Abstract
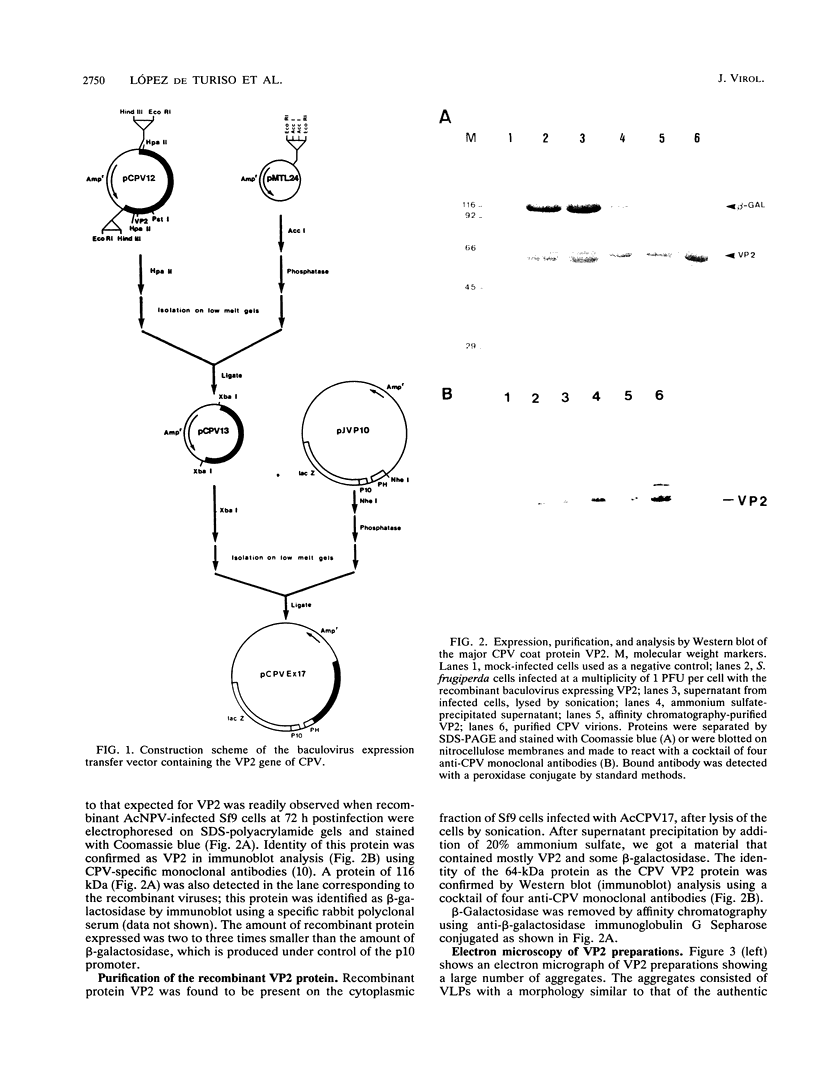
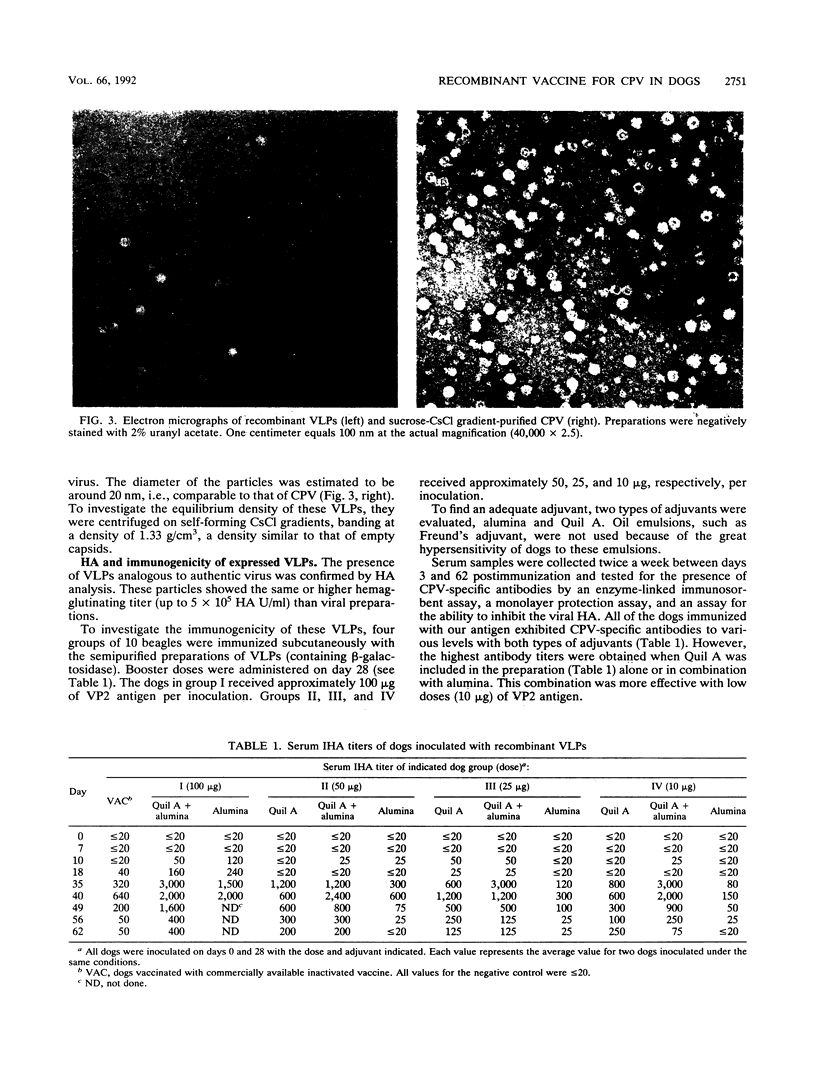
VP2 is the major component of canine parvovirus (CPV) capsids. The VP2-coding gene was engineered to be expressed by a recombinant baculovirus under the control of the polyhedrin promoter. A transfer vector that contains the lacZ gene under the control of the p10 promoter was used in order to facilitate the selection of recombinants. The expressed VP2 was found to be structurally and immunologically indistinguishable from authentic VP2. The recombinant VP2 shows also the capability to self-assemble, forming viruslike particles similar in size and appearance to CPV virions. These viruslike particles have been used to immunize dogs in different doses and combinations of adjuvants, and the anti-CPV responses have been measured by enzyme-linked immunosorbent assay, monolayer protection assays, and an assay for the inhibition of hemagglutination. A dose of ca. 10 micrograms of VP2 was able to elicit a good protective response, higher than that obtained with a commercially available, inactivated vaccine. The results indicate that these viruslike particles can be used to protect dogs from CPV infection.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appel M. J., Scott F. W., Carmichael L. E. Isolation and immunisation studies of a canine parco-like virus from dogs with haemorrhagic enteritis. Vet Rec. 1979 Aug 25;105(8):156–159. doi: 10.1136/vr.105.8.156. [DOI] [PubMed] [Google Scholar]
- Birnboim H. C., Doly J. A rapid alkaline extraction procedure for screening recombinant plasmid DNA. Nucleic Acids Res. 1979 Nov 24;7(6):1513–1523. doi: 10.1093/nar/7.6.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown C. S., Van Lent J. W., Vlak J. M., Spaan W. J. Assembly of empty capsids by using baculovirus recombinants expressing human parvovirus B19 structural proteins. J Virol. 1991 May;65(5):2702–2706. doi: 10.1128/jvi.65.5.2702-2706.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burtonboy G., Coignoul F., Delferriere N., Pastoret P. P. Canine hemorrhagic enteritis: detection of viral particles by electron microscopy. Arch Virol. 1979;61(1-2):1–11. doi: 10.1007/BF01320586. [DOI] [PubMed] [Google Scholar]
- Carlson J., Rushlow K., Maxwell I., Maxwell F., Winston S., Hahn W. Cloning and sequence of DNA encoding structural proteins of the autonomous parvovirus feline panleukopenia virus. J Virol. 1985 Sep;55(3):574–582. doi: 10.1128/jvi.55.3.574-582.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmichael L. E., Joubert J. C., Pollock R. V. Hemagglutination by canine parvovirus: serologic studies and diagnostic applications. Am J Vet Res. 1980 May;41(5):784–791. [PubMed] [Google Scholar]
- Dalsgaard K. Saponin adjuvants. 3. Isolation of a substance from Quillaja saponaria Molina with adjuvant activity in food-and-mouth disease vaccines. Arch Gesamte Virusforsch. 1974;44(3):243–254. [PubMed] [Google Scholar]
- French T. J., Marshall J. J., Roy P. Assembly of double-shelled, viruslike particles of bluetongue virus by the simultaneous expression of four structural proteins. J Virol. 1990 Dec;64(12):5695–5700. doi: 10.1128/jvi.64.12.5695-5700.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- López de Turiso J. A., Cortés E., Ranz A., García J., Sanz A., Vela C., Casal J. I. Fine mapping of canine parvovirus B cell epitopes. J Gen Virol. 1991 Oct;72(Pt 10):2445–2456. doi: 10.1099/0022-1317-72-10-2445. [DOI] [PubMed] [Google Scholar]
- Parrish C. R., Aquadro C. F., Carmichael L. E. Canine host range and a specific epitope map along with variant sequences in the capsid protein gene of canine parvovirus and related feline, mink, and raccoon parvoviruses. Virology. 1988 Oct;166(2):293–307. doi: 10.1016/0042-6822(88)90500-4. [DOI] [PubMed] [Google Scholar]
- Parrish C. R., Carmichael L. E., Antczak D. F. Antigenic relationships between canine parvovirus type 2, feline panleukopenia virus and mink enteritis virus using conventional antisera and monoclonal antibodies. Arch Virol. 1982;72(4):267–278. doi: 10.1007/BF01315223. [DOI] [PubMed] [Google Scholar]
- Pollock R. V., Carmichael L. E. Dog response to inactivated canine parvovirus and feline panleukopenia virus vaccines. Cornell Vet. 1982 Jan;72(1):16–35. [PubMed] [Google Scholar]
- Redmond M. J., Ohmann H. B., Hughes H. P., Sabara M., Frenchick P. J., Poku S. K., Ijaz M. K., Parker M. D., Laarveld B., Babiuk L. A. Rotavirus particles function as immunological carriers for the delivery of peptides from infectious agents and endogenous proteins. Mol Immunol. 1991 Mar;28(3):269–278. doi: 10.1016/0161-5890(91)90073-s. [DOI] [PubMed] [Google Scholar]
- Reed A. P., Jones E. V., Miller T. J. Nucleotide sequence and genome organization of canine parvovirus. J Virol. 1988 Jan;62(1):266–276. doi: 10.1128/jvi.62.1.266-276.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takehara K., Ireland D., Bishop D. H. Co-expression of the hepatitis B surface and core antigens using baculovirus multiple expression vectors. J Gen Virol. 1988 Nov;69(Pt 11):2763–2777. doi: 10.1099/0022-1317-69-11-2763. [DOI] [PubMed] [Google Scholar]
- Tattersall P., Shatkin A. J., Ward D. C. Sequence homology between the structural polypeptides of minute virus of mice. J Mol Biol. 1977 Apr 25;111(4):375–394. doi: 10.1016/s0022-2836(77)80060-0. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tratschin J. D., McMaster G. K., Kronauer G., Siegl G. Canine parvovirus: relationship to wild-type and vaccine strains of feline panleukopenia virus and mink enteritis virus. J Gen Virol. 1982 Jul;61(Pt 50):33–41. doi: 10.1099/0022-1317-61-1-33. [DOI] [PubMed] [Google Scholar]
- Tsao J., Chapman M. S., Agbandje M., Keller W., Smith K., Wu H., Luo M., Smith T. J., Rossmann M. G., Compans R. W. The three-dimensional structure of canine parvovirus and its functional implications. Science. 1991 Mar 22;251(5000):1456–1464. doi: 10.1126/science.2006420. [DOI] [PubMed] [Google Scholar]
- Urakawa T., Ferguson M., Minor P. D., Cooper J., Sullivan M., Almond J. W., Bishop D. H. Synthesis of immunogenic, but non-infectious, poliovirus particles in insect cells by a baculovirus expression vector. J Gen Virol. 1989 Jun;70(Pt 6):1453–1463. doi: 10.1099/0022-1317-70-6-1453. [DOI] [PubMed] [Google Scholar]
- Vialard J., Lalumière M., Vernet T., Briedis D., Alkhatib G., Henning D., Levin D., Richardson C. Synthesis of the membrane fusion and hemagglutinin proteins of measles virus, using a novel baculovirus vector containing the beta-galactosidase gene. J Virol. 1990 Jan;64(1):37–50. doi: 10.1128/jvi.64.1.37-50.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlak J. M., Keus R. J. Baculovirus expression vector system for production of viral vaccines. Adv Biotechnol Processes. 1990;14:91–128. [PubMed] [Google Scholar]
- Zuidema D., Schouten A., Usmany M., Maule A. J., Belsham G. J., Roosien J., Klinge-Roode E. C., van Lent J. W., Vlak J. M. Expression of cauliflower mosaic virus gene I in insect cells using a novel polyhedrin-based baculovirus expression vector. J Gen Virol. 1990 Oct;71(Pt 10):2201–2209. doi: 10.1099/0022-1317-71-10-2201. [DOI] [PubMed] [Google Scholar]