Abstract
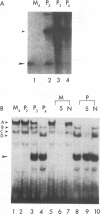
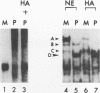
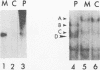
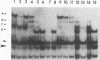
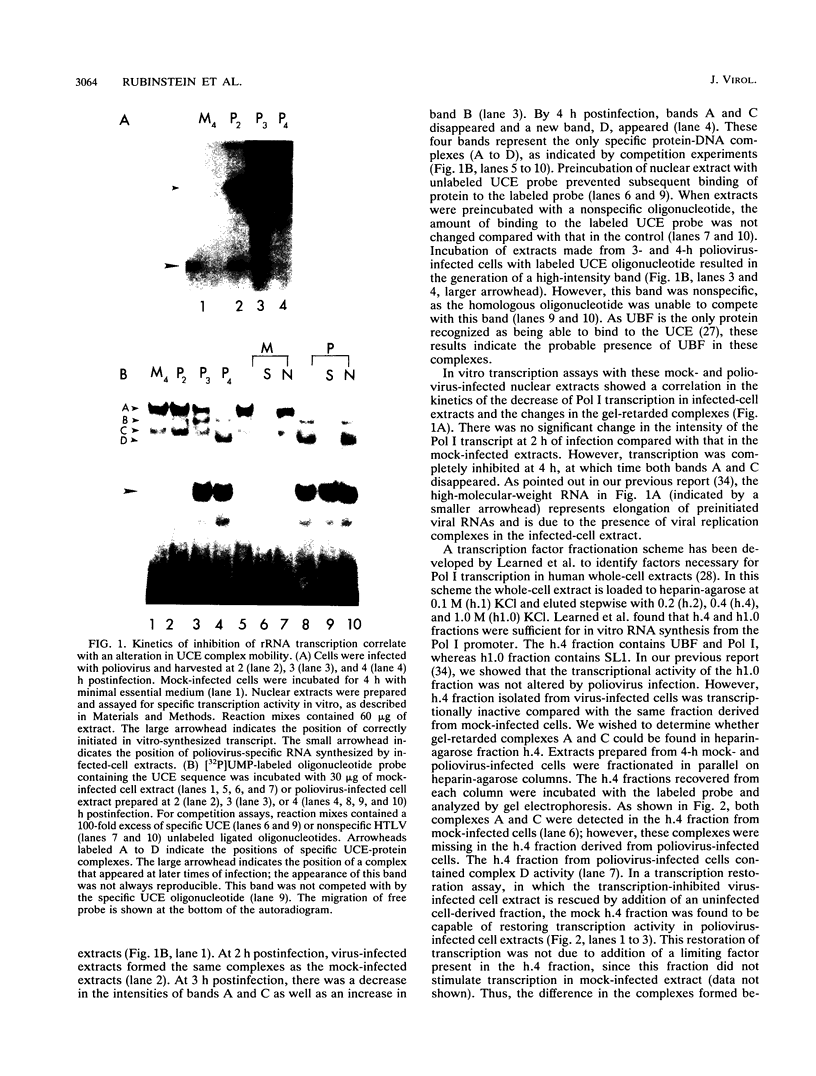
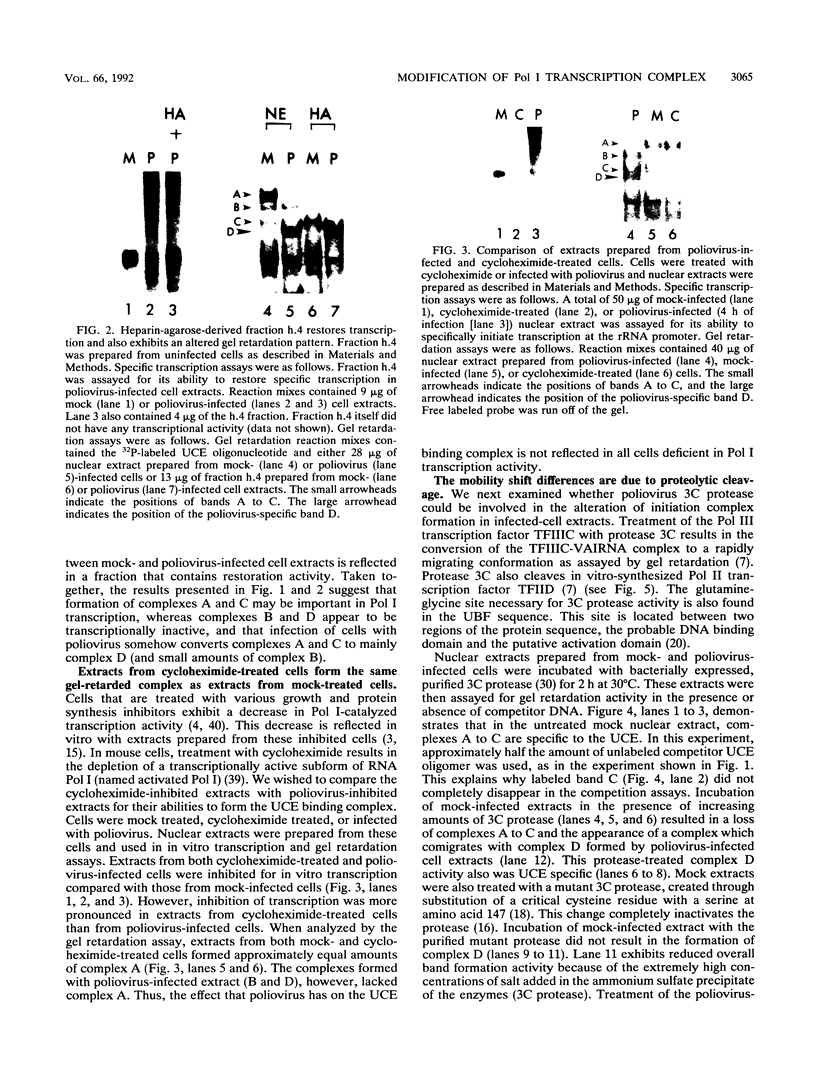
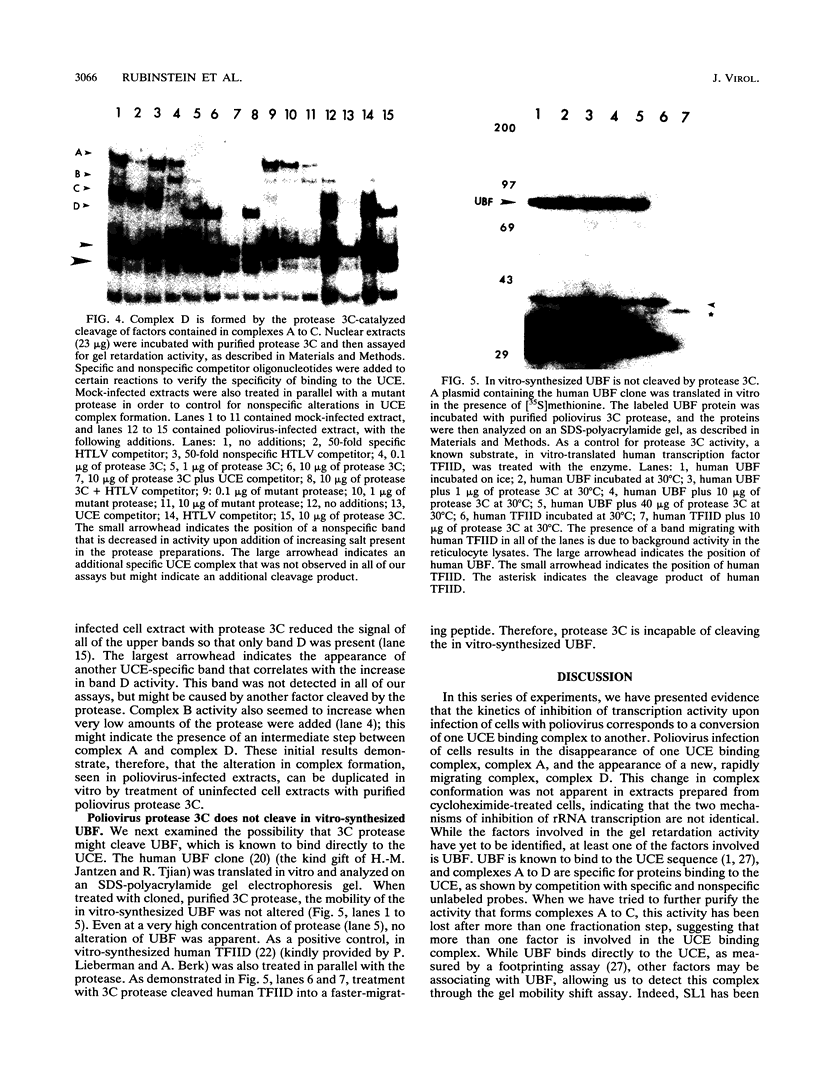
In HeLa cells, RNA polymerase I (Pol I)-mediated transcription is severely inhibited soon after infection with poliovirus. We have developed a gel retardation assay to analyze DNA-protein complexes formed at the Pol I promoter. We show here that two complexes (A and C) formed by nuclear extracts from uninfected cells disappear after infection of cells with poliovirus. In contrast, a new, rapidly migrating complex (D) is formed in virus-infected cell extract. This change in the mobility of gel-retarded complexes correlates well with the kinetics of inhibition of rRNA transcription in virus-infected cells. Incubation of nuclear extracts from mock-infected cells with bacterially expressed, purified poliovirus protease 3C results in the disappearance of complexes A and C with concomitant generation of complex D. A partially purified transcription factor fraction derived from uninfected cells that contains complex A is able to restore Pol I transcription when added to virus-infected cell extracts, suggesting that this complex plays an important role in Pol I transcription. These results suggest that poliovirus proteinase 3C may have an important role in the shutoff of Pol I transcription in cells infected with poliovirus.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bell S. P., Learned R. M., Jantzen H. M., Tjian R. Functional cooperativity between transcription factors UBF1 and SL1 mediates human ribosomal RNA synthesis. Science. 1988 Sep 2;241(4870):1192–1197. doi: 10.1126/science.3413483. [DOI] [PubMed] [Google Scholar]
- Bienz K., Egger D., Rasser Y., Bossart W. Accumulation of poliovirus proteins in the host cell nucleus. Intervirology. 1982;18(4):189–196. doi: 10.1159/000149324. [DOI] [PubMed] [Google Scholar]
- Cavanaugh A. H., Gokal P. K., Lawther R. P., Thompson E. A., Jr Glucocorticoid inhibition of initiation of transcription of the DNA encoding rRNA (rDNA) in lymphosarcoma P1798 cells. Proc Natl Acad Sci U S A. 1984 Feb;81(3):718–721. doi: 10.1073/pnas.81.3.718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavannaugh A. H., Thompson E. A., Jr Hormonal regulation of transcription of rDNA. Inhibition of transcription during glucocorticoid-mediated inhibition of proliferation of lymphosarcoma P1798 cells in culture. J Biol Chem. 1983 Aug 25;258(16):9768–9773. [PubMed] [Google Scholar]
- Clark M. E., Dasgupta A. A transcriptionally active form of TFIIIC is modified in poliovirus-infected HeLa cells. Mol Cell Biol. 1990 Oct;10(10):5106–5113. doi: 10.1128/mcb.10.10.5106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark M. E., Hämmerle T., Wimmer E., Dasgupta A. Poliovirus proteinase 3C converts an active form of transcription factor IIIC to an inactive form: a mechanism for inhibition of host cell polymerase III transcription by poliovirus. EMBO J. 1991 Oct;10(10):2941–2947. doi: 10.1002/j.1460-2075.1991.tb07844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras G., Summers D. F., Maizel J. V., Ehrenfeld E. HeLa cell nucleolar RNA synthesis after poliovirus infection. Virology. 1973 May;53(1):120–129. doi: 10.1016/0042-6822(73)90471-6. [DOI] [PubMed] [Google Scholar]
- Crawford N., Fire A., Samuels M., Sharp P. A., Baltimore D. Inhibition of transcription factor activity by poliovirus. Cell. 1981 Dec;27(3 Pt 2):555–561. doi: 10.1016/0092-8674(81)90397-4. [DOI] [PubMed] [Google Scholar]
- Dignam J. D., Lebovitz R. M., Roeder R. G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983 Mar 11;11(5):1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernández-Tomás C. The presence of viral-induced proteins in nuclei from poliovirus-infected HeLa cells. Virology. 1982 Jan 30;116(2):629–634. doi: 10.1016/0042-6822(82)90154-4. [DOI] [PubMed] [Google Scholar]
- Fradkin L. G., Yoshinaga S. K., Berk A. J., Dasgupta A. Inhibition of host cell RNA polymerase III-mediated transcription by poliovirus: inactivation of specific transcription factors. Mol Cell Biol. 1987 Nov;7(11):3880–3887. doi: 10.1128/mcb.7.11.3880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grummt I. Specific transcription of mouse ribosomal DNA in a cell-free system that mimics control in vivo. Proc Natl Acad Sci U S A. 1981 Feb;78(2):727–731. doi: 10.1073/pnas.78.2.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLLAND J. J., PETERSON J. A. NUCLEIC ACID AND PROTEIN SYNTHESIS DURING POLIOVIRUS INFECTION OF HUMAN CELLS. J Mol Biol. 1964 Apr;8:556–575. doi: 10.1016/s0022-2836(64)80011-5. [DOI] [PubMed] [Google Scholar]
- Hanecak R., Semler B. L., Anderson C. W., Wimmer E. Proteolytic processing of poliovirus polypeptides: antibodies to polypeptide P3-7c inhibit cleavage at glutamine-glycine pairs. Proc Natl Acad Sci U S A. 1982 Jul;79(13):3973–3977. doi: 10.1073/pnas.79.13.3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellen C. U., Kräusslich H. G., Wimmer E. Proteolytic processing of polyproteins in the replication of RNA viruses. Biochemistry. 1989 Dec 26;28(26):9881–9890. doi: 10.1021/bi00452a001. [DOI] [PubMed] [Google Scholar]
- Hämmerle T., Hellen C. U., Wimmer E. Site-directed mutagenesis of the putative catalytic triad of poliovirus 3C proteinase. J Biol Chem. 1991 Mar 25;266(9):5412–5416. [PubMed] [Google Scholar]
- Jantzen H. M., Admon A., Bell S. P., Tjian R. Nucleolar transcription factor hUBF contains a DNA-binding motif with homology to HMG proteins. Nature. 1990 Apr 26;344(6269):830–836. doi: 10.1038/344830a0. [DOI] [PubMed] [Google Scholar]
- Kadonaga J. T., Tjian R. Affinity purification of sequence-specific DNA binding proteins. Proc Natl Acad Sci U S A. 1986 Aug;83(16):5889–5893. doi: 10.1073/pnas.83.16.5889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao C. C., Lieberman P. M., Schmidt M. C., Zhou Q., Pei R., Berk A. J. Cloning of a transcriptionally active human TATA binding factor. Science. 1990 Jun 29;248(4963):1646–1650. doi: 10.1126/science.2194289. [DOI] [PubMed] [Google Scholar]
- Kliewer S., Dasgupta A. An RNA polymerase II transcription factor inactivated in poliovirus-infected cells copurifies with transcription factor TFIID. Mol Cell Biol. 1988 Aug;8(8):3175–3182. doi: 10.1128/mcb.8.8.3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kliewer S., Muchardt C., Gaynor R., Dasgupta A. Loss of a phosphorylated form of transcription factor CREB/ATF in poliovirus-infected cells. J Virol. 1990 Sep;64(9):4507–4515. doi: 10.1128/jvi.64.9.4507-4515.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kräusslich H. G., Nicklin M. J., Toyoda H., Etchison D., Wimmer E. Poliovirus proteinase 2A induces cleavage of eucaryotic initiation factor 4F polypeptide p220. J Virol. 1987 Sep;61(9):2711–2718. doi: 10.1128/jvi.61.9.2711-2718.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kräusslich H. G., Wimmer E. Viral proteinases. Annu Rev Biochem. 1988;57:701–754. doi: 10.1146/annurev.bi.57.070188.003413. [DOI] [PubMed] [Google Scholar]
- Learned R. M., Cordes S., Tjian R. Purification and characterization of a transcription factor that confers promoter specificity to human RNA polymerase I. Mol Cell Biol. 1985 Jun;5(6):1358–1369. doi: 10.1128/mcb.5.6.1358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Learned R. M., Learned T. K., Haltiner M. M., Tjian R. T. Human rRNA transcription is modulated by the coordinate binding of two factors to an upstream control element. Cell. 1986 Jun 20;45(6):847–857. doi: 10.1016/0092-8674(86)90559-3. [DOI] [PubMed] [Google Scholar]
- Mishima Y., Financsek I., Kominami R., Muramatsu M. Fractionation and reconstitution of factors required for accurate transcription of mammalian ribosomal RNA genes: identification of a species-dependent initiation factor. Nucleic Acids Res. 1982 Nov 11;10(21):6659–6670. doi: 10.1093/nar/10.21.6659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicklin M. J., Harris K. S., Pallai P. V., Wimmer E. Poliovirus proteinase 3C: large-scale expression, purification, and specific cleavage activity on natural and synthetic substrates in vitro. J Virol. 1988 Dec;62(12):4586–4593. doi: 10.1128/jvi.62.12.4586-4593.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicklin M. J., Kräusslich H. G., Toyoda H., Dunn J. J., Wimmer E. Poliovirus polypeptide precursors: expression in vitro and processing by exogenous 3C and 2A proteinases. Proc Natl Acad Sci U S A. 1987 Jun;84(12):4002–4006. doi: 10.1073/pnas.84.12.4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pallai P. V., Burkhardt F., Skoog M., Schreiner K., Bax P., Cohen K. A., Hansen G., Palladino D. E., Harris K. S., Nicklin M. J. Cleavage of synthetic peptides by purified poliovirus 3C proteinase. J Biol Chem. 1989 Jun 15;264(17):9738–9741. [PubMed] [Google Scholar]
- Paule M. R., Iida C. T., Perna P. J., Harris G. H., Knoll D. A., D'Alessio J. M. In vitro evidence that eukaryotic ribosomal RNA transcription is regulated by modification of RNA polymerase I. Nucleic Acids Res. 1984 Nov 12;12(21):8161–8180. doi: 10.1093/nar/12.21.8161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein S. J., Dasgupta A. Inhibition of rRNA synthesis by poliovirus: specific inactivation of transcription factors. J Virol. 1989 Nov;63(11):4689–4696. doi: 10.1128/jvi.63.11.4689-4696.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sollner-Webb B., Tower J. Transcription of cloned eukaryotic ribosomal RNA genes. Annu Rev Biochem. 1986;55:801–830. doi: 10.1146/annurev.bi.55.070186.004101. [DOI] [PubMed] [Google Scholar]
- Sun X. H., Baltimore D. Human immunodeficiency virus tat-activated expression of poliovirus protein 2A inhibits mRNA translation. Proc Natl Acad Sci U S A. 1989 Apr;86(7):2143–2146. doi: 10.1073/pnas.86.7.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesar M., Marquardt O. Foot-and-mouth disease virus protease 3C inhibits cellular transcription and mediates cleavage of histone H3. Virology. 1990 Feb;174(2):364–374. doi: 10.1016/0042-6822(90)90090-e. [DOI] [PubMed] [Google Scholar]
- Tower J., Sollner-Webb B. Transcription of mouse rDNA is regulated by an activated subform of RNA polymerase I. Cell. 1987 Sep 11;50(6):873–883. doi: 10.1016/0092-8674(87)90514-9. [DOI] [PubMed] [Google Scholar]
- Ypma-Wong M. F., Semler B. L. In vitro molecular genetics as a tool for determining the differential cleavage specificities of the poliovirus 3C proteinase. Nucleic Acids Res. 1987 Mar 11;15(5):2069–2088. doi: 10.1093/nar/15.5.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZIMMERMAN E. F., HEETER M., DARNELL J. E. RNA synthesis in poliovirus-infected cells. Virology. 1963 Mar;19:400–408. doi: 10.1016/0042-6822(63)90080-1. [DOI] [PubMed] [Google Scholar]