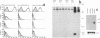Abstract
The tumorigenicity of adenovirus type 12 (Ad12)-transformed cells has been attributed to the low levels of class I major histocompatibility complex (MHC) protein expression by these cells. These levels of class I proteins are thought to be below the threshold critical for cytotoxic T-lymphocyte recognition, a process that may be involved in tumor cell immunosurveillance. We have used gene transfer experiments to investigate the role played by class I protein expression in the tumorigenicity of Ad12-transformed BALB/c mouse cells in naive, syngeneic adult mice. Our Ad12-transformed mouse cells were tumorigenic in adult mice and were similar to other Ad12-transformed mammalian cells in that they expressed low levels of class I MHC mRNA and cell surface proteins. Despite these low levels of expression, the cells were highly immunogenic in syngeneic mice and were rejected as allografts by allogeneic mice. Transfection of genomic H-2Dd or H-2Ld fragments into these cells produced a variety of cell clones that expressed increased levels of cell surface class I proteins. These cells expressing high levels of class I protein were up to 16-fold more tumorigenic than the parental cells in syngeneic adult mice. Thus, by quantitative assays, the tumorigenicity of Ad12-transformed BALB/c mouse cells is not functionally related to the low levels of class I MHC proteins they express. The increased tumorigenicity expressed by H-2Dd- and H-2Ld-transfected cells was not detected in BALB/c nu/nu mice, suggesting that a thymus-dependent mechanism that is not mediated by evasion of cytotoxic T-lymphocyte recognition could contribute to the difference in tumorigenicity of Ad12-transformed BALB/c mouse cells that express low and high levels of class I MHC proteins.
Full text
PDF

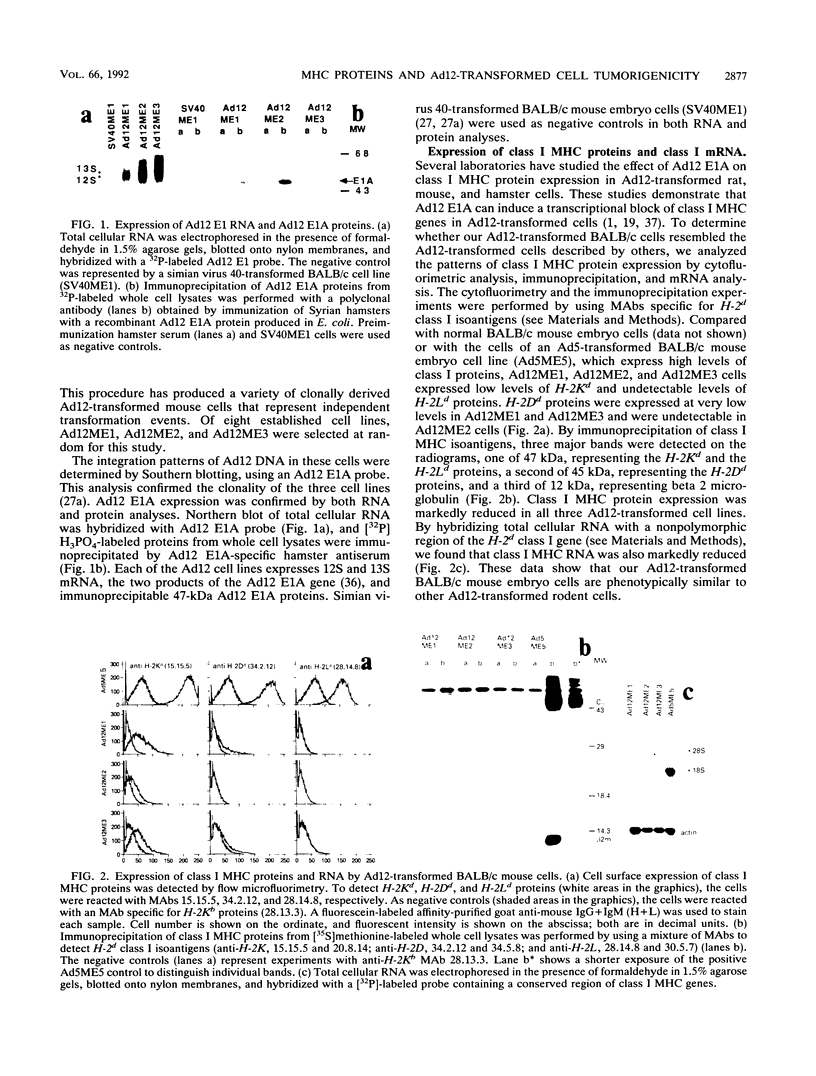
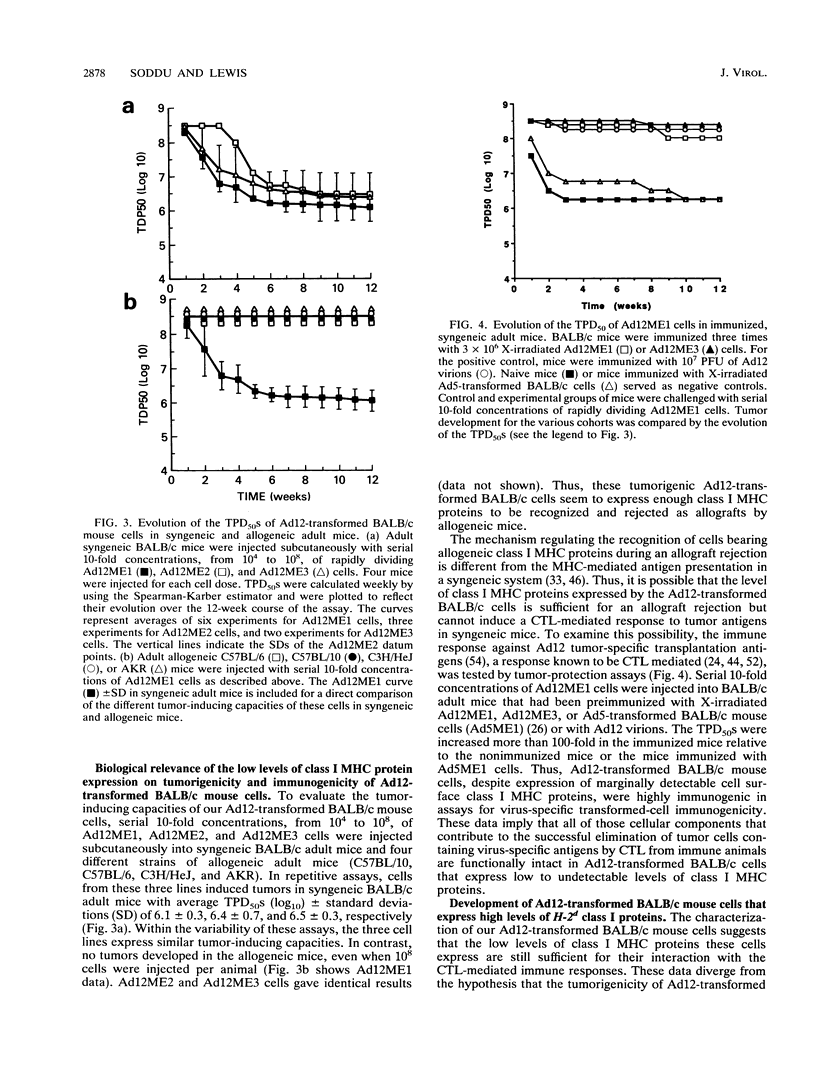
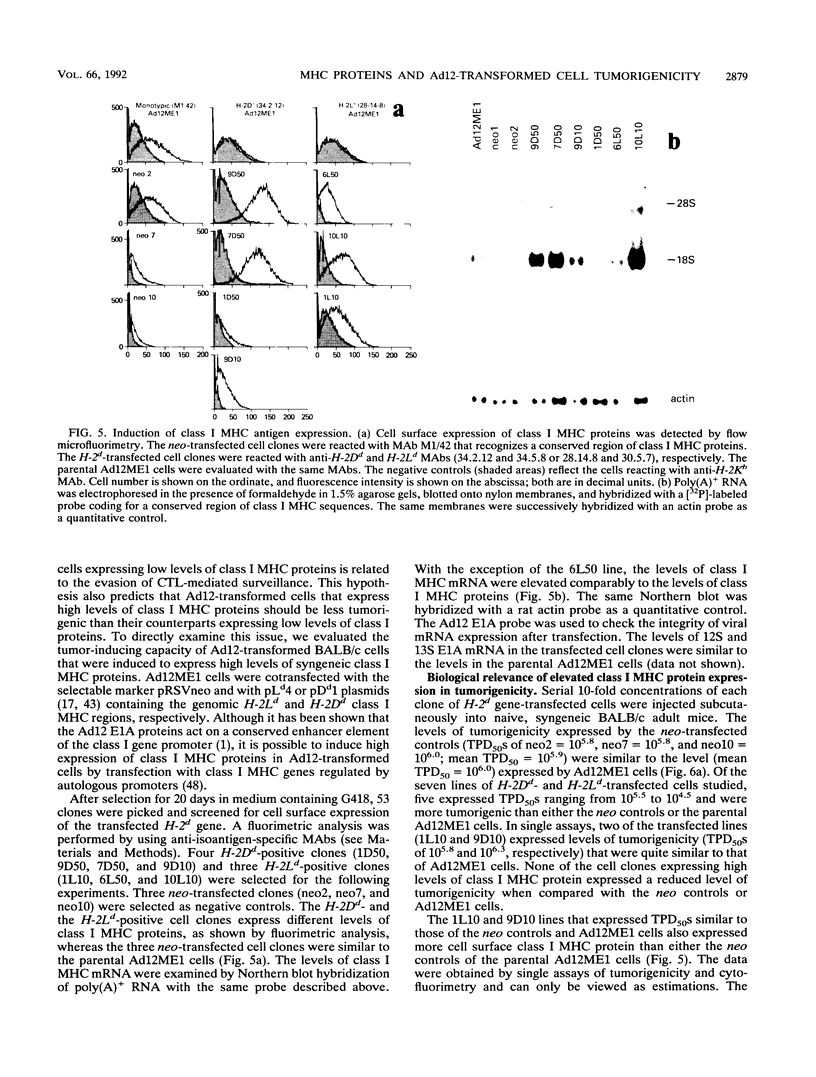
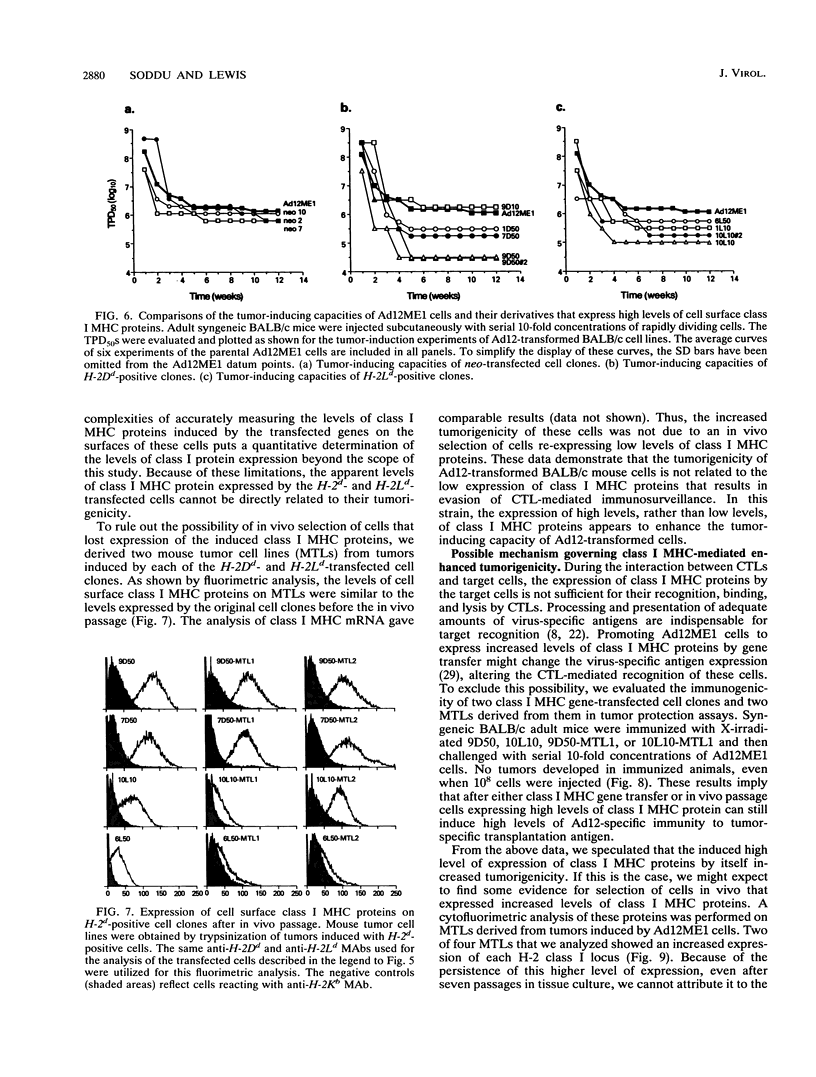
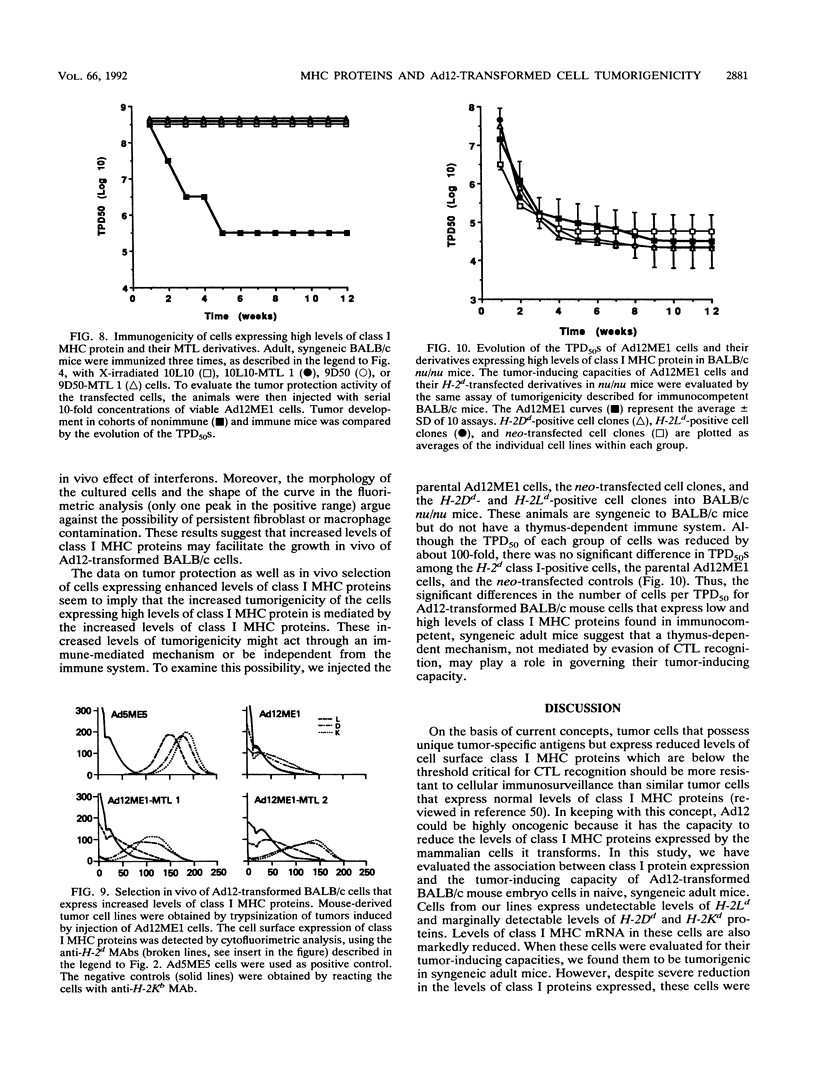



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ackrill A. M., Blair G. E. Nuclear proteins binding to an enhancer element of the major histocompatibility class I promoter: differences between highly oncogenic and nononcogenic adenovirus-transformed rat cells. Virology. 1989 Oct;172(2):643–646. doi: 10.1016/0042-6822(89)90207-9. [DOI] [PubMed] [Google Scholar]
- Akagi K., Patch C. T., Cook J. L., Kato T., Lewis A. M., Jr, Levine A. S. The level of expression of adenovirus type 2 transforming genes governs sensitivity to nonspecific immune cytolysis and other phenotypic properties of adenovirus 2-simian virus 40-transformed cell hybrids. Mol Cell Biol. 1985 Aug;5(8):1870–1877. doi: 10.1128/mcb.5.8.1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison A. C., Berman L. D., Levey R. H. Increased tumour induction by adenovirus type 12 in thymectomized mice and mice treated with anti-lymphocyte serum. Nature. 1967 Jul 8;215(5097):185–187. doi: 10.1038/215185a0. [DOI] [PubMed] [Google Scholar]
- Bernards R., Schrier P. I., Houweling A., Bos J. L., van der Eb A. J., Zijlstra M., Melief C. J. Tumorigenicity of cells transformed by adenovirus type 12 by evasion of T-cell immunity. 1983 Oct 27-Nov 2Nature. 305(5937):776–779. doi: 10.1038/305776a0. [DOI] [PubMed] [Google Scholar]
- Biassoni R., Ferrini S., Prigione I., Moretta A., Long E. O. CD3-negative lymphokine-activated cytotoxic cells express the CD3 epsilon gene. J Immunol. 1988 Mar 1;140(5):1685–1689. [PubMed] [Google Scholar]
- Bober F. J., Birk D. E., Shenk T., Raska K., Jr Tumorigenicity of adenovirus-transformed cells: collagen interaction and cell surface laminin are controlled by the serotype origin of the E1A and E1B genes. J Virol. 1988 Feb;62(2):580–585. doi: 10.1128/jvi.62.2.580-585.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braciale T. J., Morrison L. A., Sweetser M. T., Sambrook J., Gething M. J., Braciale V. L. Antigen presentation pathways to class I and class II MHC-restricted T lymphocytes. Immunol Rev. 1987 Aug;98:95–114. doi: 10.1111/j.1600-065x.1987.tb00521.x. [DOI] [PubMed] [Google Scholar]
- Byrd P. J., Chia W., Rigby P. W., Gallimore P. H. Cloning of DNA fragments from the left end of the adenovirus type 12 genome: transformation by cloned early region 1. J Gen Virol. 1982 Jun;60(Pt 2):279–293. doi: 10.1099/0022-1317-60-2-279. [DOI] [PubMed] [Google Scholar]
- Cook J. L., Hauser J., Patch C. T., Lewis A. M., Jr, Levine A. S. Adenovirus 2 early gene expression promotes susceptibility to effector cell lysis of hybrids formed between hamster cells transformed by adenovirus 2 and simian virus 40. Proc Natl Acad Sci U S A. 1983 Oct;80(19):5995–5999. doi: 10.1073/pnas.80.19.5995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook J. L., Hibbs J. B., Jr, Lewis A. M., Jr DNA virus-transformed hamster cell--host effector cell interactions: level of resistance to cytolysis correlated with tumorigenicity. Int J Cancer. 1982 Dec 15;30(6):795–803. doi: 10.1002/ijc.2910300619. [DOI] [PubMed] [Google Scholar]
- Cook J. L., Lewis A. M., Jr Host response to adenovirus 2-transformed hamster embryo cells. Cancer Res. 1979 May;39(5):1455–1461. [PubMed] [Google Scholar]
- Cook J. L., May D. L., Lewis A. M., Jr, Walker T. A. Adenovirus E1A gene induction of susceptibility to lysis by natural killer cells and activated macrophages in infected rodent cells. J Virol. 1987 Nov;61(11):3510–3520. doi: 10.1128/jvi.61.11.3510-3520.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle A., Martin W. J., Funa K., Gazdar A., Carney D., Martin S. E., Linnoila I., Cuttitta F., Mulshine J., Bunn P. Markedly decreased expression of class I histocompatibility antigens, protein, and mRNA in human small-cell lung cancer. J Exp Med. 1985 May 1;161(5):1135–1151. doi: 10.1084/jem.161.5.1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eager K. B., Williams J., Breiding D., Pan S., Knowles B., Appella E., Ricciardi R. P. Expression of histocompatibility antigens H-2K, -D, and -L is reduced in adenovirus-12-transformed mouse cells and is restored by interferon gamma. Proc Natl Acad Sci U S A. 1985 Aug;82(16):5525–5529. doi: 10.1073/pnas.82.16.5525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans G. A., Margulies D. H., Camerini-Otero R. D., Ozato K., Seidman J. G. Structure and expression of a mouse major histocompatibility antigen gene, H-2Ld. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1994–1998. doi: 10.1073/pnas.79.6.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans G. A., Margulies D. H., Shykind B., Seidman J. G., Ozato K. Exon shuffling: mapping polymorphic determinants on hybrid mouse transplantation antigens. Nature. 1982 Dec 23;300(5894):755–757. doi: 10.1038/300755a0. [DOI] [PubMed] [Google Scholar]
- Fearon E. R., Pardoll D. M., Itaya T., Golumbek P., Levitsky H. I., Simons J. W., Karasuyama H., Vogelstein B., Frost P. Interleukin-2 production by tumor cells bypasses T helper function in the generation of an antitumor response. Cell. 1990 Feb 9;60(3):397–403. doi: 10.1016/0092-8674(90)90591-2. [DOI] [PubMed] [Google Scholar]
- Friedman D. J., Ricciardi R. P. Adenovirus type 12 E1A gene represses accumulation of MHC class I mRNAs at the level of transcription. Virology. 1988 Jul;165(1):303–305. doi: 10.1016/0042-6822(88)90689-7. [DOI] [PubMed] [Google Scholar]
- Gallimore P. H., Paraskeva C. A study to determine the reasons for differences in the tumorigenicity of rat cell lines transformed by adenovirus 2 and adenovirus 12. Cold Spring Harb Symp Quant Biol. 1980;44(Pt 1):703–713. doi: 10.1101/sqb.1980.044.01.075. [DOI] [PubMed] [Google Scholar]
- Gallimore P. H. Tumour production in immunosuppressed rats with cells transformed in vitro by adenovirus type 2. J Gen Virol. 1972 Jul;16(1):99–102. doi: 10.1099/0022-1317-16-1-99. [DOI] [PubMed] [Google Scholar]
- Germain R. N. Immunology. The ins and outs of antigen processing and presentation. Nature. 1986 Aug 21;322(6081):687–689. doi: 10.1038/322687a0. [DOI] [PubMed] [Google Scholar]
- Goodenow R. S., Vogel J. M., Linsk R. L. Histocompatibility antigens on murine tumors. Science. 1985 Nov 15;230(4727):777–783. doi: 10.1126/science.2997918. [DOI] [PubMed] [Google Scholar]
- Gooding L. R. Specificities of killing by T lymphocytes generated against syngeneic SV40 transformants: studies employing recombinants within the H-2 complex. J Immunol. 1979 Mar;122(3):1002–1008. [PubMed] [Google Scholar]
- HUEBNER R. J., ROWE W. P., LANE W. T. Oncogenic effects in hamsters of human adenovirus types 12 and 18. Proc Natl Acad Sci U S A. 1962 Dec 15;48:2051–2058. doi: 10.1073/pnas.48.12.2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddada H., Lewis A. M., Jr, Sogn J. A., Coligan J. E., Cook J. L., Walker T. A., Levine A. S. Tumorigenicity of hamster and mouse cells transformed by adenovirus types 2 and 5 is not influenced by the level of class I major histocompatibility antigens expressed on the cells. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9684–9688. doi: 10.1073/pnas.83.24.9684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddada H., Sogn J. A., Coligan J. E., Carbone M., Dixon K., Levine A. S., Lewis A. M., Jr Viral gene inhibition of class I major histocompatibility antigen expression: not a general mechanism governing the tumorigenicity of adenovirus type 2-, adenovirus type 12-, and simian virus 40-transformed Syrian hamster cells. J Virol. 1988 Aug;62(8):2755–2761. doi: 10.1128/jvi.62.8.2755-2761.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi H., Tanaka K., Jay F., Khoury G., Jay G. Modulation of the tumorigenicity of human adenovirus-12-transformed cells by interferon. Cell. 1985 Nov;43(1):263–267. doi: 10.1016/0092-8674(85)90031-5. [DOI] [PubMed] [Google Scholar]
- Hosken N. A., Bevan M. J. Defective presentation of endogenous antigen by a cell line expressing class I molecules. Science. 1990 Apr 20;248(4953):367–370. doi: 10.1126/science.2326647. [DOI] [PubMed] [Google Scholar]
- Kast W. M., Offringa R., Peters P. J., Voordouw A. C., Meloen R. H., van der Eb A. J., Melief C. J. Eradication of adenovirus E1-induced tumors by E1A-specific cytotoxic T lymphocytes. Cell. 1989 Nov 17;59(4):603–614. doi: 10.1016/0092-8674(89)90006-8. [DOI] [PubMed] [Google Scholar]
- Lafferty K. J., Prowse S. J., Simeonovic C. J., Warren H. S. Immunobiology of tissue transplantation: a return to the passenger leukocyte concept. Annu Rev Immunol. 1983;1:143–173. doi: 10.1146/annurev.iy.01.040183.001043. [DOI] [PubMed] [Google Scholar]
- Lampson L. A., Fisher C. A., Whelan J. P. Striking paucity of HLA-A, B, C and beta 2-microglobulin on human neuroblastoma cell lines. J Immunol. 1983 May;130(5):2471–2478. [PubMed] [Google Scholar]
- Lewis A. M., Jr, Cook J. L. The interface between adenovirus-transformed cells and cellular immune response in the challenged host. Curr Top Microbiol Immunol. 1984;110:1–22. doi: 10.1007/978-3-642-46494-2_1. [DOI] [PubMed] [Google Scholar]
- Lillie J. W., Green M., Green M. R. An adenovirus E1a protein region required for transformation and transcriptional repression. Cell. 1986 Sep 26;46(7):1043–1051. doi: 10.1016/0092-8674(86)90704-x. [DOI] [PubMed] [Google Scholar]
- Meijer I., Jochemsen A. G., de Wit C. M., Bos J. L., Morello D., van der Eb A. J. Adenovirus type 12 E1A down regulates expression of a transgene under control of a major histocompatibility complex class I promoter: evidence for transcriptional control. J Virol. 1989 Sep;63(9):4039–4042. doi: 10.1128/jvi.63.9.4039-4042.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellow G. H., Föhring B., Dougherty J., Gallimore P. H., Raska K., Jr Tumorigenicity of adenovirus-transformed rat cells and expression of class I major histocompatibility antigen. Virology. 1984 Apr 30;134(2):460–465. doi: 10.1016/0042-6822(84)90313-1. [DOI] [PubMed] [Google Scholar]
- Ohlén C., Bejarano M. T., Grönberg A., Torsteinsdottir S., Franksson L., Ljunggren H. G., Klein E., Klein G., Kärre K. Studies of sublines selected for loss of HLA expression from an EBV-transformed lymphoblastoid cell line. Changes in sensitivity to cytotoxic T cells activated by allostimulation and natural killer cells activated by IFN or IL-2. J Immunol. 1989 May 1;142(9):3336–3341. [PubMed] [Google Scholar]
- Piontek G. E., Taniguchi K., Ljunggren H. G., Grönberg A., Kiessling R., Klein G., Kärre K. YAC-1 MHC class I variants reveal an association between decreased NK sensitivity and increased H-2 expression after interferon treatment or in vivo passage. J Immunol. 1985 Dec;135(6):4281–4288. [PubMed] [Google Scholar]
- Raska K., Jr, Gallimore P. H. An inverse relation of the oncogenic potential of adenovirus-transformed cells and their sensitivity to killing by syngeneic natural killer cells. Virology. 1982 Nov;123(1):8–18. doi: 10.1016/0042-6822(82)90290-2. [DOI] [PubMed] [Google Scholar]
- Rothstein S. J., Reznikoff W. S. The functional differences in the inverted repeats of Tn5 are caused by a single base pair nonhomology. Cell. 1981 Jan;23(1):191–199. doi: 10.1016/0092-8674(81)90284-1. [DOI] [PubMed] [Google Scholar]
- Sawada Y., Urbanelli D., Raskova J., Shenk T. E., Raska K., Jr Adenovirus tumor-specific transplantation antigen is a function of the E1A early region. J Exp Med. 1986 Mar 1;163(3):563–572. doi: 10.1084/jem.163.3.563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrier P. I., Bernards R., Vaessen R. T., Houweling A., van der Eb A. J. Expression of class I major histocompatibility antigens switched off by highly oncogenic adenovirus 12 in transformed rat cells. 1983 Oct 27-Nov 2Nature. 305(5937):771–775. doi: 10.1038/305771a0. [DOI] [PubMed] [Google Scholar]
- Schwartz R. H. T-lymphocyte recognition of antigen in association with gene products of the major histocompatibility complex. Annu Rev Immunol. 1985;3:237–261. doi: 10.1146/annurev.iy.03.040185.001321. [DOI] [PubMed] [Google Scholar]
- Storkus W. J., Alexander J., Payne J. A., Dawson J. R., Cresswell P. Reversal of natural killing susceptibility in target cells expressing transfected class I HLA genes. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2361–2364. doi: 10.1073/pnas.86.7.2361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TRENTIN J. J., YABE Y., TAYLOR G. The quest for human cancer viruses. Science. 1962 Sep 14;137(3533):835–841. doi: 10.1126/science.137.3533.835. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Hayashi H., Hamada C., Khoury G., Jay G. Expression of major histocompatibility complex class I antigens as a strategy for the potentiation of immune recognition of tumor cells. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8723–8727. doi: 10.1073/pnas.83.22.8723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K., Isselbacher K. J., Khoury G., Jay G. Reversal of oncogenesis by the expression of a major histocompatibility complex class I gene. Science. 1985 Apr 5;228(4695):26–30. doi: 10.1126/science.3975631. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Yoshioka T., Bieberich C., Jay G. Role of the major histocompatibility complex class I antigens in tumor growth and metastasis. Annu Rev Immunol. 1988;6:359–380. doi: 10.1146/annurev.iy.06.040188.002043. [DOI] [PubMed] [Google Scholar]
- Tepper R. I., Pattengale P. K., Leder P. Murine interleukin-4 displays potent anti-tumor activity in vivo. Cell. 1989 May 5;57(3):503–512. doi: 10.1016/0092-8674(89)90925-2. [DOI] [PubMed] [Google Scholar]
- Tevethia S. S. Recognition of simian virus 40 T antigen by cytotoxic T lymphocytes. Mol Biol Med. 1990 Feb;7(1):83–96. [PubMed] [Google Scholar]
- Tomita Y., Mayumi H., Eto M., Nomoto K. Tumor allograft rejection is mainly mediated by CD8+ cytotoxic T lymphocytes stimulated with class I alloantigens in cooperation with CD4+ helper T cells recognizing class II alloantigens. J Immunol. 1990 Mar 15;144(6):2425–2435. [PubMed] [Google Scholar]
- Trentin J. J., Bryan E. Virus-induced transplantation immunity to human adenovirus type 12 tumors of the hamster and mouse. Proc Soc Exp Biol Med. 1966 Apr;121(4):1216–1219. doi: 10.3181/00379727-121-31009. [DOI] [PubMed] [Google Scholar]
- Wesslén T. SV40-tumorigenesis in mouse. Acta Pathol Microbiol Scand B Microbiol Immunol. 1970;78(4):479–487. doi: 10.1111/j.1699-0463.1970.tb04331.x. [DOI] [PubMed] [Google Scholar]