Abstract
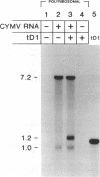
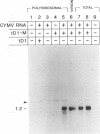
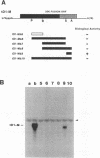
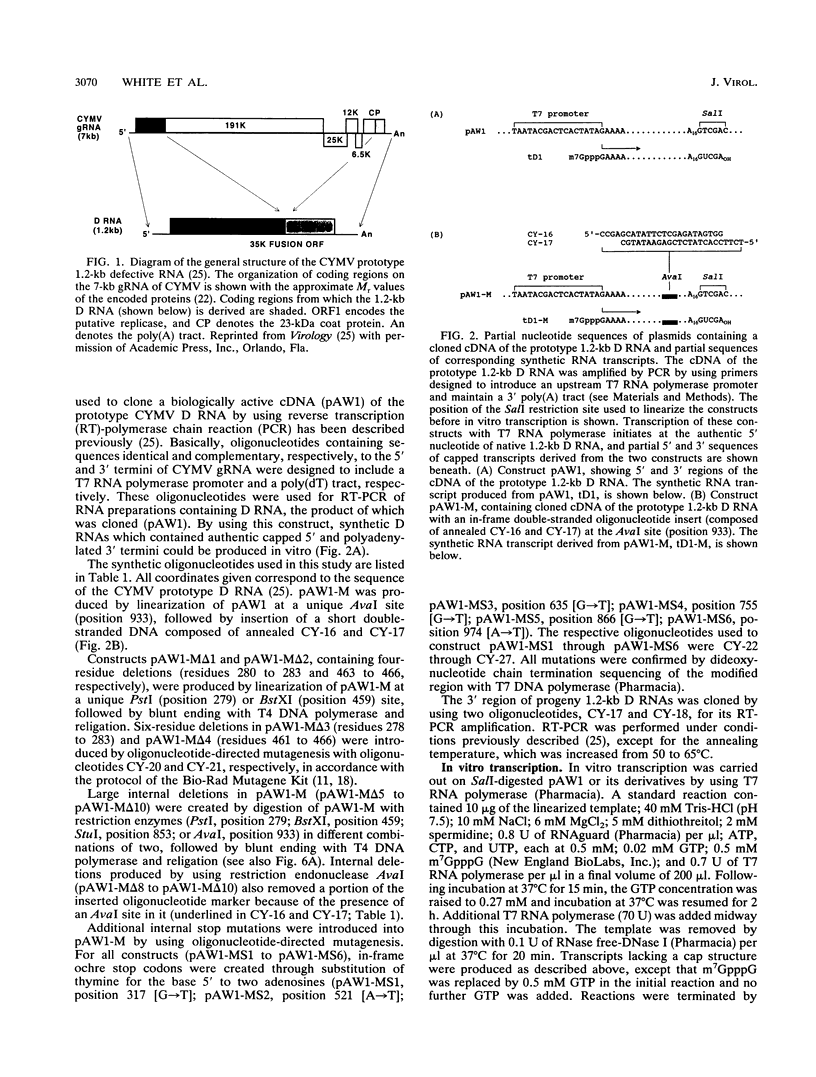
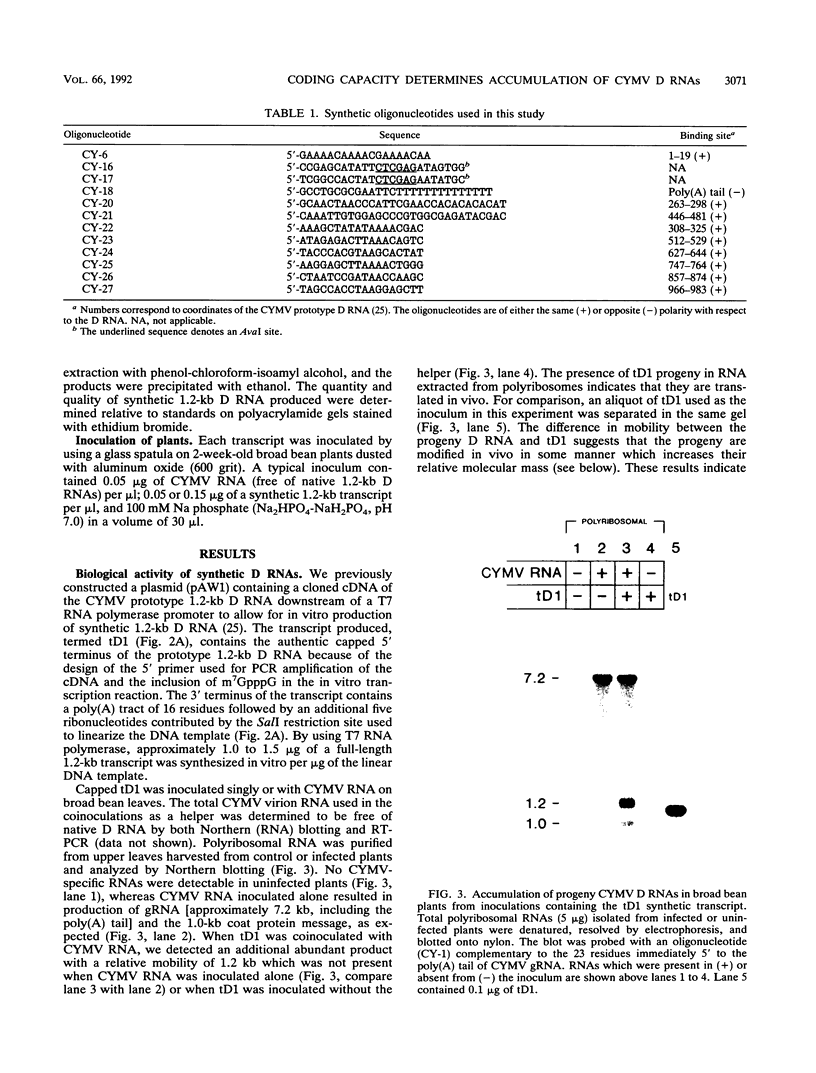
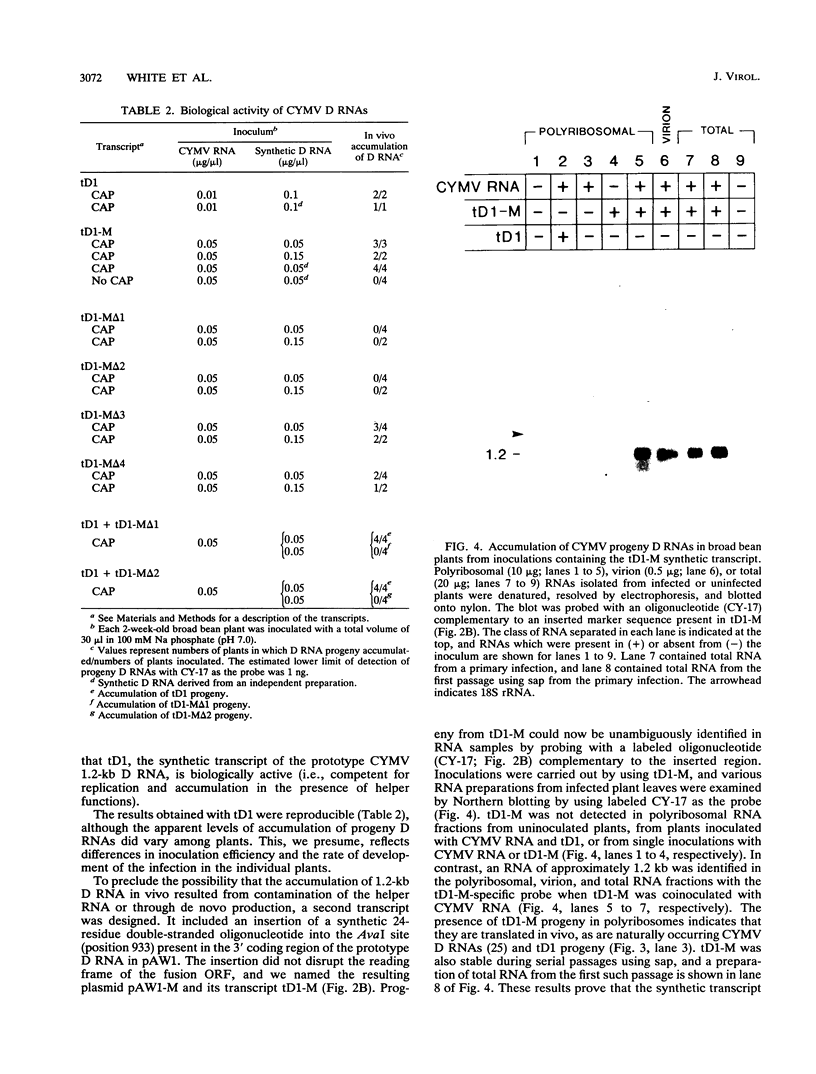
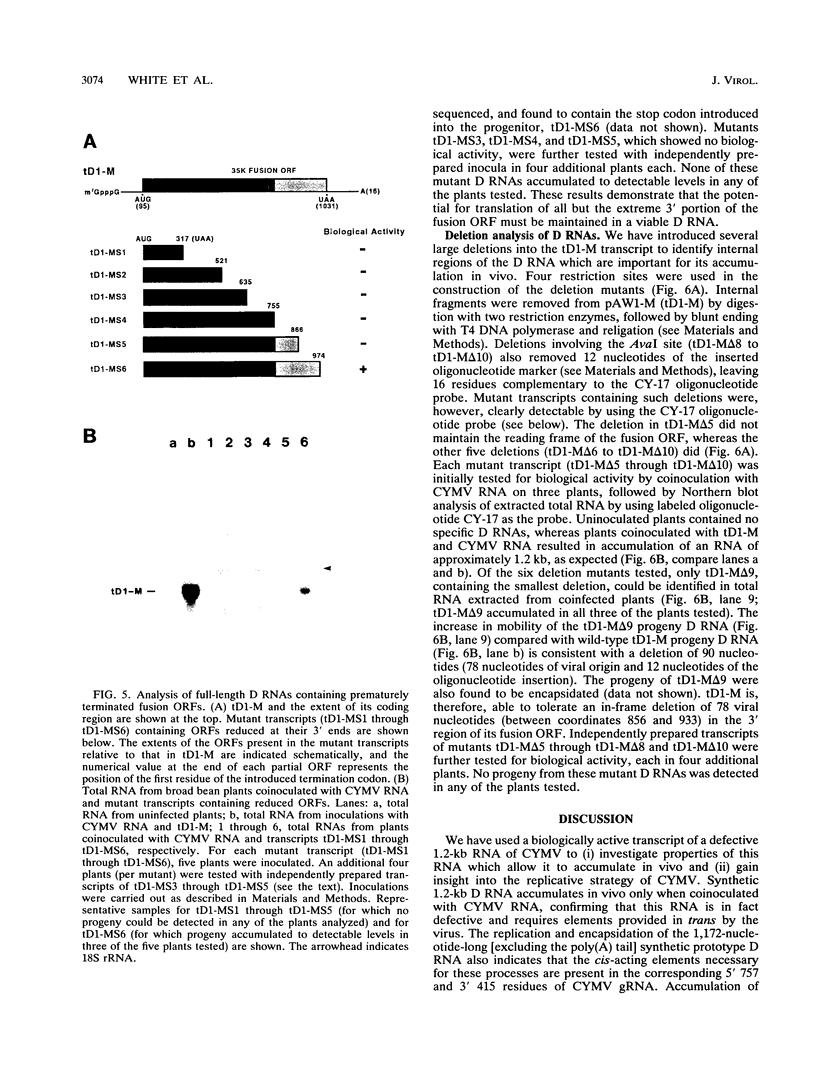
Naturally occurring defective RNAs (D RNAs) derived from the potexvirus clover yellow mosaic virus (CYMV) contain large internal deletions yet maintain a single open reading frame (ORF) representing the in-frame fusion of 5' and 3' terminal ORFs. Capped transcripts of the prototype 1.2-kb D RNA of CYMV were synthesized in vitro and used to inoculate broad bean plants. Progeny D RNA accumulated only if synthetic D RNA transcripts were coinoculated with CYMV RNA. Several experiments showed that helper-dependent accumulation of the D RNA in vivo depended on the maintenance of its encoded fusion ORF. (i) D RNAs with six-residue deletions introduced early in the fusion ORF accumulated, whereas those with four-residue out-of-frame deletions at the same sites were nonviable. (ii) Analysis of D RNAs containing termination codons at different locations showed that only the most 3' stop codon (maintaining over 93% of the fusion ORF) was permissive for D RNA accumulation. (iii) D RNAs with small in-frame deletions and insertions in their 3' coding regions were viable. (iv) Nonviable D RNAs containing disrupted fusion ORFs could not be complemented by the presence in the infection of a D RNA encoding a complete fusion ORF. Taken together, the results indicate that the process of translation, rather than the encoded product, modulates an event(s) which influences the propagation and/or accumulation of this RNA in vivo. This represents a unique requirement among plant virus D RNAs.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bancroft J. B., Rouleau M., Johnston R., Prins L., Mackie G. A. The entire nucleotide sequence of foxtail mosaic virus RNA. J Gen Virol. 1991 Sep;72(Pt 9):2173–2181. doi: 10.1099/0022-1317-72-9-2173. [DOI] [PubMed] [Google Scholar]
- Beck D. L., Forster R. L., Bevan M. W., Boxen K. A., Lowe S. C. Infectious transcripts and nucleotide sequence of cloned cDNA of the potexvirus white clover mosaic virus. Virology. 1990 Jul;177(1):152–158. doi: 10.1016/0042-6822(90)90469-8. [DOI] [PubMed] [Google Scholar]
- Dawson W. O., Beck D. L., Knorr D. A., Grantham G. L. cDNA cloning of the complete genome of tobacco mosaic virus and production of infectious transcripts. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1832–1836. doi: 10.1073/pnas.83.6.1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guilford P. J., Beck D. L., Forster R. L. Influence of the poly(A) tail and putative polyadenylation signal on the infectivity of white clover mosaic potexvirus. Virology. 1991 May;182(1):61–67. doi: 10.1016/0042-6822(91)90648-u. [DOI] [PubMed] [Google Scholar]
- Hagino-Yamagishi K., Nomoto A. In vitro construction of poliovirus defective interfering particles. J Virol. 1989 Dec;63(12):5386–5392. doi: 10.1128/jvi.63.12.5386-5392.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillman B. I., Carrington J. C., Morris T. J. A defective interfering RNA that contains a mosaic of a plant virus genome. Cell. 1987 Nov 6;51(3):427–433. doi: 10.1016/0092-8674(87)90638-6. [DOI] [PubMed] [Google Scholar]
- Kunkel T. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Proc Natl Acad Sci U S A. 1985 Jan;82(2):488–492. doi: 10.1073/pnas.82.2.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X. H., Heaton L. A., Morris T. J., Simon A. E. Turnip crinkle virus defective interfering RNAs intensify viral symptoms and are generated de novo. Proc Natl Acad Sci U S A. 1989 Dec;86(23):9173–9177. doi: 10.1073/pnas.86.23.9173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X. H., Simon A. E. In vivo accumulation of a turnip crinkle virus defective interfering RNA is affected by alterations in size and sequence. J Virol. 1991 Sep;65(9):4582–4590. doi: 10.1128/jvi.65.9.4582-4590.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackie G. A. Structure of the DNA distal to the gene for ribosomal protein S20 in Escherichia coli K12: presence of a strong terminator and an IS1 element. Nucleic Acids Res. 1986 Sep 11;14(17):6965–6981. doi: 10.1093/nar/14.17.6965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino S., Shieh C. K., Soe L. H., Baker S. C., Lai M. M. Primary structure and translation of a defective interfering RNA of murine coronavirus. Virology. 1988 Oct;166(2):550–560. doi: 10.1016/0042-6822(88)90526-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makino S., Yokomori K., Lai M. M. Analysis of efficiently packaged defective interfering RNAs of murine coronavirus: localization of a possible RNA-packaging signal. J Virol. 1990 Dec;64(12):6045–6053. doi: 10.1128/jvi.64.12.6045-6053.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMaster G. K., Carmichael G. G. Analysis of single- and double-stranded nucleic acids on polyacrylamide and agarose gels by using glyoxal and acridine orange. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4835–4838. doi: 10.1073/pnas.74.11.4835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mead D. A., Szczesna-Skorupa E., Kemper B. Single-stranded DNA 'blue' T7 promoter plasmids: a versatile tandem promoter system for cloning and protein engineering. Protein Eng. 1986 Oct-Nov;1(1):67–74. doi: 10.1093/protein/1.1.67. [DOI] [PubMed] [Google Scholar]
- Monroe S. S., Schlesinger S. Common and distinct regions of defective-interfering RNAs of Sindbis virus. J Virol. 1984 Mar;49(3):865–872. doi: 10.1128/jvi.49.3.865-872.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petty I. T., Hunter B. G., Wei N., Jackson A. O. Infectious barley stripe mosaic virus RNA transcribed in vitro from full-length genomic cDNA clones. Virology. 1989 Aug;171(2):342–349. doi: 10.1016/0042-6822(89)90601-6. [DOI] [PubMed] [Google Scholar]
- Roux L., Simon A. E., Holland J. J. Effects of defective interfering viruses on virus replication and pathogenesis in vitro and in vivo. Adv Virus Res. 1991;40:181–211. doi: 10.1016/S0065-3527(08)60279-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sit T. L., White K. A., Holy S., Padmanabhan U., Eweida M., Hiebert M., Mackie G. A., AbouHaidar M. G. Complete nucleotide sequence of clover yellow mosaic virus RNA. J Gen Virol. 1990 Sep;71(Pt 9):1913–1920. doi: 10.1099/0022-1317-71-9-1913. [DOI] [PubMed] [Google Scholar]
- Weiland J. J., Dreher T. W. Infectious TYMV RNA from cloned cDNA: effects in vitro and in vivo of point substitutions in the initiation codons of two extensively overlapping ORFs. Nucleic Acids Res. 1989 Jun 26;17(12):4675–4687. doi: 10.1093/nar/17.12.4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White K. A., Bancroft J. B., Mackie G. A. Defective RNAs of clover yellow mosaic virus encode nonstructural/coat protein fusion products. Virology. 1991 Aug;183(2):479–486. doi: 10.1016/0042-6822(91)90977-J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White K. A., Mackie G. A. Control and expression of 3' open reading frames in clover yellow mosaic virus. Virology. 1990 Dec;179(2):576–584. doi: 10.1016/0042-6822(90)90124-a. [DOI] [PubMed] [Google Scholar]
- van der Most R. G., Bredenbeek P. J., Spaan W. J. A domain at the 3' end of the polymerase gene is essential for encapsidation of coronavirus defective interfering RNAs. J Virol. 1991 Jun;65(6):3219–3226. doi: 10.1128/jvi.65.6.3219-3226.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]