Abstract
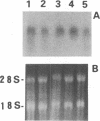
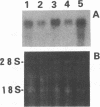
In order to clarify the protective immune responses against a newly identified herpesvirus, human herpesvirus 6 (HHV-6), we established HHV-6-specific human T-cell clones and examined their functional properties. Five CD3+CD4+CD8- T-cell clones, which proliferated in response to stimulation with two different strains of HHV-6 in the presence of autologous antigen-presenting cells but not with herpes simplex virus type 1 or human cytomegalovirus, were established from peripheral blood lymphocytes of a healthy individual. The proliferative response of all T-cell clones to HHV-6 antigen was inhibited by addition of anti-HLA-DR monoclonal antibody, indicating that these clones were human leukocyte antigen (HLA) class II DR restricted. Of the five clones, two lysed HHV-6-infected autologous lymphoblasts, but not HHV-6-infected allogeneic cells or natural killer-sensitive K562 cells (group 1); one showed cytotoxicity against HHV-6-infected autologous lymphoblasts as well as HHV-6-infected allogeneic cells and K562 cells (group 2); and the remaining two showed no cytotoxic activity (group 3). The cytotoxic activity of group 1 was inhibited by addition of anti-HLA-DR monoclonal antibody to the culture, whereas this monoclonal antibody had no effect on the cytotoxicity of group 2 and did not induce the cytotoxicity of group 3. Perforin, which is one of the mediators of cytotoxicity, was abundantly expressed in group 1 and 2 clones. Moreover, all groups of clones produced gamma interferon after culture with antigen-presenting cells followed by HHV-6 antigen stimulation. These results suggest that HHV-6-specific CD4+ T cells have heterogeneous functions.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agut H., Guetard D., Collandre H., Dauguet C., Montagnier L., Miclea J. M., Baurmann H., Gessain A. Concomitant infection by human herpesvirus 6, HTLV-I, and HIV-2. Lancet. 1988 Mar 26;1(8587):712–712. doi: 10.1016/s0140-6736(88)91520-6. [DOI] [PubMed] [Google Scholar]
- Barnstable C. J., Bodmer W. F., Brown G., Galfre G., Milstein C., Williams A. F., Ziegler A. Production of monoclonal antibodies to group A erythrocytes, HLA and other human cell surface antigens-new tools for genetic analysis. Cell. 1978 May;14(1):9–20. doi: 10.1016/0092-8674(78)90296-9. [DOI] [PubMed] [Google Scholar]
- Briggs M., Fox J., Tedder R. S. Age prevalence of antibody to human herpesvirus 6. Lancet. 1988 May 7;1(8593):1058–1059. doi: 10.1016/s0140-6736(88)91883-1. [DOI] [PubMed] [Google Scholar]
- Brown N. A., Sumaya C. V., Liu C. R., Ench Y., Kovacs A., Coronesi M., Kaplan M. H. Fall in human herpesvirus 6 seropositivity with age. Lancet. 1988 Aug 13;2(8607):396–396. doi: 10.1016/s0140-6736(88)92864-4. [DOI] [PubMed] [Google Scholar]
- Carrigan D. R., Drobyski W. R., Russler S. K., Tapper M. A., Knox K. K., Ash R. C. Interstitial pneumonitis associated with human herpesvirus-6 infection after marrow transplantation. Lancet. 1991 Jul 20;338(8760):147–149. doi: 10.1016/0140-6736(91)90137-e. [DOI] [PubMed] [Google Scholar]
- Cher D. J., Mosmann T. R. Two types of murine helper T cell clone. II. Delayed-type hypersensitivity is mediated by TH1 clones. J Immunol. 1987 Jun 1;138(11):3688–3694. [PubMed] [Google Scholar]
- Cherwinski H. M., Schumacher J. H., Brown K. D., Mosmann T. R. Two types of mouse helper T cell clone. III. Further differences in lymphokine synthesis between Th1 and Th2 clones revealed by RNA hybridization, functionally monospecific bioassays, and monoclonal antibodies. J Exp Med. 1987 Nov 1;166(5):1229–1244. doi: 10.1084/jem.166.5.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Downing R. G., Sewankambo N., Serwadda D., Honess R., Crawford D., Jarrett R., Griffin B. E. Isolation of human lymphotropic herpesviruses from Uganda. Lancet. 1987 Aug 15;2(8555):390–390. doi: 10.1016/s0140-6736(87)92403-2. [DOI] [PubMed] [Google Scholar]
- Dubedat S., Kappagoda N. Hepatitis due to human herpesvirus-6. Lancet. 1989 Dec 16;2(8677):1463–1464. doi: 10.1016/s0140-6736(89)92077-1. [DOI] [PubMed] [Google Scholar]
- Flamand L., Gosselin J., D'Addario M., Hiscott J., Ablashi D. V., Gallo R. C., Menezes J. Human herpesvirus 6 induces interleukin-1 beta and tumor necrosis factor alpha, but not interleukin-6, in peripheral blood mononuclear cell cultures. J Virol. 1991 Sep;65(9):5105–5110. doi: 10.1128/jvi.65.9.5105-5110.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray P. W., Leung D. W., Pennica D., Yelverton E., Najarian R., Simonsen C. C., Derynck R., Sherwood P. J., Wallace D. M., Berger S. L. Expression of human immune interferon cDNA in E. coli and monkey cells. Nature. 1982 Feb 11;295(5849):503–508. doi: 10.1038/295503a0. [DOI] [PubMed] [Google Scholar]
- Hayward A. R., Pontesilli O., Herberger M., Laszlo M., Levin M. Specific lysis of varicella zoster virus-infected B lymphoblasts by human T cells. J Virol. 1986 Apr;58(1):179–184. doi: 10.1128/jvi.58.1.179-184.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirayama K., Matsushita S., Kikuchi I., Iuchi M., Ohta N., Sasazuki T. HLA-DQ is epistatic to HLA-DR in controlling the immune response to schistosomal antigen in humans. Nature. 1987 Jun 4;327(6121):426–430. doi: 10.1038/327426a0. [DOI] [PubMed] [Google Scholar]
- Ikeda H., Kasahara M., Ogasawara K., Takenouchi T., Okuyama T., Ishikawa N., Wakisaka A., Kikuchi Y., Aizawa M. Evidence for polymorphism of MB3 antigens among three HLA-D clusters associated with HLA-DR4. Immunogenetics. 1984;19(5):381–390. doi: 10.1007/BF00364642. [DOI] [PubMed] [Google Scholar]
- Jacobson S., Richert J. R., Biddison W. E., Satinsky A., Hartzman R. J., McFarland H. F. Measles virus-specific T4+ human cytotoxic T cell clones are restricted by class II HLA antigens. J Immunol. 1984 Aug;133(2):754–757. [PubMed] [Google Scholar]
- Kaplan D. R., Griffith R., Braciale V. L., Braciale T. J. Influenza virus-specific human cytotoxic T cell clones: heterogeneity in antigenic specificity and restriction by class II MHC products. Cell Immunol. 1984 Oct 1;88(1):193–206. doi: 10.1016/0008-8749(84)90064-9. [DOI] [PubMed] [Google Scholar]
- Kawasaki A., Shinkai Y., Kuwana Y., Furuya A., Iigo Y., Hanai N., Itoh S., Yagita H., Okumura K. Perforin, a pore-forming protein detectable by monoclonal antibodies, is a functional marker for killer cells. Int Immunol. 1990;2(7):677–684. doi: 10.1093/intimm/2.7.677. [DOI] [PubMed] [Google Scholar]
- Kikuta H., Nakane A., Lu H., Taguchi Y., Minagawa T., Matsumoto S. Interferon induction by human herpesvirus 6 in human mononuclear cells. J Infect Dis. 1990 Jul;162(1):35–38. doi: 10.1093/infdis/162.1.35. [DOI] [PubMed] [Google Scholar]
- Knowles W. A., Gardner S. D. High prevalence of antibody to human herpesvirus-6 and seroconversion associated with rash in two infants. Lancet. 1988 Oct 15;2(8616):912–913. doi: 10.1016/s0140-6736(88)92522-6. [DOI] [PubMed] [Google Scholar]
- Kojima H., Fukasawa Y., Ishikawa N., Tajima Y., Wakisaka A., Aizawa M. Detection of a novel HLA-DQ specificity. II. Cellular analysis by cytotoxic T-cell clones. Immunogenetics. 1988;27(2):145–147. doi: 10.1007/BF00351090. [DOI] [PubMed] [Google Scholar]
- Kondo K., Kondo T., Okuno T., Takahashi M., Yamanishi K. Latent human herpesvirus 6 infection of human monocytes/macrophages. J Gen Virol. 1991 Jun;72(Pt 6):1401–1408. doi: 10.1099/0022-1317-72-6-1401. [DOI] [PubMed] [Google Scholar]
- Levy J. A., Ferro F., Greenspan D., Lennette E. T. Frequent isolation of HHV-6 from saliva and high seroprevalence of the virus in the population. Lancet. 1990 May 5;335(8697):1047–1050. doi: 10.1016/0140-6736(90)92628-u. [DOI] [PubMed] [Google Scholar]
- Lindsley M. D., Torpey D. J., 3rd, Rinaldo C. R., Jr HLA-DR-restricted cytotoxicity of cytomegalovirus-infected monocytes mediated by Leu-3-positive T cells. J Immunol. 1986 Apr 15;136(8):3045–3051. [PubMed] [Google Scholar]
- Maggi E., Del Prete G., Macchia D., Parronchi P., Tiri A., Chrétien I., Ricci M., Romagnani S. Profiles of lymphokine activities and helper function for IgE in human T cell clones. Eur J Immunol. 1988 Jul;18(7):1045–1050. doi: 10.1002/eji.1830180712. [DOI] [PubMed] [Google Scholar]
- Misko I. S., Pope J. H., Hütter R., Soszynski T. D., Kane R. G. HLA-DR-antigen-associated restriction of EBV-specific cytotoxic T-cell colonies. Int J Cancer. 1984 Feb 15;33(2):239–243. doi: 10.1002/ijc.2910330212. [DOI] [PubMed] [Google Scholar]
- Morrison L. A., Lukacher A. E., Braciale V. L., Fan D. P., Braciale T. J. Differences in antigen presentation to MHC class I-and class II-restricted influenza virus-specific cytolytic T lymphocyte clones. J Exp Med. 1986 Apr 1;163(4):903–921. doi: 10.1084/jem.163.4.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosmann T. R., Cherwinski H., Bond M. W., Giedlin M. A., Coffman R. L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986 Apr 1;136(7):2348–2357. [PubMed] [Google Scholar]
- Paliard X., de Waal Malefijt R., Yssel H., Blanchard D., Chrétien I., Abrams J., de Vries J., Spits H. Simultaneous production of IL-2, IL-4, and IFN-gamma by activated human CD4+ and CD8+ T cell clones. J Immunol. 1988 Aug 1;141(3):849–855. [PubMed] [Google Scholar]
- Podack E. R., Hengartner H., Lichtenheld M. G. A central role of perforin in cytolysis? Annu Rev Immunol. 1991;9:129–157. doi: 10.1146/annurev.iy.09.040191.001021. [DOI] [PubMed] [Google Scholar]
- Quint D. J., Bolton E. J., McNamee L. A., Solari R., Hissey P. H., Champion B. R., MacKenzie A. R., Zanders E. D. Functional and phenotypic analysis of human T-cell clones which stimulate IgE production in vitro. Immunology. 1989 May;67(1):68–74. [PMC free article] [PubMed] [Google Scholar]
- Reinherz E. L., Kung P. C., Goldstein G., Schlossman S. F. Separation of functional subsets of human T cells by a monoclonal antibody. Proc Natl Acad Sci U S A. 1979 Aug;76(8):4061–4065. doi: 10.1073/pnas.76.8.4061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salahuddin S. Z., Ablashi D. V., Markham P. D., Josephs S. F., Sturzenegger S., Kaplan M., Halligan G., Biberfeld P., Wong-Staal F., Kramarsky B. Isolation of a new virus, HBLV, in patients with lymphoproliferative disorders. Science. 1986 Oct 31;234(4776):596–601. doi: 10.1126/science.2876520. [DOI] [PubMed] [Google Scholar]
- Salmon M., Kitas G. D., Bacon P. A. Production of lymphokine mRNA by CD45R+ and CD45R- helper T cells from human peripheral blood and by human CD4+ T cell clones. J Immunol. 1989 Aug 1;143(3):907–912. [PubMed] [Google Scholar]
- Schmid D. S. The human MHC-restricted cellular response to herpes simplex virus type 1 is mediated by CD4+, CD8- T cells and is restricted to the DR region of the MHC complex. J Immunol. 1988 May 15;140(10):3610–3616. [PubMed] [Google Scholar]
- Shinkai Y., Yoshida M. C., Maeda K., Kobata T., Maruyama K., Yodoi J., Yagita H., Okumura K. Molecular cloning and chromosomal assignment of a human perforin (PFP) gene. Immunogenetics. 1989;30(6):452–457. doi: 10.1007/BF02421177. [DOI] [PubMed] [Google Scholar]
- Smyth M. J., Ortaldo J. R., Shinkai Y., Yagita H., Nakata M., Okumura K., Young H. A. Interleukin 2 induction of pore-forming protein gene expression in human peripheral blood CD8+ T cells. J Exp Med. 1990 Apr 1;171(4):1269–1281. doi: 10.1084/jem.171.4.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steeper T. A., Horwitz C. A., Ablashi D. V., Salahuddin S. Z., Saxinger C., Saltzman R., Schwartz B. The spectrum of clinical and laboratory findings resulting from human herpesvirus-6 (HHV-6) in patients with mononucleosis-like illnesses not resulting from Epstein-Barr virus or cytomegalovirus. Am J Clin Pathol. 1990 Jun;93(6):776–783. doi: 10.1093/ajcp/93.6.776. [DOI] [PubMed] [Google Scholar]
- Takahashi K., Sonoda S., Higashi K., Kondo T., Takahashi H., Takahashi M., Yamanishi K. Predominant CD4 T-lymphocyte tropism of human herpesvirus 6-related virus. J Virol. 1989 Jul;63(7):3161–3163. doi: 10.1128/jvi.63.7.3161-3163.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedder R. S., Briggs M., Cameron C. H., Honess R., Robertson D., Whittle H. A novel lymphotropic herpesvirus. Lancet. 1987 Aug 15;2(8555):390–392. doi: 10.1016/s0140-6736(87)92404-4. [DOI] [PubMed] [Google Scholar]
- Yakushijin Y., Yasukawa M., Kobayashi Y. T-cell immune response to human herpesvirus-6 in healthy adults. Microbiol Immunol. 1991;35(8):655–660. doi: 10.1111/j.1348-0421.1991.tb01597.x. [DOI] [PubMed] [Google Scholar]
- Yamanishi K., Okuno T., Shiraki K., Takahashi M., Kondo T., Asano Y., Kurata T. Identification of human herpesvirus-6 as a causal agent for exanthem subitum. Lancet. 1988 May 14;1(8594):1065–1067. doi: 10.1016/s0140-6736(88)91893-4. [DOI] [PubMed] [Google Scholar]
- Yasukawa M., Inatsuki A., Horiuchi T., Kobayashi Y. Functional heterogeneity among herpes simplex virus-specific human CD4+ T cells. J Immunol. 1991 Feb 15;146(4):1341–1347. [PubMed] [Google Scholar]
- Yasukawa M., Inatsuki A., Kobayashi Y. Differential in vitro activation of CD4+CD8- and CD8+CD4- herpes simplex virus-specific human cytotoxic T cells. J Immunol. 1989 Sep 15;143(6):2051–2057. [PubMed] [Google Scholar]
- Yasukawa M., Inatsuki A., Kobayashi Y. Helper activity in antigen-specific antibody production mediated by CD4+ human cytotoxic T cell clones directed against herpes simplex virus. J Immunol. 1988 May 15;140(10):3419–3425. [PubMed] [Google Scholar]
- Yasukawa M., Zarling J. M. Human cytotoxic T cell clones directed against herpes simplex virus-infected cells. I. Lysis restricted by HLA class II MB and DR antigens. J Immunol. 1984 Jul;133(1):422–427. [PubMed] [Google Scholar]
- Yasukawa M., Zarling J. M. Human cytotoxic T cell clones directed against herpes simplex virus-infected cells. II. Bifunctional clones with cytotoxic and virus-induced proliferative activities exhibit herpes simplex virus type 1 and 2 specific or type common reactivities. J Immunol. 1984 Nov;133(5):2736–2742. [PubMed] [Google Scholar]
- Yoshikawa T., Suga S., Asano Y., Yazaki T., Kodama H., Ozaki T. Distribution of antibodies to a causative agent of exanthem subitum (human herpesvirus-6) in healthy individuals. Pediatrics. 1989 Oct;84(4):675–677. [PubMed] [Google Scholar]
- Young J. D., Cohn Z. A., Podack E. R. The ninth component of complement and the pore-forming protein (perforin 1) from cytotoxic T cells: structural, immunological, and functional similarities. Science. 1986 Jul 11;233(4760):184–190. doi: 10.1126/science.2425429. [DOI] [PubMed] [Google Scholar]