Abstract
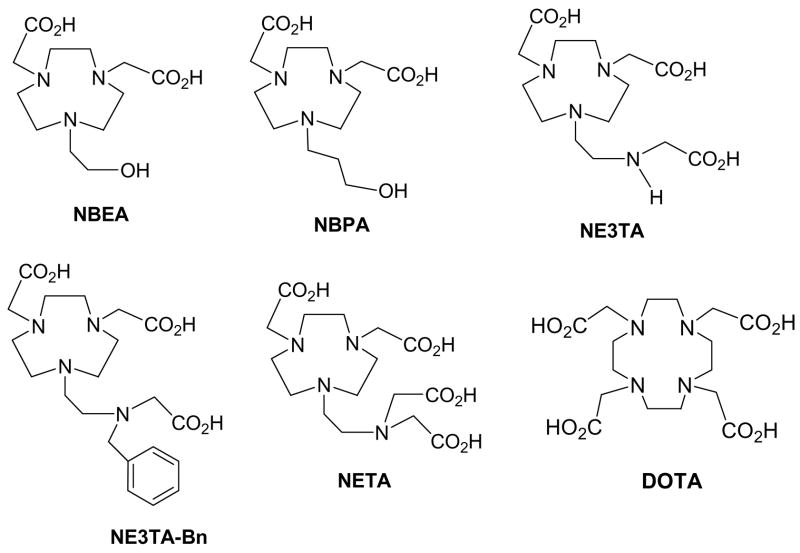
Novel ligands, NBEA, NBPA, NETA, NE3TA, and NE3TA-Bn were synthesized and evaluated as potential chelators of copper radioisotopes for use in targeted positron emission tomography (PET) imaging or radiation therapy. The new ligands were radiolabeled with 64Cu, and in vitro stability of the radiolabeled complexes was assessed in rat serum. Serum stability results suggest that among the ligands tested, NETA, NE3TA, and NE3TA-Bn form stable complexes with 64Cu.
Introduction
Among the available copper radioisotpes, 64 Cu (t1/2 = 12.7 h, Emaxβ+ = 656 keV; Emaxβ− is proven to be effective for use in positron emission tomography (PET) imaging and targeted radiation therapy applicable to many types of cancer.1–2 Bifunctional ligands that possess both binding moieties of Cu(II) and a functional group for conjugation to a targeting moiety are required for the modalities. Research efforts have been directed towards the development of optimal bifunctional ligands that can rapidly form stable complexes with the short-lived 64Cu while being conjugated to a targeting moiety, either peptide or antibody, to provides an efficient way of generating stable and safe copper radioisotope-labeled drugs for cancer therapy and imaging.3
Recently, we have developed a structurally novel ligand, NETA, and its analogues.4 NETA features a parent macrocyclic ligand with a flexible acyclic multidentate pendant arm. DOTA (and DTPA are the frequently explored chelators of biologically important metals for biomedical applications. In general, the acyclic ligands such as DTPA possess rapid complex formation kinetics, but weak binding to the metal, whereas the less dynamic macrocyclic ligands such as DOTA display strong binding to the metal, but with relatively slower complex formation kinetics. The idea of designing NETA was to integrate the advantage of both the macrocyclic and acyclic frameworks, i.e., both thermodynamic stability and favorable formation kinetics. The bimodal octadentate NETA was found to effectively bind the metallic radionuclides for targeted radiotherapy such as 90Y, 177Lu, and 205/6Bi.4–5 Given the capability of octadentate DOTA to tightly bind Cu(II) in vitro and in vivo,2 we wanted to explore complexation of NETA and NETA analogues, NE3TA and NE3TA-Bn with Cu(II). NE3TA contains four amines and three caroxylates as potential donor groups. NE3TA-Bn is a heptadentate ligand with a benzyl group which can be further modified for conjugation to a targeting moiety. Hexadentate NBEA and NBPA possess three amines, two carboxylates, and a hydroxyl group as the donor groups. Our hypothesis for the design of NBEA and NBPA was that the size-fit between the macrocyclic cavity in NBEA and NBPA and the ionic radius of Cu(II)6 might provide enhanced radioisotope complex stability while producing a neutral Cu(II) complex that would have an advantage of less protein interaction and a potentially more favorable in vivo tissue distribution. NBPA possesses a longer propylene bridge between one of the amino groups and the hydroxyl group compared to the analogous ethylene bridged ligand, NBEA.
The structures of the novel ligands, NETA, NE3TA, NE3TA-Bn, NBEA, and NBPA are shown in Scheme 1. These ligands are not functionalized for conjugation to a targeting peptide or antibody. Preparation of the non-functionalized parent ligands is generally relatively straightforward as compared to the bifunctional analogues wherein introduction of an element of asymmetry frequently complicates synthetic efforts. The generally valid assumption is that the bifunctionalization of the ligands usually makes little difference in their metal binding ability. In vitro serum stability is a useful and qualitative screening procedure for identifying unstable metal complexes of non-functionalized chelates by assessment of their potential to maintain stable complexes without dissociation of the metal ion in vitro.4a The radiolabeled non-functionalized chelates that demonstrate acceptable in vitro stability qualify for preparation of their respective bifunctional analogues for further evaluation post-conjugation to targeting molecules.
Scheme 1.
Potential Ligands for use in PET imaging and Radiotherapy of Copper
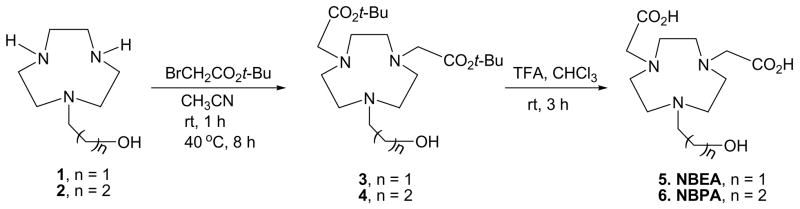
The new ligands NBEA and NBPA are efficiently synthesized starting from 1 and 2, respectively. We previously reported the efficient and convenient synthetic route to the starting materials 1 and 2 from the readily available ditosylate4b using a convenient cyclization and detosylation process.7 The base-promoted reaction of 1 or 2 with tert-butylbromoacetate afforded alkylated ligand 3 or 4 in good yield (~50%).8 Removal of tert-butyl groups in 3 and 4 was efficient with TFA (room temperapture, 4 h) to provide NBEA and NBPA in high yields (> 90%) without any further purification.9 Synthesis of NE3TA and NETA, and NE3TA-Bn involves a coupling reaction between two precursor molecules, a pre-alkylated amino ethyl bromide and a bisubstituted 1,4,7-triazatricyclononane (tacn) derivative. The key coupling step was very efficiently achieved while minimizing formation of polyalkylated byproducts.5
The radiolabeling reactions of the new ligands with 64Cu were performed at elevated temperatures to ensure complete complex formation.10 At the no-carrier-added (NCA) level, all ligands were successfully labeled with 64Cu in quantitative radiochemical yields (100%) as determined by radio-TLC. The ligands displayed rapid labeling reaction kinetics with 64Cu. The 64Cu complexes of NE3TA, NETA, NE3TA-Bn, NBEA, and NBPA possess the respective Rf values of 0.63, 0.61, 0.53, 0.62 and 0.59 (Table 1. Rf2). The 64Cu-labeled complexes were freshly prepared for their serum stability evaluation, which was assessed by measuring the transfer of radionuclide from the complex to serum proteins over 24 h.11 The results determined by radio-TLC are shown in Table 1. The stability of the complexes is comparable to that of 64Cu-DOTA, which was used as a positive control. DOTA is known to bind Cu(II) using the four nitrogens in the macrocyclic ring and two pendant carboxylate oxygens as demonstrated by a X-ray crystallography.2
Table 1.
In Vitro Serum Stability of 64Cu-Labeled New Ligands in Rat Serum
| Complex | Incubation Time | Rf1 | Rf2 | Purity (%) |
|---|---|---|---|---|
| 64Cu-NBEA | 1 h | 0.6 | 100 ± 0.0 | |
| 4 h | 0.6 | 100 ± 0.0 | ||
| 24 h | 0.12 | 0.28 | 45.34 ± 9.8 | |
| 64Cu-NBPA | 1 h | 0.55 | 99.59 ± 0.72 | |
| 4 h | 0.55 | 97.48 ± 4.4 | ||
| 24 h | 0.09 | 0.67 | 70.52 ± 6.54 | |
| 64Cu-NE3TA | 1 h | 0.60 | 100 ± 0.0 | |
| 4 h | 0.60 | 100 ± 0.0 | ||
| 24 h | 0.58 | 100 ± 0.0 | ||
| 64Cu-NE3T-Bn | 1 h | 0.39 | 100 ± 0.0 | |
| 4 h | 0.41 | 100 ± 0.0 | ||
| 24 h | 0.37 | 100 ± 0.0 | ||
| 64Cu-NETA | 1 h | 0.39 | 100 ± 0.0 | |
| 4 h | 0.42 | 100 ± 0.0 | ||
| 24 h | 0.39 | 100 ± 0.0 |
Among the ligands tested, 64Cu-labeled complexes of NETA, NE3TA, NE3TA-Bn were stable in rat serum for 24 h with no measurable loss of radioactivity. However, the 64Cu complexes of NBPA and NBEA appear to be less stable in rat serum. Significant amounts of 64Cu dissociated from the NBPA and NBEA complexes (64Cu-NBPA: 29%; 64Cu-NBEA: 54%. See Rf1 values in Table 1) in 24 h as determined by radio-TLC. It appears that the serum stability of the Cu(II) complexes has some dependency on the length of the carbon chain between the pendant donor groups and the macrocyclic ring. It is interesting to note that NBPA possessing the hydroxyl group connected to the amino group via a longer propyl chain that can form six-membered chelate ring with the metal is more effective in holding 64Cu compared to NBEA. The serum stability data suggest that the bidentate aminocarboxylate groups in NETA, NE3TA, and NE3TA-Bn more effectively serve as the donor group, tightly holding 64Cu in serum than the monodentate hydroxyl group in NBEA and NBPA, and that the introduction of a benzyl group into one of the tertiary amines in the side arm (NE3TA-Bn) does not impact the complex stability. That no measurable loss of radioactivity from 64Cu-NE3TA-Bn was recorded out to 24 h (nearly 2 half lives of 64Cu) demonstrates the potential of utilizing the NE3TA-Bn backbone as a basis for bifunctional chelators for targeted PET imaging or radiation therapy.
In summary, novel ligands, NBEA, NBPA, NETA, NE3TA, and NE3TA-Bn have been evaluated as potential chelators of 64Cu for targeted PET imaging or radiation cancer therapy. NBEA, NBPA, NETA, NE3TA, and NE3TA-Bn possess flexible carboxylate coordination groups, while NBEA and NBPA contain a hydroxyl pendant arm and form neutral complexes with Cu(II). The new ligands were efficiently synthesized and radiolabeled with 64Cu, and in vitro stability of these radiolabeled complexes were evaluated in rat serum. In vitro studies indicate that NETA, NE3TA, and NE3TA-Bn complexes with 64Cu stayed intact in rat serum for 2 days, while NBEA and NBPA compounds were not so effective in binding 64Cu. The results suggest that NETA, NE3TA, and NE3TA-Bn are a new group of chelating agents for copper radiopharmaceuticals that may find applications in PET imaging or targeted radiation therapy. Exploration of the bifunctional versions of these new ligands is underway.
List of Potential Reviewers
We would like to recommend any of the following experts in the field as potential reviewers:
Prof. Buck E. Rogers, Department of Radiation Oncology, Washington University, 4511 Forest Park Blvd, Suite 411, St Louis, MO 63108. Tel: 314) 362-9787, Fax: 314) 362-9790. E-mail: rogers@radonc.wustl.edu
Prof. Qi-Huang Zheng, Department of Radiology, School of Medicine, Indiana University, Phone: 317-278-4671; Email: qzheng@iupui.edu
Prof. Zhude Tu, Department of Radiology, School of Medicine, Washington University, Phone: 314-362-8487; Email: tuz@wustl.edu
Dr. Saed Mirzadeh, Nuclear Medicine Group, Oak Ridge National Laboratories, P.O. Box 2008, Oak Ridge, TN 37831-6229, Tel: 865) 574-8399, Fax: 865) 574-6226, E-mail: mirzadehs@ornl.gov
Scheme 2.
Synthesis of new hexadentate ligands, NBEA and NBPA
Acknowledgments
This research was supported in part by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research.
References
- 1.Wadas TJ, Wong EH, Weisman GR, Anderson CJ. Current Pharmaceutical Design. 2007;13:3–16. doi: 10.2174/138161207779313768. [DOI] [PubMed] [Google Scholar]
- 2.Smith SVJ. Inorg Biochem. 2004;98:1874–1901. doi: 10.1016/j.jinorgbio.2004.06.009. [DOI] [PubMed] [Google Scholar]
- 3.(a) Cai W, Wu Y, Chen K, Cao Q, Tice DA, Chen X. Cancer Res. 2006;66:9673–9681. doi: 10.1158/0008-5472.CAN-06-1480. [DOI] [PubMed] [Google Scholar]; (b) Chen X, Park R, Tohme M, Shahinian AH, Bading JR, Conti PS. Bioconjug Chem. 2004;15:41–9. doi: 10.1021/bc0300403. [DOI] [PubMed] [Google Scholar]
- 4.(a) Chong HS, Milenic DE, Garmestani K, Brady ED, Arora H, Pfiester C, Brechbiel MW. Nucl Med Biol. 2006;33:459–67. doi: 10.1016/j.nucmedbio.2006.03.004. [DOI] [PubMed] [Google Scholar]; (b) Chong HS, Garmestani K, Millenic D, Brechbiel MW. J Med Chem. 2002;45:3458–3464. doi: 10.1021/jm0200759. [DOI] [PubMed] [Google Scholar]
- 5.The fully detailed synthesis and characterization of NE3TA and NE3TA-Bn is described elsewhere.
- 6.Shannon RD. Acta Crys. 1976;A32:751–767. [Google Scholar]
- 7.Chong HS, Brechbiel MW. Syn Comm. 2003;33:1147. [Google Scholar]
- 8.General synthetic procedure to compound 3 and 4: To a suspension of alcohol 1 or 2 (1mmol) and K2CO3 (2 mmol) in CH3CN (5 mL) under argon was added dropwise tert-butyl bromoacetate (2 mmol), and the resulting mixture was stirred at room temperature for 1h, followed by heating at 40 °C for 8h. The reaction mixture was allowed to cool gradually to ambient temperature and was filtered, and the filtrate was concentrated in vacuo. The residue was purified via column chromatography on silica gel (220–400 mesh) eluting with 8% CH3OH in CH2Cl2. Compound 3 or 4 was obtained as a waxy white solid: Compound 3 (Yield: 65%) 1H NMR (300 MHz, CDCl3) δ 4.51 (t, J = 6.5 Hz, 2H), 3.77 (t, J = 6.32 Hz, 2H), 3.45 (br, 8H), 3.2 (m, 6H), 2.99 (s, 2H), 1.27 (s, 18H). 13C NMR (300 MHz, CDCl3) δ 170.82, 81.72, 61.72, 56.25, 54.67, 52.09, 47.91, 28.14. HRMS (positive ion FAB) Calcd for C22H42O6: [M + H]+ m/z 402.2692. Found: [M + H]+ m/z 402.2968. Compound 4 (Yield: 50%) 1H NMR (300 MHz, CDCl3) δ 1.42 (s, 18H), 2.10–2.25 (m, 2H), 2.60–2.85 (m, 4H), 2.90–3.20 (m, 4H), 3.30–3.55 (m, 10H), 4.15 (t, 2H); 13C NMR (300 MHz, CDCl3) δ 28.15, 48.23, 51.58, 52.29, 53.61, 56.92, 64.24, 81.76, 170.84. HRMS (positive ion FAB) Calcd for C23H44O6: [M + H]+ m/z 416.3129. Found: [M + H]+ m/z 416.3124.
- 9.General procedure for deprotection of tert-Butyl groups in 3 and 4: To either compound 3 or 4 (2 mmol) was added TFA (3mL) at room temperature. The reaction mixture was stirred for 3h. TFA was removed under vacuo and the residue obtained was treated with diethyl ether (10 mL). The precipitated product was filtered, washed with diethyl ether (30 mL) and immediately dissolved in water (5mL). The aqueous layer was washed with CH2Cl2 (3 × 10 mL) and concentrated under vacuo to obtain the desired product 5 or 6: (Compound 5: Yield 86%) 1H-NMR(300 MHz, D2O) δ 4.51(t, J = 6.5 Hz, 2H), 3.87 (s, J = 6.3 Hz, 4H), 3.75 (br, 6H), 3.54 (m, 4H), 3.22 (s, 4H); 13C NMR (300 MHz, D2O) δ 172.70, 62.44, 56.50, 56.21, 51.34, 50.31, 49.25. HRMS (positive ion FAB) Calcd for C14H26O6: [M +H]+ m/z 290.1716. Found: [M + H]+ m/z 290.1715. (Compound 6: Yield 92%) 1H NMR (300 MHz, CDCl3) δ 2.05–2.20 (m, 2H), 3.13–3.25 (m, 4H), 3.26–3.44 (m, 6H), 3.45–3.60 (m, 4H), 3.70–3.85 (m, 4H), 4.05 (t, 2H); 13C NMR (300 MHz, CDCl3) δ 23.80, 49.22, 50.59, 50.98, 54.88, 56.89, 65.66, 173.13. HRMS (positive ion FAB) Calcd for C15H28O6: [M + H]+ m/z 304.1872. Found: [M + H]+ m/z 304.1880.
- 10.General radiolabeling procedure: Nitric acid (10 – 20%) used for acid wash was prepared by diluting 70% nitric acid with mini-Q water (18 Mω-cm). The ammonium acetate buffer (0.4 M, pH 7.0) was pretreated with Chelex 100 resin (Bio-Rad, Hercules, CA) before use. Silica gel 60 F254 plates were purchased from Merck & Co (Whitehouse Station, NJ). Copper-64 (64CuCl2 in 0.1N HCl) was purchased from Trace Life Sciences (Denton, TX). Radio-TLC analysis was performed on a Rita Star Radioisotope TLC Analyzer (Straubenhardt, Germany). Prior to labeling, all reaction vials were acid washed with 10–20% nitric acid overnight._ To 100 μL of each ligand solution (5 mM in 0.4 M NH4OAc buffer, pH 7.0), 0.5 μL of 64CuCl2 (420–450 μCi) was added. The resulting solutions were incubated at 60 °C for 1 h in an Eppendorf thermomixer with 1,000 rpm. The radiochemical yields and purity were determined by radio-TLC (Raytest, VA) with silica gel plate as the static phase and 10% NH4OAc/MeOH = 1/1 (v/v) as the mobile phase. Under this TLC condition, free 64Cu2+ or 64Cu-associated proteins stay at the origin.
- 11.In vitro serum stability: To 100 μL of rat serum, 10 μL of each 64Cu-labeled complex was added (n = 3 per complex). DOTA labeled with64Cu was used as a positive control. The resulting solutions were incubated at 37°C in a water-bath. At 1 h, 4 h, 24 h and 48 h after the complex addition to rat serum, the solutions were sampled and analyzed by radio-TLC.