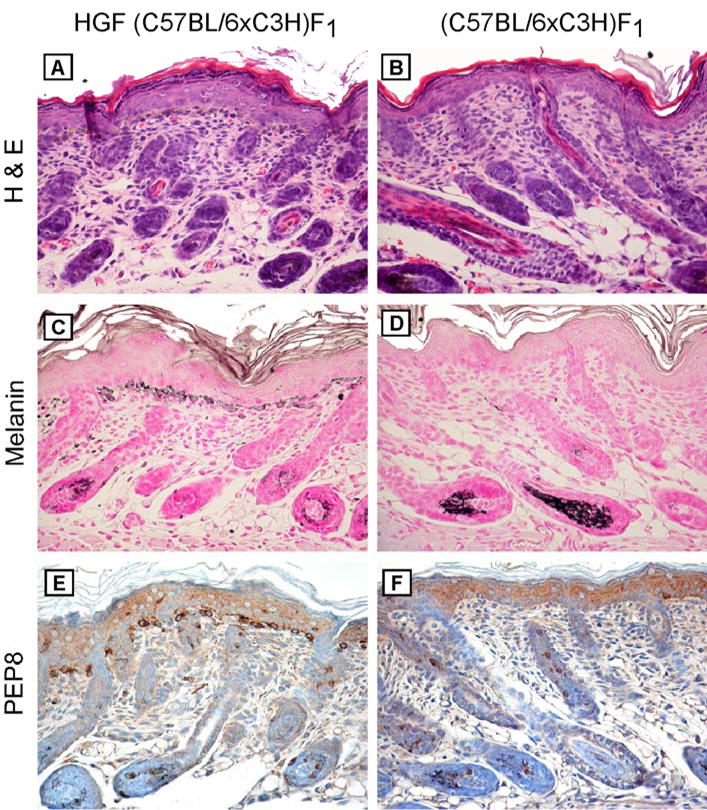
Melanocyte distribution in neonatal HGF (C57BL/6xC3H)F1 skin. Skin was harvested from 2-day-old HGF (C57BL/6xC3H)F1 (left panel) and (C57BL/6xC3H)F1 (right panel) mice and stained as indicated. (A, B) H&E-stained sections. Original magnification, x100. (C, D) Fontana-Masson. Original magnification, x100. (E, F) PEP8 staining of separate bleached sections. Original magnification, x100.
DISCUSSION
In this study, we examined the histologic patterns of UV-induced pigmented cutaneous lesions arising in transgenic HGF (C57BL/6 x C3H/HeN)F1 mice. The HGF mouse model of UV-induced melanoma has attracted considerable attention because in contrast to previously developed models, it represents the sole genetic mouse model that consistently produces lesions arising from the epidermal-dermal junction with a radial growth phase pattern similar to most human melanomas (Noonan et al., 2001; Noonan et al., 2003). We were surprised to find that UV-induced tumors in HGF (C57BL/6 x C3H/HeN)F1 mice did not recapitulate the histologic pattern seen in HGF/FVB mice (Noonan et al., 2001), but rather the vast majority (91%) of lesions were exclusively dermal tumors composed of heavily pigmented epithelioid melanocytes without a significant epidermal component. Though in several of these cases we did observe occasional epidermal pigmented cells, it is most likely that these represent epidermotropism associated with dermal tumors.
The histology of these dermal melanocytomas is strikingly similar to the animal-type melanoma (Crowson et al., 1999), EBN (Carney and Ferreiro, 1996), and PEM (Zembowicz et al., 2004), described in humans. These tumors represent a diagnostic and therapeutic dilemma for clinicians because they frequently spread to regional lymph nodes but generally do not metastasize systemically (Zembowicz et al., 2004). We have not examined UV-treated HGF (C57BL/6 x C3H/HeN)F1 mice for longer time periods (beyond 43 weeks) to assess fully the metastatic potential of these tumors. It is presently unclear why HGF (C57BL/6 x C3H/HeN)F1 mice develop this pattern of UV-induced melanocytic tumors, but they might serve as an animal model for studying PEM and EBN. The role of HGF signaling in the development and lymphatic spread of PEM and EBN has not been investigated.
Although we did observe several tumors with clear epidermal involvement, they were in the minority and did not resemble typical human malignant melanoma with a radial growth phase component (Reed, 1985), nor the UV-induced melanomas described (Noonan et al., 2001) in HGF/FVB mice. It is likely that the difference in histologic pattern of UV-induced tumors in HGF (C57BL/6 x C3H/HeN)F1 mice reported here and that in HGF/FVB mice reported previously (Noonan et al., 2001) relates to differences in genetic background. While earlier mouse models of melanoma were primarily developed using four strains: C57BL/6, C3H/HeN, BALB/C and FVB, there are no studies (to our knowledge) simultaneously evaluating a particular genetic model on more than one pure genetic background to assess background effects (Walker and Hayward, 2002). Initial studies with the Mt1-Ret mouse, which was generated on a mixed (BALB/C x C57BL/6) background, were carried out on separate sublines generated from single backcrosses with C57BL/6, BCF1 aguouti, and BALB/C strains (Iwamoto et al., 1991). Interestingly, the incidence of melanocytic tumors was reduced in progeny of backcrosses with BALB/C compared to those of backcrosses with C57BL/6, but there was no discernable difference in melanocyte populations in the non-tumorous skin of these animals (Iwamoto et al., 1991). In addition, there was no difference in tumor incidence between albino and non-albino progeny from backcrosses with BALB/C (Iwamoto et al., 1991). These results suggest that genetic factors other than pigmentation may playa role in this model of spontaneous melanomagenesis.
The role of pigmentation in the HGF model of UV-induced melanoma, as well as other genetic factors, has not been elucidated. Although very little melanin was apparent in the skin of HGF (C57BL/6 x C3H/HeN)F1 mice 2 days after birth (time point of UV irradiation), it is tempting nevertheless to speculate that the presence of melanin may affect melanomagenesis. Although melanocytes may be protected by endogenous melanin which can directly absorb UV-generated photons and oxygen radicals, melanin may also be oxidized and lead to the generation of reactive oxygen species (ROS) that can damage many cellular substrates including DNA (Riley, 1997). Melanocytes appear particularly sensitive to oxidative stress (Jimbow et al., 2001), possibly as a result of ROS generated in the process of melanin biosynthesis (Urabe et al., 1994). Differences in melanin composition (eumelanin and pheomelanin) might be important. Strain-related differences may also relate to HGF levels and signaling, which have not been evaluated in HGF mice on different backgrounds. It is possible that there could be strain-related cis- or trans-acting genetic factors that affect transcription of the HGF transgene. It is also unclear in this model exactly how constitutive HGF expression promotes melanomagenesis. Since HGF expression in this model is driven off a metallothionein I promoter (Takayama et al., 1996), we may expect HGF expression in additional cell types in the skin and stroma that may impact melanomagenesis. We do not know how UV-induced melanoma susceptibility or tumor phenotype would differ in an animal with melanocyte-specific expression of HGF. Many of these questions can hopefully be answered in future studies.
Acknowledgments
We thank Glenn Merlino, Frances Noonan, and Miriam Anver for the HGF mice, assistance with immunohistochemistry, and numerous helpful discussions. D.G. was supported by NIH grant AR050102 and the Huntsman Cancer Foundation, and S.R.F. was supported by NIH grant RR17525.
Abbreviations
- EBN
epithelioid blue nevi
- HGF
hepatocyte growth factor
- PEM
pigmented epithelioid melanocytomas
References
- Berkelhammer J, Oxenhandler RW, Hook RR, Jr, Hennessy JM. Development of a new melanoma model in C57BL/6 mice. Cancer Res. 1982;42:3157–3163. [PubMed] [Google Scholar]
- Bradl M, Klein-Szanto A, Porter S, Mintz B. Malignant melanoma in transgenic mice. Proc Natl Acad Sci U S A. 1991;88:164–168. doi: 10.1073/pnas.88.1.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broome Powell M, Gause PR, Hyman P, Gregus J, Lluria-Prevatt M, Nagle R, Bowden GT. Induction of melanoma in TPras transgenic mice. Carcinogenesis. 1999;20:1747–1453. doi: 10.1093/carcin/20.9.1747. [DOI] [PubMed] [Google Scholar]
- Carney JA, Ferreiro JA. The epithelioid blue nevus. A multicentric familial tumor with important associations, including cardiac myxoma and psammomatous melanotic schwannoma. Am J Surg Pathol. 1996;20:259–272. doi: 10.1097/00000478-199603000-00001. [DOI] [PubMed] [Google Scholar]
- Chin L, Pomerantz J, Polsky D, Jacobson M, Cohen C, Cordon-Cardo C, Horner JW, 2nd, DePinho RA. Cooperative effects of INK4a and ras in melanoma susceptibility in vivo. Genes Dev. 1997;11:2822–2834. doi: 10.1101/gad.11.21.2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowson AN, Magro CM, Mihm MC., Jr Malignant melanoma with prominent pigment synthesis: “animal type” melanoma--a clinical and histological study of six cases with a consideration of other melanocytic neoplasms with prominent pigment synthesis. Hum Pathol. 1999;30:543–550. doi: 10.1016/s0046-8177(99)90199-5. [DOI] [PubMed] [Google Scholar]
- Cruz J, Reis-Filho JS, Silva P, Lopes JM. Expression of c-met tyrosine kinase receptor is biologically and prognostically relevant for primary cutaneous malignant melanomas. Oncology. 2003;65:72–82. doi: 10.1159/000071207. [DOI] [PubMed] [Google Scholar]
- Di Renzo MF, Olivero M, Martone T, Maffe A, Maggiora P, Stefani AD, Valente G, Giordano S, Cortesina G, et al. Somatic mutations of the MET oncogene are selected during metastatic spread of human HNSC carcinomas. Oncogene. 2000;19:1547–1555. doi: 10.1038/sj.onc.1203455. [DOI] [PubMed] [Google Scholar]
- Epstein JH, Epstein WL, Nakai T. Production of melanomas from DMBA-induced “blue nevi” in hairless mice with ultraviolet light. J Natl Cancer Inst. 1967;38:19–30. [PubMed] [Google Scholar]
- Ferracini R, Di Renzo MF, Scotlandi K, Baldini N, Olivero M, Lollini P, Cremona O, Campanacci M, Comoglio PM. The Met/HGF receptor is over-expressed in human osteosarcomas and is activated by either a paracrine or an autocrine circuit. Oncogene. 1995;10:739–749. [PubMed] [Google Scholar]
- Gentile A, Comoglio PM. Invasive growth: a genetic program. Int J Dev Biol. 2004;48:451–456. doi: 10.1387/ijdb.041799ag. [DOI] [PubMed] [Google Scholar]
- Hamoen KE, Borel Rinkes IH, Morgan JR. Hepatocyte growth factor and melanoma: gene transfer studies in human melanocytes. Melanoma Res. 2001;11:89–97. doi: 10.1097/00008390-200104000-00002. [DOI] [PubMed] [Google Scholar]
- Iwamoto T, Takahashi M, Ito M, Hamatani K, Ohbayashi M, Wajjwalku W, Isobe K, Nakashima I. Aberrant melanogenesis and melanocytic tumour development in transgenic mice that carry a metallothionein/ret fusion gene. Embo J. 1991;10:3167–3175. doi: 10.1002/j.1460-2075.1991.tb04878.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, Thun MJ. Cancer statistics, 2006. CA Cancer J Clin. 2006;56:106–130. doi: 10.3322/canjclin.56.2.106. [DOI] [PubMed] [Google Scholar]
- Jimbow K, Chen H, Park JS, Thomas PD. Increased sensitivity of melanocytes to oxidative stress and abnormal expression of tyrosinase-related protein in vitiligo. Br J Dermatol. 2001;144:55–65. doi: 10.1046/j.1365-2133.2001.03952.x. [DOI] [PubMed] [Google Scholar]
- Kelsall SR, Mintz B. Metastatic cutaneous melanoma promoted by ultraviolet radiation in mice with transgene-initiated low melanoma susceptibility. Cancer Res. 1998;58:4061–4065. [PubMed] [Google Scholar]
- Kramer TR, Powell MB, Wilson MM, Salvatore J, Grossniklaus HE. Pigmented uveal tumours in a transgenic mouse model. Br J Ophthalmol. 1998;82:953–960. doi: 10.1136/bjo.82.8.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krimpenfort P, Quon KC, Mooi WJ, Loonstra A, Berns A. Loss of p16Ink4a confers susceptibility to metastatic melanoma in mice. Nature. 2001;413:83–86. doi: 10.1038/35092584. [DOI] [PubMed] [Google Scholar]
- Levene A. Equine melanotic disease. Tumori. 1971;57:133–168. doi: 10.1177/030089167105700303. [DOI] [PubMed] [Google Scholar]
- Natali PG, Nicotra MR, Di Renzo MF, Prat M, Bigotti A, Cavaliere R, Comoglio PM. Expression of the c-Met/HGF receptor in human melanocytic neoplasms: demonstration of the relationship to malignant melanoma tumour progression. Br J Cancer. 1993;68:746–750. doi: 10.1038/bjc.1993.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noonan FP, Dudek J, Merlino G, De Fabo EC. Animal models of melanoma: an HGF/SF transgenic mouse model may facilitate experimental access to UV initiating events. Pigment Cell Res. 2003;16:16–25. doi: 10.1034/j.1600-0749.2003.00014.x. [DOI] [PubMed] [Google Scholar]
- Noonan FP, Recio JA, Takayama H, Duray P, Anver MR, Rush WL, De Fabo EC, Merlino G. Neonatal sunburn and melanoma in mice. Nature. 2001;413:271–272. doi: 10.1038/35095108. [DOI] [PubMed] [Google Scholar]
- Park WS, Dong SM, Kim SY, Na EY, Shin MS, Pi JH, Kim BJ, Bae JH, Hong YK, et al. Somatic mutations in the kinase domain of the Met/hepatocyte growth factor receptor gene in childhood hepatocellular carcinomas. Cancer Res. 1999;59:307–310. [PubMed] [Google Scholar]
- Penna D, Schmidt A, Beermann F. Tumors of the retinal pigment epithelium metastasize to inguinal lymph nodes and spleen in tyrosinase-related protein 1/SV40 T antigen transgenic mice. Oncogene. 1998;17:2601–2607. doi: 10.1038/sj.onc.1202196. [DOI] [PubMed] [Google Scholar]
- Recio JA, Noonan FP, Takayama H, Anver MR, Duray P, Rush WL, Lindner G, De Fabo EC, DePinho RA, et al. Ink4a/arf deficiency promotes ultraviolet radiation-induced melanomagenesis. Cancer Res. 2002;62:6724–6730. [PubMed] [Google Scholar]
- Reed RJ. The histological variance of malignant melanoma: the interrelationship of histological subtype, neoplastic progression, and biological behaviour. Pathology. 1985;17:301–312. doi: 10.3109/00313028509063772. [DOI] [PubMed] [Google Scholar]
- Riley PA. Melanin. Int J Biochem Cell Biol. 1997;29:1235–1239. doi: 10.1016/s1357-2725(97)00013-7. [DOI] [PubMed] [Google Scholar]
- Romerdahl CA, Stephens LC, Bucana C, Kripke ML. The role of ultraviolet radiation in the induction of melanocytic skin tumors in inbred mice. Cancer Commun. 1989;1:209–216. [PubMed] [Google Scholar]
- Saitoh K, Takahashi H, Sawada N, Parsons PG. Detection of the c-met proto-oncogene product in normal skin and tumours of melanocytic origin. J Pathol. 1994;74:191–199. doi: 10.1002/path.1711740308. [DOI] [PubMed] [Google Scholar]
- Schmidt A, Tief K, Yavuzer U, Beermann F. Ectopic expression of RET results in microphthalmia and tumors in the retinal pigment epithelium. Int J Cancer. 1999;80:600–605. doi: 10.1002/(sici)1097-0215(19990209)80:4<600::aid-ijc19>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Serrano M, Lee H, Chin L, Cordon-Cardo C, Beach D, DePinho RA. Role of the INK4a locus in tumor suppression and cell mortality. Cell. 1996;85:27–37. doi: 10.1016/s0092-8674(00)81079-x. [DOI] [PubMed] [Google Scholar]
- Strickland FM, Muller HK, Stephens LC, Bucana CD, Donawho CK, Sun Y, Pelley RP. Induction of primary cutaneous melanomas in C3H mice by combined treatment with ultraviolet radiation, ethanol and aloe emodin. Photochem Photobiol. 2000;72:407–414. doi: 10.1562/0031-8655(2000)072<0407:iopcmi>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Takayama H, La Rochelle WJ, Anver M, Bockman DE, Merlino G. Scatter factor/hepatocyte growth factor as a regulator of skeletal muscle and neural crest development. Proc Natl Acad Sci U S A. 1996;93:5866–5871. doi: 10.1073/pnas.93.12.5866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao H, Atkins MB, Sober AJ. Management of cutaneous melanoma. N Engl J Med. 2004;351:998–1012. doi: 10.1056/NEJMra041245. [DOI] [PubMed] [Google Scholar]
- Urabe K, Aroca P, Tsukamoto K, Mascagna D, Palumbo A, Prota G, Hearing VJ. The inherent cytotoxicity of melanin precursors: a revision. Biochim Biophys Acta. 1994;1221:272–278. doi: 10.1016/0167-4889(94)90250-x. [DOI] [PubMed] [Google Scholar]
- Walker GJ, Hayward NK. Pathways to melanoma development: lessons from the mouse. J Invest Dermatol. 2002;119:783–792. doi: 10.1046/j.1523-1747.2002.00217.x. [DOI] [PubMed] [Google Scholar]
- Zembowicz A, Carney JA, Mihm MC. Pigmented epithelioid melanocytoma: a low-grade melanocytic tumor with metastatic potential indistinguishable from animal-type melanoma and epithelioid blue nevus. Am J Surg Pathol. 2004;28:31–40. doi: 10.1097/00000478-200401000-00002. [DOI] [PubMed] [Google Scholar]


