Abstract
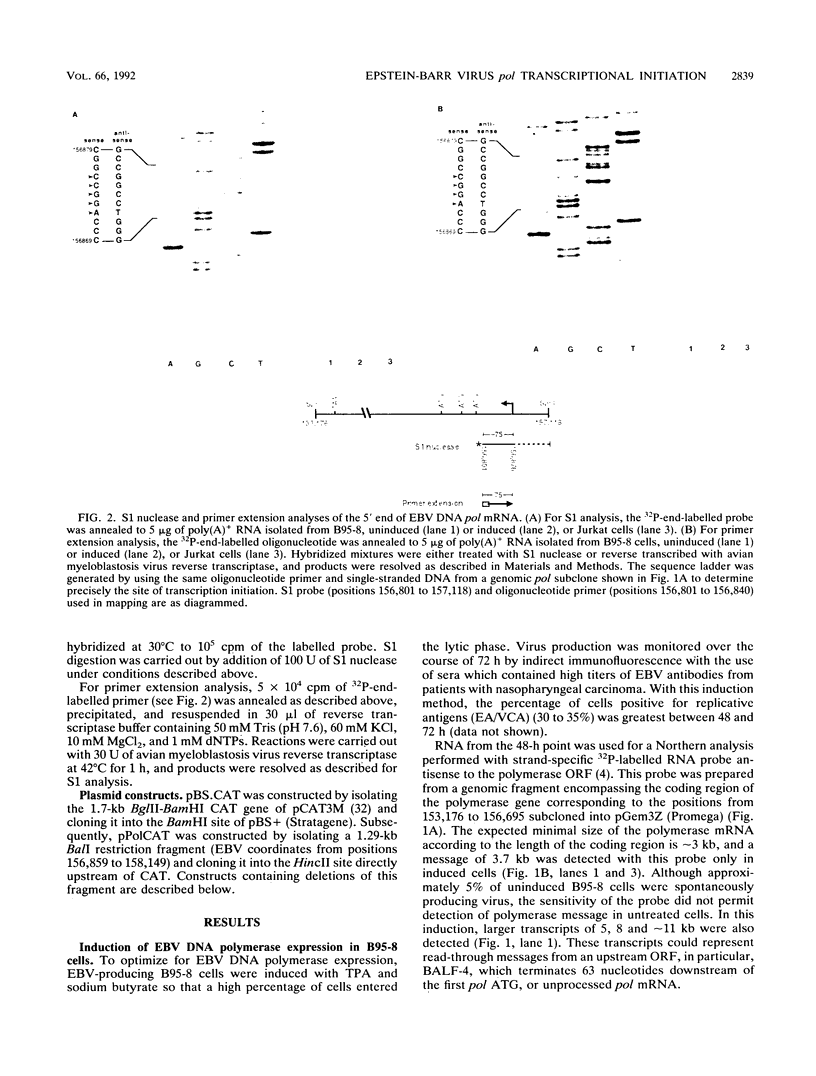
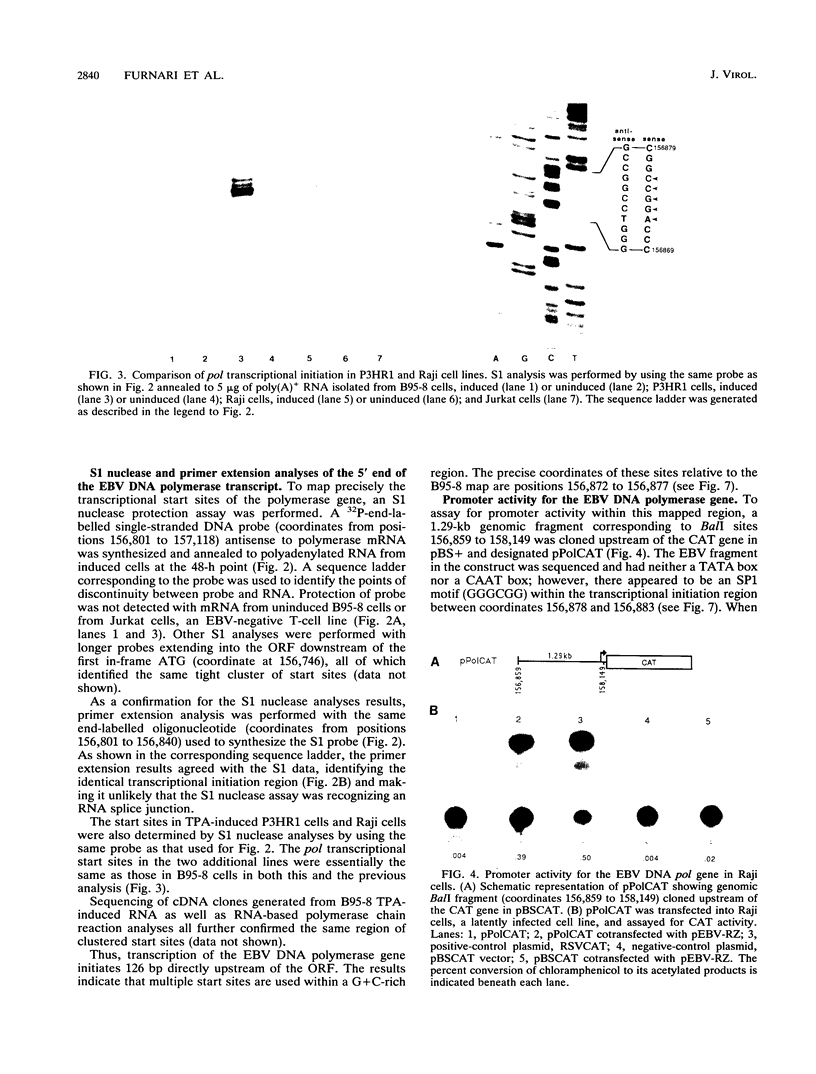
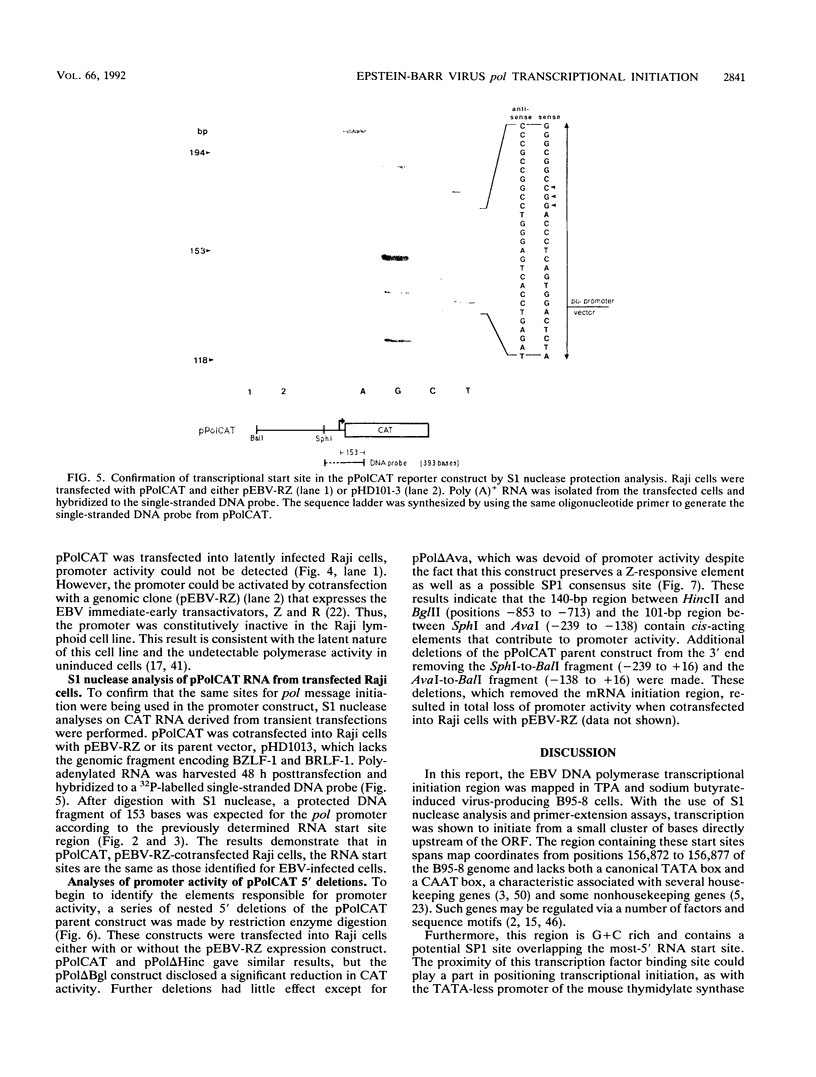
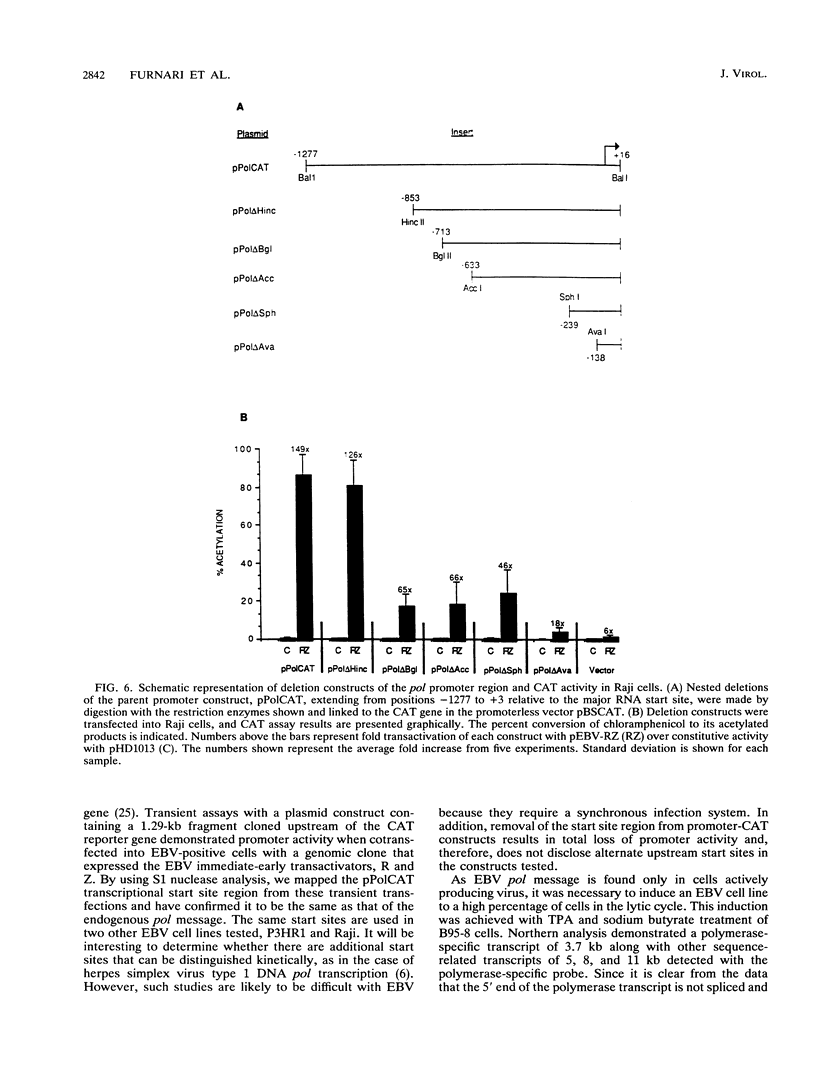
The gene (pol) encoding the Epstein-Barr virus (EBV) DNA polymerase is a member of the "early" class of viral genes which are expressed shortly after activation of latent virus infection. First, mRNA from the EBV-producing cell line, B95-8, treated with 12-O-tetradecanoylphorbol-13-acetate and sodium butyrate to induce lytic replication and expression of this gene was analyzed. Northern (RNA) analysis revealed a message of 3.7 kb found only in induced cells. 5' mapping of pol mRNA by S1 nuclease and primer extension analyses indicates that transcription initiates at tightly clustered sites within a G + C-rich region 126 bp upstream of the open reading frame. The same initiation region was identified in two other EBV-infected cell lines, P3HR1 and Raji, after induction. Second, a 1.29-kb genomic fragment containing this region, when cloned upstream of the chloramphenicol acetyltransferase reporter gene, demonstrated promoter activity in lymphoid cells cotransfected with pEBV-RZ, a genomic expression construct that includes genes for the EBV immediate-early transactivator proteins, BZLF-1 and BRLF-1. Within the upstream 1.29-kb sequence, two regions of 140 bp and 101 bp appear to be needed for promoter activity. These results demonstrate that unlike most EBV genes studied thus far, the pol gene contains multiple transcriptional start sites. The upstream regulatory region of the promoter for the pol gene does not contain canonical promoter elements such as TATA and CAAT boxes and, furthermore, is not constitutively active but requires transactivation by two or more viral proteins.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ayer D. E., Dynan W. S. Simian virus 40 major late promoter: a novel tripartite structure that includes intragenic sequences. Mol Cell Biol. 1988 May;8(5):2021–2033. doi: 10.1128/mcb.8.5.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azizkhan J. C., Vaughn J. P., Christy R. J., Hamlin J. L. Nucleotide sequence and nuclease hypersensitivity of the Chinese hamster dihydrofolate reductase gene promoter region. Biochemistry. 1986 Oct 7;25(20):6228–6236. doi: 10.1021/bi00368a059. [DOI] [PubMed] [Google Scholar]
- Baer R., Bankier A. T., Biggin M. D., Deininger P. L., Farrell P. J., Gibson T. J., Hatfull G., Hudson G. S., Satchwell S. C., Séguin C. DNA sequence and expression of the B95-8 Epstein-Barr virus genome. Nature. 1984 Jul 19;310(5974):207–211. doi: 10.1038/310207a0. [DOI] [PubMed] [Google Scholar]
- Blasband A. J., Rogers K. T., Chen X. R., Azizkhan J. C., Lee D. C. Characterization of the rat transforming growth factor alpha gene and identification of promoter sequences. Mol Cell Biol. 1990 May;10(5):2111–2121. doi: 10.1128/mcb.10.5.2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bludau H., Freese U. K. Analysis of the HSV-1 strain 17 DNA polymerase gene reveals the expression of four different classes of pol transcripts. Virology. 1991 Aug;183(2):505–518. doi: 10.1016/0042-6822(91)90980-p. [DOI] [PubMed] [Google Scholar]
- Buisson M., Manet E., Trescol-Biemont M. C., Gruffat H., Durand B., Sergeant A. The Epstein-Barr virus (EBV) early protein EB2 is a posttranscriptional activator expressed under the control of EBV transcription factors EB1 and R. J Virol. 1989 Dec;63(12):5276–5284. doi: 10.1128/jvi.63.12.5276-5284.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M. R., Hsu T. Y., Chen J. Y., Yang C. S. Molecular characterization of a cDNA clone encoding the Epstein-Barr virus (EBV) DNase. J Virol Methods. 1990 Aug;29(2):127–141. doi: 10.1016/0166-0934(90)90107-q. [DOI] [PubMed] [Google Scholar]
- Chevallier-Greco A., Manet E., Chavrier P., Mosnier C., Daillie J., Sergeant A. Both Epstein-Barr virus (EBV)-encoded trans-acting factors, EB1 and EB2, are required to activate transcription from an EBV early promoter. EMBO J. 1986 Dec 1;5(12):3243–3249. doi: 10.1002/j.1460-2075.1986.tb04635.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Countryman J., Miller G. Activation of expression of latent Epstein-Barr herpesvirus after gene transfer with a small cloned subfragment of heterogeneous viral DNA. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4085–4089. doi: 10.1073/pnas.82.12.4085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta A. K., Colby B. M., Shaw J. E., Pagano J. S. Acyclovir inhibition of Epstein-Barr virus replication. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5163–5166. doi: 10.1073/pnas.77.9.5163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta A. K., Feighny R. J., Pagano J. S. Induction of Epstein-Barr virus-associated DNA polymerase by 12-O-tetradecanoylphorbol-13-acetate. Purification and characterization. J Biol Chem. 1980 Jun 10;255(11):5120–5125. [PubMed] [Google Scholar]
- DeLeon D. V., Cox K. H., Angerer L. M., Angerer R. C. Most early-variant histone mRNA is contained in the pronucleus of sea urchin eggs. Dev Biol. 1983 Nov;100(1):197–206. doi: 10.1016/0012-1606(83)90211-7. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dynan W. S., Tjian R. The promoter-specific transcription factor Sp1 binds to upstream sequences in the SV40 early promoter. Cell. 1983 Nov;35(1):79–87. doi: 10.1016/0092-8674(83)90210-6. [DOI] [PubMed] [Google Scholar]
- Farrell P. J., Rowe D. T., Rooney C. M., Kouzarides T. Epstein-Barr virus BZLF1 trans-activator specifically binds to a consensus AP-1 site and is related to c-fos. EMBO J. 1989 Jan;8(1):127–132. doi: 10.1002/j.1460-2075.1989.tb03356.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feighny R. J., Henry B. E., 2nd, Datta A. K., Pagano J. S. Induction of DNA polymerase activity after superinfection of Raji cells with Epstein-Barr virus. Virology. 1980 Dec;107(2):415–423. doi: 10.1016/0042-6822(80)90308-6. [DOI] [PubMed] [Google Scholar]
- Gorman C. M., Moffat L. F., Howard B. H. Recombinant genomes which express chloramphenicol acetyltransferase in mammalian cells. Mol Cell Biol. 1982 Sep;2(9):1044–1051. doi: 10.1128/mcb.2.9.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruffat H., Manet E., Rigolet A., Sergeant A. The enhancer factor R of Epstein-Barr virus (EBV) is a sequence-specific DNA binding protein. Nucleic Acids Res. 1990 Dec 11;18(23):6835–6843. doi: 10.1093/nar/18.23.6835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardwick J. M., Lieberman P. M., Hayward S. D. A new Epstein-Barr virus transactivator, R, induces expression of a cytoplasmic early antigen. J Virol. 1988 Jul;62(7):2274–2284. doi: 10.1128/jvi.62.7.2274-2284.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinuma Y., Konn M., Yamaguchi J., Wudarski D. J., Blakeslee J. R., Jr, Grace J. T., Jr Immunofluorescence and herpes-type virus particles in the P3HR-1 Burkitt lymphoma cell line. J Virol. 1967 Oct;1(5):1045–1051. doi: 10.1128/jvi.1.5.1045-1051.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holley-Guthrie E. A., Quinlivan E. B., Mar E. C., Kenney S. The Epstein-Barr virus (EBV) BMRF1 promoter for early antigen (EA-D) is regulated by the EBV transactivators, BRLF1 and BZLF1, in a cell-specific manner. J Virol. 1990 Aug;64(8):3753–3759. doi: 10.1128/jvi.64.8.3753-3759.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii S., Kadonaga J. T., Tjian R., Brady J. N., Merlino G. T., Pastan I. Binding of the Sp1 transcription factor by the human Harvey ras1 proto-oncogene promoter. Science. 1986 Jun 13;232(4756):1410–1413. doi: 10.1126/science.3012774. [DOI] [PubMed] [Google Scholar]
- Iwasaki K., Temin H. M. The efficiency of RNA 3'-end formation is determined by the distance between the cap site and the poly(A) site in spleen necrosis virus. Genes Dev. 1990 Dec;4(12B):2299–2307. doi: 10.1101/gad.4.12b.2299. [DOI] [PubMed] [Google Scholar]
- Jolliff K., Li Y., Johnson L. F. Multiple protein-DNA interactions in the TATAA-less mouse thymidylate synthase promoter. Nucleic Acids Res. 1991 May 11;19(9):2267–2274. doi: 10.1093/nar/19.9.2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kallin B., Sternås L., Saemundssen A. K., Luka J., Jörnvall H., Eriksson B., Tao P. Z., Nilsson M. T., Klein G. Purification of Epstein-Barr virus DNA polymerase from P3HR-1 cells. J Virol. 1985 May;54(2):561–568. doi: 10.1128/jvi.54.2.561-568.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney S., Kamine J., Holley-Guthrie E., Lin J. C., Mar E. C., Pagano J. The Epstein-Barr virus (EBV) BZLF1 immediate-early gene product differentially affects latent versus productive EBV promoters. J Virol. 1989 Apr;63(4):1729–1736. doi: 10.1128/jvi.63.4.1729-1736.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenney S., Kamine J., Holley-Guthrie E., Mar E. C., Lin J. C., Markovitz D., Pagano J. The Epstein-Barr virus immediate-early gene product, BMLF1, acts in trans by a posttranscriptional mechanism which is reporter gene dependent. J Virol. 1989 Sep;63(9):3870–3877. doi: 10.1128/jvi.63.9.3870-3877.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiehl A., Dorsky D. I. Cooperation of EBV DNA polymerase and EA-D(BMRF1) in vitro and colocalization in nuclei of infected cells. Virology. 1991 Sep;184(1):330–340. doi: 10.1016/0042-6822(91)90849-7. [DOI] [PubMed] [Google Scholar]
- Kouzarides T., Bankier A. T., Satchwell S. C., Weston K., Tomlinson P., Barrell B. G. Sequence and transcription analysis of the human cytomegalovirus DNA polymerase gene. J Virol. 1987 Jan;61(1):125–133. doi: 10.1128/jvi.61.1.125-133.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Influences of mRNA secondary structure on initiation by eukaryotic ribosomes. Proc Natl Acad Sci U S A. 1986 May;83(9):2850–2854. doi: 10.1073/pnas.83.9.2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laimins L. A., Gruss P., Pozzatti R., Khoury G. Characterization of enhancer elements in the long terminal repeat of Moloney murine sarcoma virus. J Virol. 1984 Jan;49(1):183–189. doi: 10.1128/jvi.49.1.183-189.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman P. M., Berk A. J. In vitro transcriptional activation, dimerization, and DNA-binding specificity of the Epstein-Barr virus Zta protein. J Virol. 1990 Jun;64(6):2560–2568. doi: 10.1128/jvi.64.6.2560-2568.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lieberman P. M., Hardwick J. M., Sample J., Hayward G. S., Hayward S. D. The zta transactivator involved in induction of lytic cycle gene expression in Epstein-Barr virus-infected lymphocytes binds to both AP-1 and ZRE sites in target promoter and enhancer regions. J Virol. 1990 Mar;64(3):1143–1155. doi: 10.1128/jvi.64.3.1143-1155.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J. C., Shaw J. E., Smith M. C., Pagano J. S. Effect of 12-O-tetradecanoyl-phorbol-13-acetate on the replication of Epstein-Barr virus. I. Characterization of viral DNA. Virology. 1979 Nov;99(1):183–187. doi: 10.1016/0042-6822(79)90052-7. [DOI] [PubMed] [Google Scholar]
- Lin J. C., Sista N. D., Besençon F., Kamine J., Pagano J. S. Identification and functional characterization of Epstein-Barr virus DNA polymerase by in vitro transcription-translation of a cloned gene. J Virol. 1991 May;65(5):2728–2731. doi: 10.1128/jvi.65.5.2728-2731.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luka J., Kallin B., Klein G. Induction of the Epstein-Barr virus (EBV) cycle in latently infected cells by n-butyrate. Virology. 1979 Apr 15;94(1):228–231. doi: 10.1016/0042-6822(79)90455-0. [DOI] [PubMed] [Google Scholar]
- Miller G., Shope T., Lisco H., Stitt D., Lipman M. Epstein-Barr virus: transformation, cytopathic changes, and viral antigens in squirrel monkey and marmoset leukocytes. Proc Natl Acad Sci U S A. 1972 Feb;69(2):383–387. doi: 10.1073/pnas.69.2.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller R. L., Glaser R., Rapp F. Studies of an Epstein-Barr virus-induced DNA polymerase. Virology. 1977 Feb;76(2):494–502. doi: 10.1016/0042-6822(77)90232-x. [DOI] [PubMed] [Google Scholar]
- Neumann E., Schaefer-Ridder M., Wang Y., Hofschneider P. H. Gene transfer into mouse lyoma cells by electroporation in high electric fields. EMBO J. 1982;1(7):841–845. doi: 10.1002/j.1460-2075.1982.tb01257.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nutter L. M., Grill S. P., Li J. S., Tan R. S., Cheng Y. C. Induction of virus enzymes by phorbol esters and n-butyrate in Epstein-Barr virus genome-carrying Raji cells. Cancer Res. 1987 Aug 15;47(16):4407–4412. [PubMed] [Google Scholar]
- PULVERTAFT J. V. A STUDY OF MALIGNANT TUMOURS IN NIGERIA BY SHORT-TERM TISSUE CULTURE. J Clin Pathol. 1965 May;18:261–273. doi: 10.1136/jcp.18.3.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkin N. T., Cohen E. A., Darveau A., Rosen C., Haseltine W., Sonenberg N. Mutational analysis of the 5' non-coding region of human immunodeficiency virus type 1: effects of secondary structure on translation. EMBO J. 1988 Sep;7(9):2831–2837. doi: 10.1002/j.1460-2075.1988.tb03139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier J., Sonenberg N. Insertion mutagenesis to increase secondary structure within the 5' noncoding region of a eukaryotic mRNA reduces translational efficiency. Cell. 1985 Mar;40(3):515–526. doi: 10.1016/0092-8674(85)90200-4. [DOI] [PubMed] [Google Scholar]
- Smale S. T., Baltimore D. The "initiator" as a transcription control element. Cell. 1989 Apr 7;57(1):103–113. doi: 10.1016/0092-8674(89)90176-1. [DOI] [PubMed] [Google Scholar]
- Takada K., Ono Y. Synchronous and sequential activation of latently infected Epstein-Barr virus genomes. J Virol. 1989 Jan;63(1):445–449. doi: 10.1128/jvi.63.1.445-449.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada K., Shimizu N., Sakuma S., Ono Y. trans activation of the latent Epstein-Barr virus (EBV) genome after transfection of the EBV DNA fragment. J Virol. 1986 Mar;57(3):1016–1022. doi: 10.1128/jvi.57.3.1016-1022.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urier G., Buisson M., Chambard P., Sergeant A. The Epstein-Barr virus early protein EB1 activates transcription from different responsive elements including AP-1 binding sites. EMBO J. 1989 May;8(5):1447–1453. doi: 10.1002/j.1460-2075.1989.tb03527.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valerio D., Duyvesteyn M. G., Dekker B. M., Weeda G., Berkvens T. M., van der Voorn L., van Ormondt H., van der Eb A. J. Adenosine deaminase: characterization and expression of a gene with a remarkable promoter. EMBO J. 1985 Feb;4(2):437–443. doi: 10.1002/j.1460-2075.1985.tb03648.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weichs an der Glon C., Monks J., Proudfoot N. J. Occlusion of the HIV poly(A) site. Genes Dev. 1991 Feb;5(2):244–253. doi: 10.1101/gad.5.2.244. [DOI] [PubMed] [Google Scholar]
- Yager D. R., Coen D. M. Analysis of the transcript of the herpes simplex virus DNA polymerase gene provides evidence that polymerase expression is inefficient at the level of translation. J Virol. 1988 Jun;62(6):2007–2015. doi: 10.1128/jvi.62.6.2007-2015.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]