Abstract
Homeostasis of internal carbon dioxide (CO2) and oxygen (O2) levels is fundamental to all animals. Here we examine the CO2 response of the nematode Caenorhabditis elegans. This species inhabits rotting material, which typically has a broad CO2 concentration range. We show that well fed C. elegans avoid CO2 levels above 0.5%. Animals can respond to both absolute CO2 concentrations and changes in CO2 levels within seconds. Responses to CO2 do not reflect avoidance of acid pH but appear to define a new sensory response. Sensation of CO2 is promoted by the cGMP-gated ion channel subunits TAX-2 and TAX-4, but other pathways are also important. Robust CO2 avoidance in well fed animals requires inhibition of the DAF-16 forkhead transcription factor by the insulin-like receptor DAF-2. Starvation, which activates DAF-16, strongly suppresses CO2 avoidance. Exposure to hypoxia (<1% O2) also suppresses CO2 avoidance via activation of the hypoxia-inducible transcription factor HIF-1. The npr-1 215V allele of the naturally polymorphic neuropeptide receptor npr-1, besides inhibiting avoidance of high ambient O2 in feeding C. elegans, also promotes avoidance of high CO2. C. elegans integrates competing O2 and CO2 sensory inputs so that one response dominates. Food and allelic variation at NPR-1 regulate which response prevails. Our results suggest that multiple sensory inputs are coordinated by C. elegans to generate different coherent foraging strategies.
Keywords: carbon dioxide sensing, natural variation, oxygen sensing
CO2 is an important sensory cue for many organisms. Insects can use elevated CO2 as part of an alarm signal or to find food (1–3). In fungi, high CO2 can induce filamentation (4) and regulate sporulation (5). Nematode parasites of plants and animals can follow CO2 gradients to locate their hosts (6, 7). Internal CO2 levels also provide important signals. For example, insects and mammals monitor internal CO2 to modulate respiratory exchange (8–10). This homeostatic function prevents respiratory poisoning and pH changes in body fluids, which can occur if CO2 levels rise above 5% (11).
Several mechanisms have been implicated in sensing CO2. In Drosophila, avoidance of high CO2 is mediated by a pair of odorant receptors (2, 12, 13). Artificially activating neurons expressing these receptors elicits the escape response (14). Less is known about how insects monitor internal CO2 to control opening of spiracles (15). In mammals internal CO2 levels regulate breathing, diuresis, blood pH, and blood flow (8). In most cases the molecular sensors involved are unclear although pH changes associated with hydration of CO2 are thought to be important. Carbonic anhydrases, which catalyze the hydration of CO2 to produce H+ and HCO3−, are widely expressed in mammals. HCO3− has been shown to regulate the activity of a family of adenylate cyclases that is conserved from bacteria to man (16). However, the role of these enzymes in CO2 signaling in animals is unclear. In fungi an HCO3−-regulated adenylate cyclase modulates development in response to elevated CO2 (4).
Caenorhabditis elegans belongs to the Nematoda, one of the largest phyla. Little is known, at a mechanistic level, about how these animals respond to CO2. Nematodes lack specialized respiratory structures, and gaseous exchange is thought to occur through their cuticle. Previous studies have described C. elegans chemotaxis to HCO3− but have not examined responses to gradients of CO2 (17, 18). C. elegans thrives in compost, mushroom beds, and decaying fruit, where it feeds on bacteria (19, 20). Broad ranges in O2 and CO2 concentrations exist in such environments depending on microbial growth, temperature, aeration, and moisture, and CO2 levels can rise to 10% (21, 22). Here we investigate how C. elegans responds to CO2.
Results
C. elegans Avoids Elevated CO2.
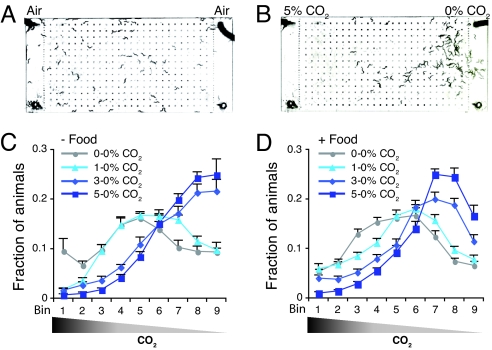
To investigate how C. elegans responds to CO2, we first exposed N2 (Bristol) wild-type animals to spatial CO2 gradients. Gas gradients were set up over worms on agar surfaces using microfluidic chambers connected to defined gas mixtures (Fig. 1 A and B and ref. 23; see Methods). Within these chambers laminar flow operates such that a linear gas gradient is generated by simple diffusion between the two ends of the chamber. Unless otherwise indicated, O2 was kept at 21% in these mixtures: CO2 was increased at the expense of N2. When only air was pumped into the chamber, N2 animals distributed equally to both sides of the chamber space (Fig. 1A). However, on introduction of a 5% to 0% CO2 gradient, animals rapidly (<10 min) vacated areas of the chamber where CO2 levels were high (Fig. 1B). To examine the concentration dependence of C. elegans CO2 avoidance, we also assayed animals in gradients of 0.25% to 0%, 0.5% to 0%, 1% to 0%, and 3% to 0% CO2. Avoidance of CO2 was concentration-dependent, and animals avoided high CO2 both in the presence and in the absence of a lawn of Escherichia coli food [Fig. 1 C and D and supporting information (SI) Fig. S1]. However, bacteria slightly but significantly reduced the strength of the avoidance response (Fig. 1 C and D and Fig. S1). The significance threshold for C. elegans CO2 response was 1% CO2 on food and 0.5% CO2 off food at the 0.01% significance level (Fig. 1 C and D and Fig. S1). Thus, CO2 is a potent repellent for N2 animals.
Fig. 1.
C. elegans avoids elevated levels of CO2. (A and B) Distribution of N2 animals in microfluidic devices after 10 min without a CO2 gradient (A) or with a 5% to 0% CO2 gradient (B). Assays are in the absence of food. Gases pumped into the chamber are indicated at the top. (C and D) Distribution of N2 animals in CO2 gradients in the absence (C and Table S1) or presence (D) of E. coli food (see also Fig. S1). Bin numbers refer to different portions of the microfluidic chamber. High CO2 is to the left, as indicated by the wedge. Distribution of animals in all CO2 gradients shown was significantly different from 0–0% CO2 (P < 0.0001). Distribution of animals in all CO2 gradients shown on food was significantly different from that off food (P < 0.0001). In this and all subsequent figures measurements were taken 10 min after the assay began.
To provide a simple measure for the CO2 response we calculated a chemotaxis index by subtracting the number of animals in the low CO2 half of the chamber from the number in the high CO2 half and dividing by the total number of animals in the assay. Chemotaxis indices of +1, 0, and −1 indicate perfect attraction, indifference, and perfect avoidance of CO2, respectively. The chemotaxis indices for CO2 gradients of 1% to 0%, 3% to 0%, and 5% to 0% were −0.28, −0.66, and −0.80, respectively (see Fig. S1C, Assays without food).
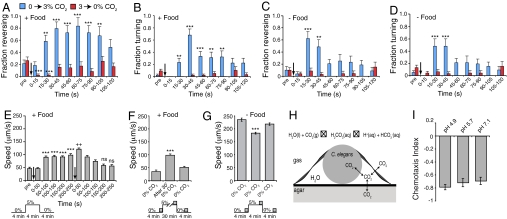
To examine how C. elegans avoids CO2, we exposed N2 animals to temporal CO2 gradients by pumping defined gas mixtures at set rates into a behavioral arena. We subjected animals to both increases (0% to 3% or 5%) and decreases (from 3% or 5% to 0%) in CO2. Animals subjected to a 0–3% rise in CO2 responded within 10 s of the gas switch; forward movement briefly ceased, animals reversed, and by ≈25 s after the switch most animals had committed a near to 180° turn (Movie S1, Animals on food). To quantify this response we took a reversal to be backward movement of an animal by greater than one-quarter of its body length and a turn to be when an animal brings its head close to its tail to create the shape of the Greek letter omega (Ω). Raising CO2 levels transiently stimulated reversals and turns both in the presence and absence of bacterial food (Fig. 2 A–D). Reversals were sustained for longer in the presence of food, suggesting that bacterial signals modify C. elegans CO2 response pathways (Fig. 2 A–D). Because the responses do not persist after CO2 levels plateau at 3%, they are likely evoked by a neural circuit that responds to changes in CO2 concentration rather than absolute concentrations.
Fig. 2.
Behavioral mechanisms involved in avoidance of CO2. (A–D) Fraction of animals reversing (A and C) or executing a turn (B and D) after a switch in CO2 concentration. A and B show responses on food, and C and D show responses off food. Events are binned into 15-s time intervals. Gas switches (indicated by an arrow) occur at time 0. Blue bars represent animals subjected to an increase in CO2, from 0% to 3%; red bars represent animals subjected to a decrease in CO2 from 3% to 0%. “pre” indicates responses in a 15-s interval immediately before the gas switch. Asterisks indicate significances compared with responses before the gas switch (pre). In this and all subsequent figures, *** or +++ indicates P < 0.001, ** or ++ indicates P < 0.01, and * or + indicates P < 0.05. (E) Feeding N2 animals respond to high CO2 by increasing their movement. Animals were subjected to a rise in CO2 (indicated by the first arrow) from 0% to 5% followed by a fall in CO2 (indicated by the second arrow) from 5% to 0%. “pre” refers to speed before the first gas switch. The gas stimulus regime is indicated below the graph. Speed was measured for each animal every second and then binned into 50-s intervals. Asterisks indicate the significance compared with speed before the up step (“pre”). + indicates significance compared with the 50-s interval before the down step. (F) The average speed of feeding N2 animals exposed to 5% CO2 remains elevated as long as CO2 levels are high. Animals were exposed to 0% CO2 for 4 min, switched to 5% CO2 for 30 min, and then returned to 0% CO2 for 4 min. Bars represent the average speed of animals during 50-s intervals just before increasing CO2 levels, just before decreasing CO2 levels, and 3 min after return of CO2 levels to 0%. Fifty-second intervals are indicated by shaded boxes in the gas stimulus regime displayed below the graph. Asterisks indicate significance compared with speed at 0% CO2. (G) In the absence of food, N2 animals respond to a rise in CO2 by reducing their speed. Speeds were averaged over the 50-s intervals indicated by shaded boxes in the gas stimulus regime displayed below the graph. (H) CO2 is potentially a complex stimulus. Aqueous CO2 as well H+ and HCO3− could be sensory cues for the nematode. Because nematodes are gas-permeable, CO2 detection could involve both external and internal sensors. Double-headed arrows indicate equilibration of CO2 among gas, liquid, worm, and agar phases. (I) Avoidance of 5% CO2 persists with little or no change in magnitude across a broad range of external pH. All pairwise comparisons of chemotaxis indices at different pH values are not significantly different.
CO2 Stimulates C. elegans Locomotory Activity on Food but Not off Food.
The speed an animal moves at influences how rapidly it can escape an aversive cue. This led us to examine whether elevated CO2 stimulated movement in C. elegans. Raising CO2 from 0% to 5% led to a doubling of the average speed of feeding N2 animals, from 46 to 92 μm/s (Fig. 2E). Unlike the increase in reversals and turns, which lasted for only 1–2 min (Fig. 2 A–D), the increased rate of movement was sustained as long as CO2 levels remained high (>30 min; Fig. 2F). This perdurance suggests that absolute levels of CO2, rather than change in its concentration, can signal to control speed of movement.
When returned from 5% CO2 to 0%, feeding animals showed a further transient increase in speed before slowing down to the speed they exhibited before the CO2 rise (Fig. 2E). In contrast to our observations in the presence of food, raising CO2 levels from 0% to 5% in the absence of food caused a decrease in the average speed of movement, from 235 to 183 μm/s (Fig. 2G). Returning animals to atmospheric CO2 levels reversed this inhibition. In summary, our data suggest that C. elegans can respond both to absolute levels of CO2, which can regulate speed, and to changes in CO2 levels, which modulate reversals and turns and, to some extent, speed too.
CO2 Avoidance Is Distinct from Avoidance of Acid pH.
CO2 is potentially a complex sensory stimulus. C. elegans lives in aqueous films and responds to chemical stimuli dissolved in these films. CO2 is highly soluble in water, reacting to form carbonic acid that dissociates to yield H+ and HCO3− (Fig. 2H). HCO3− can dissociate further to yield H+ and CO32−, but CO32− concentrations are negligible at physiological pH. Thus at the air–water interface an equilibrium is set up between gaseous CO2 and its solvation products (Fig. 2H).
Previous studies have indicated that C. elegans avoids acid pH (24). This raised the possibility that CO2 avoidance reflects escape from acid pH. We therefore examined how a 5% to 0% CO2 gradient changed agar pH across the microfluidic chamber (Fig. S2). We observed a pH change of <0.1 pH units across the chamber, from pH 6.22 to pH 6.29. The small size of the pH change was expected because the agar substrate is buffered (see Methods). This small pH change and the previous observation that C. elegans avoids acid only below pH 4 (24) suggest that changes in external pH are unlikely to explain CO2 avoidance.
C. elegans could also avoid CO2 by responding to changes in HCO3− levels in the medium. To test this we examined CO2 responses on agars buffered at different pH values, from 4.9 to 7.1. The concentrations of HCO3− generated by any given partial pressure of CO2 should vary 100-fold across this pH range. We saw no substantial differences in avoidance of 5% CO2 at different pH values (Fig. 2I). These data suggest that changes in external H+ and HCO3− are unlikely to be the sensory stimuli that trigger CO2 avoidance. However, the permeability of CO2 across lipid bilayers is high (≈0.35 cm s−1) (25), and the C. elegans genome encodes several genes with homology to carbonic anhydrases, the enzymes that catalyze hydration of CO2 (www.wormbase.org). C. elegans could therefore sense CO2 fluctuations by monitoring internal (extracellular or intracellular) H+ or HCO3− levels. Alternatively, C. elegans could respond to molecular CO2.
Signaling Through cGMP-Gated Ion Channels Contributes to CO2 Avoidance.
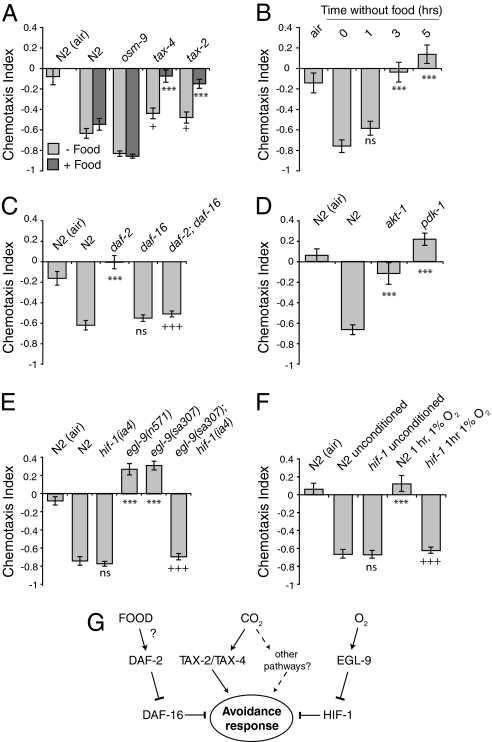
Two major chemosensory pathways have been defined in C. elegans. One is mediated by a cGMP-gated ion channel encoded by the tax-2 and tax-4 genes (26, 27). A second is mediated by transient receptor potential V-like (TRPV-like) ion channels encoded by osm-9 and its associated subunits encoded by ocr genes (28, 29). We tested whether mutations in these genes disrupted CO2 avoidance. Loss of osm-9 did not cause a carbon dioxide avoidance defective (Cdad) phenotype in the presence or absence of food (Fig. 3A). In contrast, mutations in tax-2 or tax-4 completely disrupted CO2 avoidance on food but only partially disrupted avoidance off food (Fig. 3A). Thus, cGMP pathways contribute to CO2 avoidance, but other signal transduction pathways may also be important.
Fig. 3.
Multiple signal transduction pathways contribute to CO2 avoidance. (A) Disrupting the cGMP-gated ion channel encoding genes tax-2 and tax-4 reduces avoidance of 5% CO2 both on and off food, whereas loss of osm-9, which encodes a TRPV-related channel, does not. In this and all other panels of this figure, a 5% to 0% CO2 gradient was used to test behavior. * and + indicate significance compared with N2. Alleles used were tax-4(p678), tax-2(p691), and osm-9(ky10). “N2 (air)” represents a negative control with no CO2 gradient. (B) N2 animals deprived of food gradually reduce CO2 avoidance. Asterisks indicate significance compared with unstarved N2. (C) Disrupting DAF-2 insulin-like receptor signaling results in strong defects in CO2 avoidance. Reduced DAF-2 signaling inhibits CO2 responses by activating the DAF-16 Forkhead transcription factor. Alleles used were daf-2(e1370) and daf-16(mgDf47). Because daf-2(e1370) is a temperature-sensitive allele, animals were grown at 15°C and assayed at 22°C. *, significance compared with N2; ns, not significant compared with N2; +, significance compared with daf-2. (D) Mutations that disrupt pdk-1 3-phosphoinositide-dependent protein kinase 1 or akt-1 protein kinase B also disrupt avoidance of CO2. PDK-1 and AKT-1 link activation of DAF-2 to inhibition of DAF-16. Alleles used were pdk-1(sa709) and akt-1(mg306). *, significance compared with N2. (E) egl-9 mutants grown in 21% O2 exhibit attraction to high CO2. This switch in CO2 response requires HIF-1. ns, not significant compared with N2; *, significance compared with N2; +, significance compared with egl-9 (sa307). (F) Exposing feeding N2 animals to hypoxia (1% O2) for 1 h inhibits CO2 avoidance in a HIF-1-dependent manner. ns, not significant compared with N2; *, significance compared with N2; +, significance compared with N2 conditioned in 1% O2 for 1 h on food. (G) Genetic pathways contributing to CO2 avoidance and its modulation.
Starvation Suppresses CO2 Avoidance.
C. elegans thrives in decaying organic matter where microbial activity can significantly raise local CO2 levels (21, 22). It was therefore surprising that N2 animals avoided CO2. Studies of other nematodes, both free-living bacteriophagous species (e.g., Panagrellus silusiae) and plant (e.g., Meloidogyne incognita) and animal (e.g., Steinernema sp.) parasites, have reported chemoattraction not chemorepulsion to CO2 (6, 30, 31). This led us to examine whether C. elegans avoidance of CO2 is context-dependent. We began by asking whether starvation alters CO2 avoidance. We removed N2 animals from food for 1, 3, or 5 h and then tested their responses in a 5% to 0% CO2 gradient off food. Food deprivation suppressed CO2 avoidance: N2 animals showed no significant CO2 avoidance after 3 h without food and weak attraction toward CO2 after 5 h without food (Fig. 3B). Thus, whereas well fed or feeding animals strongly avoid CO2, starved animals do not.
Insulin-Like Signaling Sustains CO2 Avoidance.
Several neuroendocrine pathways signal feeding state in C. elegans (32–35). These include the daf-2 insulin-like receptor pathway: high DAF-2 signaling is associated with the well fed state, whereas low signaling is associated with food deprivation. We speculated that starvation might suppress CO2 avoidance by inhibiting DAF-2 signaling. This hypothesis predicts that mutants in this pathway would behave like starved wild-type animals even when they are well fed. Consistent with this, mutants in the insulin-like signaling pathway, including the daf-2 insulin-like receptor, the 3-phosphoinositide-dependent kinase pdk-1, and the protein kinase B serine/threonine kinase akt-1 showed reduced CO2 avoidance or even weak attraction (Fig. 3 C and D). Insulin-like signaling thus sustains avoidance of high CO2.
The effects of food deprivation on CO2 responses occurred over several hours (Fig. 3B), a timescale consistent with a transcriptional reconfiguration of CO2-sensing circuits. Reduced DAF-2 signaling activates the DAF-16 Forkhead transcription factor (32, 36). We therefore asked whether DAF-16 was responsible for suppressing CO2 avoidance in daf-2 mutants. Consistent with such a scenario, daf-2; daf-16 double mutants strongly avoided high CO2 and behaved indistinguishably from N2 animals (Fig. 3C). Together these data are consistent with a model in which starvation reconfigures CO2 responses, at least in part, by down-regulating insulin-like signaling and activating the DAF-16 forkhead transcription factor.
Hypoxia Suppresses CO2 Avoidance via Activation of HIF-1.
Because CO2 is the by-product of aerobic respiration, we speculated that O2-sensing pathways might regulate CO2 responses. One pathway regulated by O2 is the hypoxia-inducible pathway. In both C. elegans and mammals, severe hypoxia (<1% O2) induces hypoxia-inducible factor (HIF) transcription factors. In high O2 HIFs are targeted for degradation by prolyl hydroxylases. These enzymes use molecular O2 as a cosubstrate and are active in high, but not low, O2. C. elegans encodes a single HIF, called HIF-1 (37), which is targeted for degradation by the prolyl hydroxylase EGL-9 (38). Loss of egl-9 leads to high levels of HIF-1 irrespective of ambient O2. egl-9 mutants were attracted to CO2 (Fig. 3E). To investigate whether this reversal of CO2 chemotaxis was due to high HIF-1 activity, we examined the behavior of egl-9; hif-1 double mutants. Loss of hif-1 restored strong CO2 avoidance to egl-9 mutant animals (Fig. 3E). Finally, we asked whether wild-type animals suppress CO2 avoidance after experiencing hypoxia. After 1 h in 1% O2, N2 animals, but not hif-1 mutant animals, suppressed CO2 avoidance (Fig. 3F). Taken together, these data suggest that hypoxia signals through HIF-1 to reconfigure CO2-sensing circuits, leading to indifference or even attraction to high CO2.
The NPR-1 Neuropeptide Receptor Promotes CO2 Avoidance.
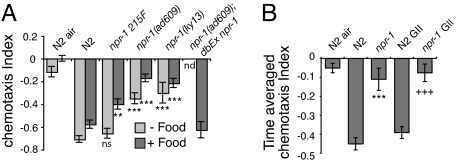
We chose to extend our studies on the interplay between O2 and CO2 sensing. Previous work has shown that natural variation in the neuropeptide receptor npr-1 alters C. elegans foraging behavior (39). Strains expressing the less active NPR-1 215F isoform avoid high ambient O2 and burrow as they feed. Strains bearing the more active NPR-1 215V isoform do not avoid high O2 as they feed and do not burrow (23, 39). We asked whether npr-1 regulated not only O2 but also CO2 responses. Consistent with this hypothesis, npr-1 loss-of-function mutants showed striking defects in CO2 avoidance both on and off food (Fig. 4A). This CO2 avoidance defect could be rescued by an npr-1 215V transgene (Fig. 4A).
Fig. 4.
The naturally polymorphic NPR-1 receptor promotes CO2 avoidance. (A) Mutations in npr-1 reduce avoidance of 5% CO2 both in the presence and in the absence of E. coli; this phenotype is rescued by an npr-1 215V transgene. N2 animals carrying the npr-1 215F natural allele also exhibit reduced avoidance of CO2 in the presence of food but maintain avoidance in its absence. ns, not significantly different compared with N2; nd, not determined. (B) The CO2 avoidance defect in npr-1 mutants is not a consequence of their aggregation behavior. npr-1 animals grown in isolation (GII) retain a strong defect in avoidance of 5% CO2 compared with similarly reared N2 animals. The weighted chemotaxis index was calculated by recording the position of each animal in a CO2 gradient at 1-s intervals for 5 min and weighting this according to location in the CO2 gradient (see Methods). “N2 air” represents a negative control with no CO2 gradient. *, significance for comparisons between N2 and npr-1; +, significance between N2 GII and npr-1 GII.
To test whether the natural npr-1 215F allele also modified CO2 avoidance, we compared the behavior of N2 animals to a near isogenic strain, AX613, which bears the npr-1 215F allele from the German wild strain RC301 backcrossed 20 times into N2. Animals bearing npr-1 215F showed a significant reduction in CO2 avoidance compared with N2 on food but not off food (Fig. 4A). Thus, animals having high npr-1 activity strongly avoid CO2 whereas animals with low npr-1 activity exhibit weaker avoidance.
Under normal cultivation conditions npr-1 mutant animals aggregate strongly. One explanation for their reduced CO2 avoidance is a difference in experience compared with N2. To explore this possibility we grew N2 and npr-1 animals in isolation. npr-1(ad609) animals grown in isolation retained a strong defect in CO2 avoidance (Fig. 4B). Together, these data suggest that signaling from the NPR-1 neuropeptide receptor promotes CO2 avoidance, particularly when food is present.
Sensory Integration of CO2 and O2 Signals in C. elegans.
In our previous experiments we exposed animals to gradients of CO2 in a background of 21% O2. However, in nature C. elegans is likely to encounter simultaneous gradients of O2 and CO2. To explore how C. elegans navigates these more complex situations, we placed animals in combined gradients of O2 and CO2 with 11% O2 and 5% CO2 at one end of the chamber and 21% O2 and 0% CO2 at the other. As controls we tested animals in identical gradients of only CO2 or O2. Integration of CO2 and O2 stimuli was particularly interesting in the context of different alleles of npr-1 because natural variation at this receptor modifies both CO2 and O2 responses. We therefore tested strains carrying npr-1 215V (which occurs in N2 and all dispersing wild isolates), npr-1 215F (which occurs in all aggregating wild isolates), and the loss-of-function mutant npr-1(ad609).
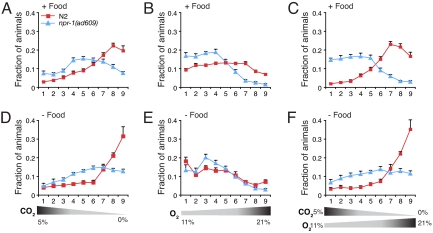
The response of N2 animals in the crossed gradient was dominated by CO2 avoidance: both on and off food animals accumulated at 21% O2/0% CO2 (Fig. 5). Thus, the avoidance of high O2 by N2 animals when food is absent was suppressed by avoidance of high CO2. By contrast, the response of npr-1(ad609) and npr-1 215F animals in the crossed gradient depended on context (Fig. 5 and Fig. S3). On food, the behavior of these animals was dominated by the O2 response: animals ignored high CO2 to accumulate at low O2. Conversely, off food it was the response to CO2 that dominated: animals behaved as if they were in a gradient that consisted only of CO2 (compare Fig. 5 D–F).
Fig. 5.
C. elegans integrates antagonistic gradients of O2 and CO2 according to food availability and genotype at the npr-1 locus. Data show distribution of N2 and npr-1(ad609) animals in simple and mixed gradients of O2 and CO2 when food is present (A–C) or absent (D–F). The gas gradients are indicated below each set of panels: 5% to 0% CO2 in A and D; 11% to 21% O2 in B and E; and a combined gradient of 5% to 0% CO2 and 11–21% O2 in C and F. N2 animals strongly avoid CO2 both on and off food, even if this requires migration to high-O2 environments. In contrast, the behavior of npr-1 mutants and animals bearing the npr-1 215F allele (see Fig. S3) depends on context. These animals accumulate at low O2/high CO2 if food is present (C): an adverse CO2 gradient does not appear to affect their avoidance of high O2. Conversely, if food is absent, they tend to migrate to high O2/low CO2.
Thus, C. elegans integrates antagonistic inputs from CO2- and O2-sensing pathways to generate a coherent behavioral response in which one input dominates. The activity of the NPR-1 receptor reconfigures which of the two sensory responses dominates within the context of food availability.
Discussion
Well fed C. elegans avoid elevated CO2, even though they seek environments where O2 levels are between 11% and 7% (23, 40). The threshold we observed for CO2 response is ≈0.5%. This is >10-fold higher than atmospheric CO2 levels, but decaying organic matter can have much higher CO2 concentrations, of 10% or more. C. elegans can respond both to absolute levels of CO2, by modifying speed, and to change in CO2 concentration, by altering direction of movement. Interestingly, C. elegans responses to O2 are also coupled to changes in both concentration and absolute levels (40).
Behavioral and genetic dissection of the C. elegans CO2 response reveals surprising complexity. Several observations are most easily explained if C. elegans has several pathways that respond to changes in CO2. First, single mutations in known sensory transduction pathways are not sufficient to abolish CO2 avoidance under all feeding conditions. Second, CO2 responses are switched from repulsion to attraction by mutations in some genes. Third, the effects of CO2 on speed of movement are complex. Although we have not identified CO2-responsive sensory neurons in this study, one set of candidate neurons is those expressing the TAX-2/TAX-4 cGMP-gated ion channel.
Avoidance of CO2 is modulated by contextual cues such as feeding state, exposure to hypoxia, and bacteria (Fig. 3G). Starvation completely suppresses CO2 avoidance. This may represent a tradeoff in which food-deprived animals ignore an aversive cue to explore a wider range of environments. Previous work has shown that starvation inhibits signaling from the insulin-like receptor daf-2 and promotes entry of the DAF-16 forkhead transcription factor into the nucleus (32). Our data are consistent with high DAF-2 signaling in well fed animals sustaining avoidance of high CO2 and low DAF-2 signaling in starved animals reducing CO2 avoidance by activating DAF-16. DAF-2 has been implicated in modulating behavior previously, notably in studies of salt chemotaxis and thermotaxis (33, 35, 41). The daf-2 pathway may therefore act globally to reset behavioral state according to feeding conditions. Suppression of CO2 avoidance in hypoxia may enable animals to migrate through CO2-rich environments to reach more aerobic environments. Suggestions for how HIF-1 might alter CO2 responses come from microarray studies. In both mammals and C. elegans, HIF regulates expression of carbonic anhydrases (42).
Bacterial signals also modulate CO2 sensing: the CO2 responses of well fed animals, both wild type and mutant, differ depending on whether food is present or not. Perhaps different combinations of sensory neurons mediate responses to CO2 on and off food. Such a scenario has been described for the response of C. elegans to the aversive odorant octanol (43).
Sensory responses to CO2 and O2 are integrated by the worm in ways that depend on context and genotype at the naturally varying npr-1 locus. Previous data have shown that NPR-1 215V suppresses avoidance of high O2 in feeding animals. Here we show that NPR-1 215V also promotes CO2 avoidance. By coordinately stimulating avoidance of high CO2 and inhibiting avoidance of high O2, npr-1 215V is likely to promote migration to surface environments. In contrast, the npr-1 215F allele permits strong avoidance of high O2 and weak avoidance of CO2, promoting migration to subsurface environments. We speculate that these niche preferences may favor speciation.
Why does C. elegans avoid CO2? One reason may be that high external CO2 can acidify the body fluid of C. elegans. However, there are other possibilities. Comparison of local O2 and CO2 levels may allow the animal to monitor aeration and escape from an environment before it becomes anaerobic.
In summary, C. elegans CO2 avoidance defines a novel behavior. CO2 avoidance is highly integrated with other sensory cues of natural importance to the worm, such as food and ambient O2. One exciting challenge for the future will be to identify the neuronal substrates of CO2 avoidance in C. elegans and to examine how contextual changes alter cellular behavior, leading to the alterations in organismal behavior patterns that we have observed in this study.
Methods
Strains.
Strains were maintained at 22°C by using standard methods unless otherwise indicated (44). Strains used in this study are listed in SI Materials and Methods.
Behavioral Assays.
Spatial CO2 gradients were generated by using custom-made 33 × 15 × 0.4-mm microfluidic devices fabricated from polydimethylsiloxane (PDMS). Design was modified from ref. 23. Devices were placed over 50–150 nematodes on nematode growth medium (NGM) agar. CO2 gradients were formed by pumping a high percentage of CO2 at one end of the chamber and 0% CO2 at the other end with a syringe pump (PhD 2000; Harvard Apparatus). Flow rate through each inlet was 2 ml/min. A 5% to 0% CO2 gradient was used in most assays; the background O2 level was 21%. Assays were run for 10 min. The distribution of nematodes was recorded by counting animals in each of nine equal divisions of the chamber as well as in the two spaces at either end of the chamber (Fig. 1A). For assays in the absence of food, animals were washed with M9 Buffer before assay. Details of the wash method, which was designed to avoid giving animals a hypoxic shock, are in SI Materials and Methods. Assays in the presence of food were performed on NGM plates on lawns seeded 2 days earlier with OP50 (44). Defined CO2::O2::N2 gas mixtures were obtained from The BOC Group.
Measurements of speed were performed by using the Digital Image Analysis System (DIAS) software as described previously (40). Each data point represents at least six assays. In all bar graphs, statistical significance was determined by using the two-tailed t test. In all worm distribution plots, significance was determined by pairwise comparison between different strains and conditions using Pearson's χ2 test at the P < 0.0001 level. In all figures, error bars denote SEM.
Environmental Manipulations.
In Fig. 2I, the pH of the nematode substrate was varied by using different buffers as follows: pH 4.9 (40 mM sodium acetate, pH 4.75), pH 5.7 (40 mM malate, pH 5.33), and pH 7.1 (40 mM phosphate, pH 7.2).
In starvation experiments (Fig. 3B), two culture plates of N2 animals were washed three times in M9 before transfer to conditioning plates (6 or 9 cm of unseeded NGM). Animals were left for 0, 1, 3, or 5 h and then washed once before being assayed off food for CO2 avoidance.
In the hypoxia conditioning experiments (Fig. 3F), C. elegans cultures were placed in a glove box (Coy Laboratory Products) at 1% O2 for 1 h before being assayed off food for CO2 avoidance.
In Fig. 4B three animals per plate were grown from the L2/L3 larval stage to adulthood. Pools of 25 animals were then assayed in CO2 gradients in the presence of food. The position of each worm in the PDMS chamber was recorded over a 5-min period, beginning 10 min after the onset of the assay, with a CCD camera mounted on a dissecting microscope. Resulting films were analyzed, and the positions of the worms in the chamber were determined with DIAS (Soll Technologies). See SI Materials and Methods for further details.
pH Measurements.
We measured CO2-induced pH changes using NGM containing 500 μM pH-sensitive chromophore 8-hydroxypyrene-1,3,6-trisulphonic acid (HPTS; Sigma). For the HPTS fluorescence (F) measurement method, see SI Materials and Methods.
Supplementary Material
Acknowledgments.
We thank the Caenorhabditis Genetics Center for strains, Christof Schwiening for help with HPTS measurements, and Ian Johnston and Christabel Tan for making microfluidic devices. We are grateful to Robyn Branicky, Africa Couto, Marina Ezcurra, Christien Merrifield, and Bill Schafer for comments on the manuscript. Funding for this work came from the Medical Research Council and the Human Frontier Science Program. K.E.B. acknowledges European Molecular Biology Organization and Marie Curie Fellowships.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0707607105/DCSupplemental.
References
- 1.Nicolas G, Sillans D. Immediate and latent effects of carbon dioxide on insects. Annu Rev Entomol. 1989;34:97–116. [Google Scholar]
- 2.Suh GS, et al. A single population of olfactory sensory neurons mediates an innate avoidance behaviour in Drosophila. Nature. 2004;431:854–859. doi: 10.1038/nature02980. [DOI] [PubMed] [Google Scholar]
- 3.Stange G, Stowe S. Carbon-dioxide sensing structures in terrestrial arthropods. Microsc Res Tech. 1999;47:416–427. doi: 10.1002/(SICI)1097-0029(19991215)47:6<416::AID-JEMT5>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 4.Klengel T, et al. Fungal adenylyl cyclase integrates CO2 sensing with cAMP signaling and virulence. Curr Biol. 2005;15:2021–2026. doi: 10.1016/j.cub.2005.10.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dahlberg KR, Van Etten JL. Physiology and biochemistry of fungal sporulation. Annu Rev Phytopathol. 1982;20:281–301. [Google Scholar]
- 6.Pline M, Dusenbury DB. Responses of plant parasitic nematode Meloidogyne incognita to carbon dioxide determined by videocamera computer tracking. J Chem Ecol. 1987;13:873–888. doi: 10.1007/BF01020167. [DOI] [PubMed] [Google Scholar]
- 7.Gaugler R, LeBeck L, Nakagaki B, Boush GM. Orientation of entomogenous nematode Neoaplectana carpocapsae to carbon dioxide. Environ Entomol. 1980;9:649–652. [Google Scholar]
- 8.Lahiri S, Forster RE., II CO2/H(+) sensing: Peripheral and central chemoreception. Int J Biochem Cell Biol. 2003;35:1413–1435. doi: 10.1016/s1357-2725(03)00050-5. [DOI] [PubMed] [Google Scholar]
- 9.Feldman JL, Mitchell GS, Nattie EE. Breathing: Rhythmicity, plasticity, chemosensitivity. Annu Rev Neurosci. 2003;26:239–266. doi: 10.1146/annurev.neuro.26.041002.131103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bustami HP, Harrison JF, Hustert R. Evidence for oxygen and carbon dioxide receptors in insect CNS influencing ventilation. Comp Biochem Physiol A Mol Integr Physiol. 2002;133:595–604. doi: 10.1016/s1095-6433(02)00155-1. [DOI] [PubMed] [Google Scholar]
- 11.Richerson GB. Serotonergic neurons as carbon dioxide sensors that maintain pH homeostasis. Nat Rev Neurosci. 2004;5:449–461. doi: 10.1038/nrn1409. [DOI] [PubMed] [Google Scholar]
- 12.Jones WD, Cayirlioglu P, Kadow IG, Vosshall LB. Two chemosensory receptors together mediate carbon dioxide detection in Drosophila. Nature. 2007;445:86–90. doi: 10.1038/nature05466. [DOI] [PubMed] [Google Scholar]
- 13.Kwon JY, Dahanukar A, Weiss LA, Carlson JR. The molecular basis of CO2 reception in Drosophila. Proc Natl Acad Sci USA. 2007;104:3574–3578. doi: 10.1073/pnas.0700079104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suh GS, et al. Light activation of an innate olfactory avoidance response in Drosophila. Curr Biol. 2007;17:905–908. doi: 10.1016/j.cub.2007.04.046. [DOI] [PubMed] [Google Scholar]
- 15.Harrison J, Hadley N, Quinlan M. Acid-base status and spiracular control during discontinuous ventilation in grasshoppers. J Exp Biol. 1995;198:1755–1763. doi: 10.1242/jeb.198.8.1755. [DOI] [PubMed] [Google Scholar]
- 16.Chen Y, et al. Soluble adenylyl cyclase as an evolutionarily conserved bicarbonate sensor. Science. 2000;289:625–628. doi: 10.1126/science.289.5479.625. [DOI] [PubMed] [Google Scholar]
- 17.Dusenbery DB. Analysis of chemotaxis in the nematode Caenorhabditis elegans by countercurrent separation. J Exp Zool. 1974;188:41–47. doi: 10.1002/jez.1401880105. [DOI] [PubMed] [Google Scholar]
- 18.Dusenbery DB. Video camera-computer tracking of nematode Caenorhabditis elegans to record behavioral responses. J Chem Ecol. 1985;11:1239–1247. doi: 10.1007/BF01024112. [DOI] [PubMed] [Google Scholar]
- 19.Barriere A, Felix MA. High local genetic diversity and low outcrossing rate in Caenorhabditis elegans natural populations. Curr Biol. 2005;15:1176–1184. doi: 10.1016/j.cub.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 20.Hodgkin J, Doniach T. Natural variation and copulatory plug formation in Caenorhabditis elegans. Genetics. 1997;146:149–164. doi: 10.1093/genetics/146.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gea T, Barrena R, Artola A, Sanchez A. Monitoring the biological activity of the composting process: Oxygen uptake rate (OUR), respirometric index (RI), and respiratory quotient (RQ) Biotechnol Bioeng. 2004;88:520–527. doi: 10.1002/bit.20281. [DOI] [PubMed] [Google Scholar]
- 22.Sojka RE, Scott HD. Aeration measurement. In: Lal R, editor. Encyclopedia of Soil Sci. Boca Raton, FL: Taylor & Francis; 2006. pp. 33–35. [Google Scholar]
- 23.Gray JM, et al. Oxygen sensation and social feeding mediated by a C. elegans guanylate cyclase homologue. Nature. 2004;430:317–322. doi: 10.1038/nature02714. [DOI] [PubMed] [Google Scholar]
- 24.Sambongi Y, et al. Caenorhabditis elegans senses protons through amphid chemosensory neurons: Proton signals elicit avoidance behavior. NeuroReport. 2000;11:2229–2232. doi: 10.1097/00001756-200007140-00033. [DOI] [PubMed] [Google Scholar]
- 25.Gutknecht J, Bisson MA, Tosteson FC. Diffusion of carbon dioxide through lipid bilayer membranes: Effects of carbonic anhydrase, bicarbonate, and unstirred layers. J Gen Physiol. 1977;69:779–794. doi: 10.1085/jgp.69.6.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coburn CM, Bargmann CI. A putative cyclic nucleotide-gated channel is required for sensory development and function in C. elegans. Neuron. 1996;17:695–706. doi: 10.1016/s0896-6273(00)80201-9. [DOI] [PubMed] [Google Scholar]
- 27.Komatsu H, Mori I, Rhee JS, Akaike N, Ohshima Y. Mutations in a cyclic nucleotide-gated channel lead to abnormal thermosensation and chemosensation in C. elegans. Neuron. 1996;17:707–718. doi: 10.1016/s0896-6273(00)80202-0. [DOI] [PubMed] [Google Scholar]
- 28.Colbert HA, Smith TL, Bargmann CI. OSM-9, a novel protein with structural similarity to channels, is required for olfaction, mechanosensation, and olfactory adaptation in Caenorhabditis elegans. J Neurosci. 1997;17:8259–8269. doi: 10.1523/JNEUROSCI.17-21-08259.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tobin D, et al. Combinatorial expression of TRPV channel proteins defines their sensory functions and subcellular localization in C. elegans neurons. Neuron. 2002;35:307–318. doi: 10.1016/s0896-6273(02)00757-2. [DOI] [PubMed] [Google Scholar]
- 30.Powell JR, Webster JM. Target host finding by Steinernema feltiae and Heterorhabditis bacteriophora in the presence of a non-target insect host. J Nematol. 2004;36:285–289. [PMC free article] [PubMed] [Google Scholar]
- 31.Viglierchio DR. Carbon dioxide sensing by Panagrellus silusiae and Ditylenchus dipsaci. Rev Nematol. 1990;13:425–432. [Google Scholar]
- 32.Henderson ST, Johnson TE. daf-16 integrates developmental and environmental inputs to mediate aging in the nematode Caenorhabditis elegans. Curr Biol. 2001;11:1975–1980. doi: 10.1016/s0960-9822(01)00594-2. [DOI] [PubMed] [Google Scholar]
- 33.Kodama E, et al. Insulin-like signaling and the neural circuit for integrative behavior in C. elegans. Genes Dev. 2006;20:2955–2960. doi: 10.1101/gad.1479906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Riddle DL, Albert PS. Genetic and environmental regulation of dauer larva development. In: Riddle DL, Blumenthal T, Meyer B, Priess JR, editors. C. elegans II. Cold Spring Harbor, NY: Cold Spring Harbor Lab Press; 1997. pp. 739–768. [PubMed] [Google Scholar]
- 35.Tomioka M, et al. The insulin/PI 3-kinase pathway regulates salt chemotaxis learning in Caenorhabditis elegans. Neuron. 2006;51:613–625. doi: 10.1016/j.neuron.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 36.Ogg S, et al. The Fork head transcription factor DAF-16 transduces insulin-like metabolic and longevity signals in C. elegans. Nature. 1997;389:994–999. doi: 10.1038/40194. [DOI] [PubMed] [Google Scholar]
- 37.Jiang H, Guo R, Powell-Coffman JA. The Caenorhabditis elegans hif-1 gene encodes a bHLH-PAS protein that is required for adaptation to hypoxia. Proc Natl Acad Sci USA. 2001;98:7916–7921. doi: 10.1073/pnas.141234698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Epstein AC, et al. C. elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell. 2001;107:43–54. doi: 10.1016/s0092-8674(01)00507-4. [DOI] [PubMed] [Google Scholar]
- 39.de Bono M, Bargmann CI. Natural variation in a neuropeptide Y receptor homolog modifies social behavior and food response in C. elegans. Cell. 1998;94:679–689. doi: 10.1016/s0092-8674(00)81609-8. [DOI] [PubMed] [Google Scholar]
- 40.Cheung BH, Cohen M, Rogers C, Albayram O, de Bono M. Experience-dependent modulation of C. elegans behavior by ambient oxygen. Curr Biol. 2005;15:905–917. doi: 10.1016/j.cub.2005.04.017. [DOI] [PubMed] [Google Scholar]
- 41.Mohri A, et al. Genetic control of temperature preference in the nematode Caenorhabditis elegans. Genetics. 2005;169:1437–1450. doi: 10.1534/genetics.104.036111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bishop T, et al. Genetic analysis of pathways regulated by the von hippel-lindau tumor suppressor in Caenorhabditis elegans. PLoS Biol. 2004;2:e289. doi: 10.1371/journal.pbio.0020289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chao MY, Komatsu H, Fukuto HS, Dionne HM, Hart AC. Feeding status and serotonin rapidly and reversibly modulate a Caenorhabditis elegans chemosensory circuit. Proc Natl Acad Sci USA. 2004;101:15512–15517. doi: 10.1073/pnas.0403369101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sulston J, Hodgkin J. In: The Nematode Caenorhabditis elegans. Wood WB, editor. Cold Spring Harbor, NY: Cold Spring Harbor Lab Press; 1988. pp. 587–606. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.