Abstract
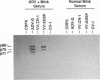
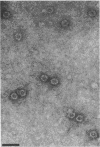
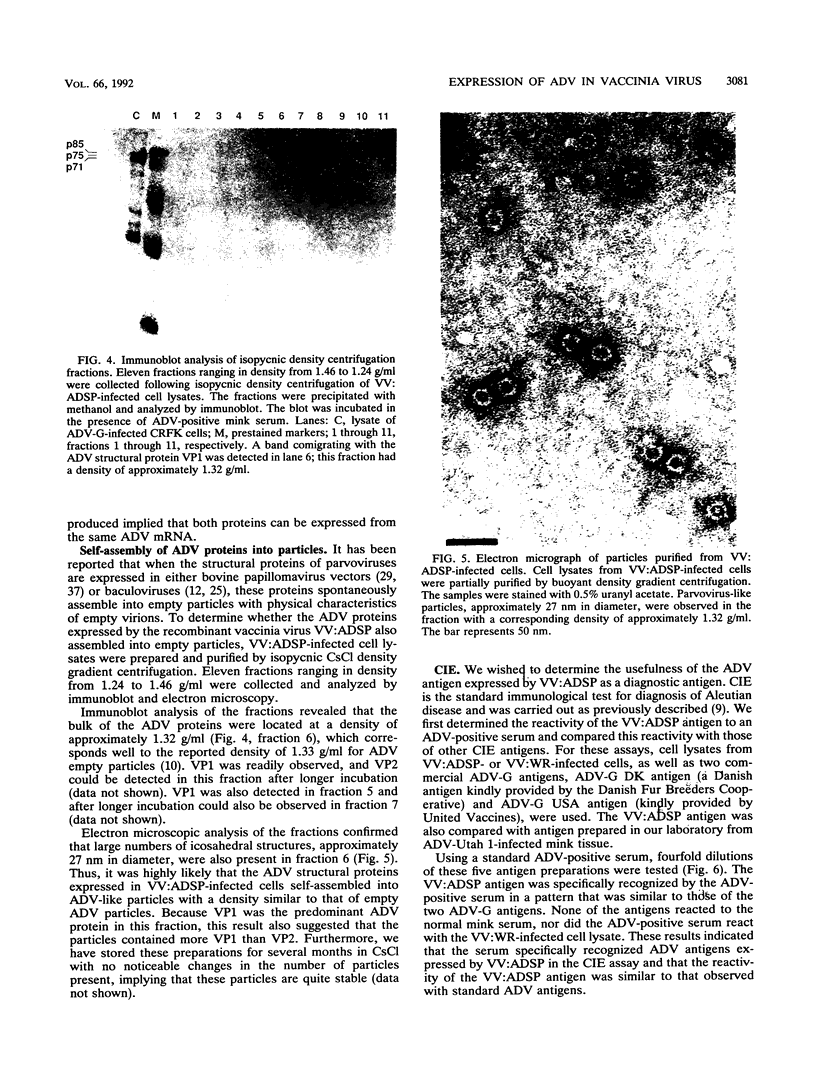
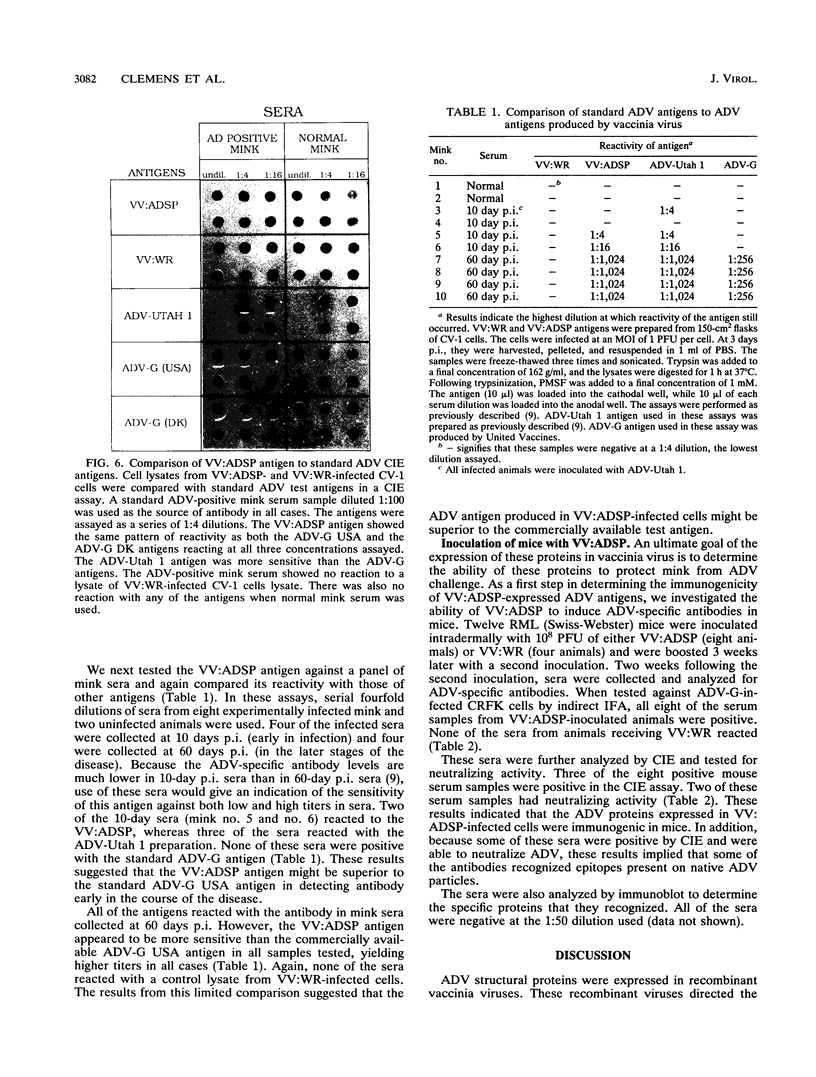
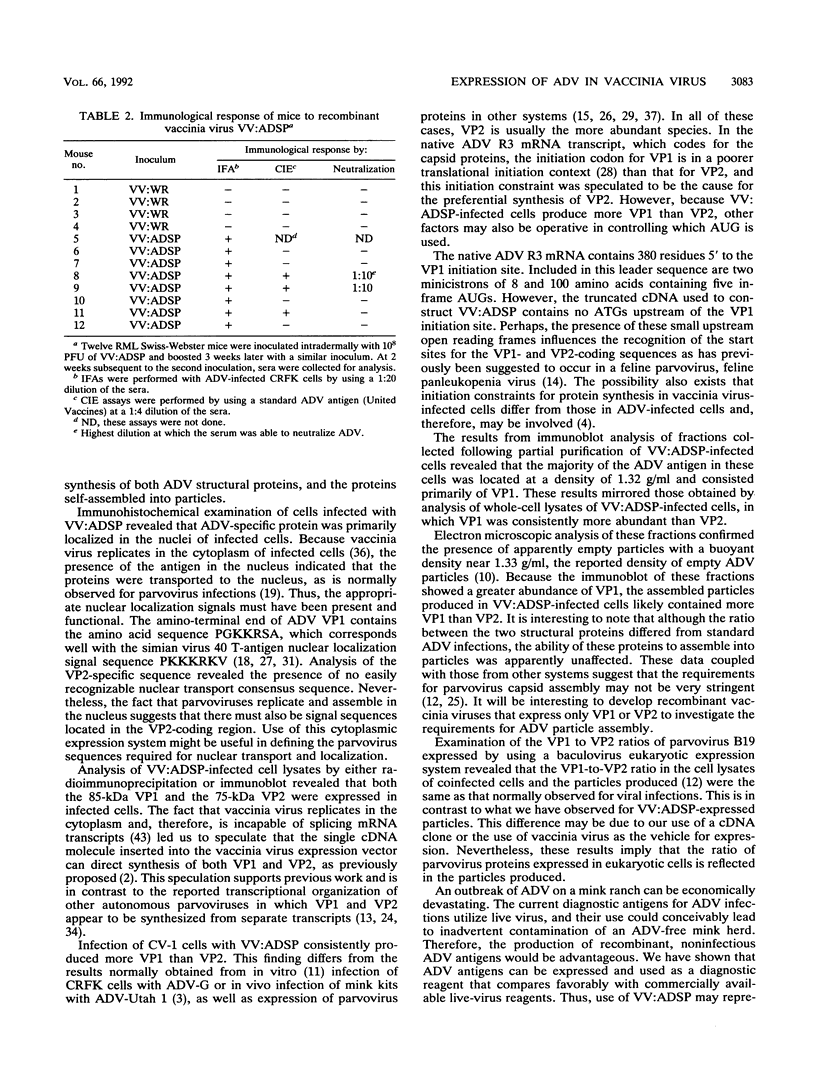
A portion of a cDNA clone containing coding sequences for both structural proteins (VP1 and VP2) of Aleutian mink disease parvovirus (ADV) was inserted into recombinant vaccinia viruses, VV:ADSP. Immunohistochemical staining of VV:ADSP-infected cells revealed that the ADV antigen was readily detected and localized in the nuclei of infected cells. Analysis of VV:ADSP-infected cell lystates indicated that both VP1 and VP2 were produced and comigrated with authentic VP1 and VP2 from ADV-infected Crandell feline kidney cells. These results suggested, therefore, that both VP1 and VP2 were synthesized from a single cloned transcript. CsCl density gradient centrifugation of partially purified VV:ADSP-infected cell lysates indicated that the majority of the antigen was located in a fraction with a density near 1.33 g/ml, indicative of empty ADV particles. Subsequent electron microscopic examination revealed the presence of 27-nm icosahedral virion-like structures at the same density, suggesting that the proteins self-assembled into empty virions. Furthermore, sera from eight of eight mice inoculated with VV:ADSP contained ADV-specific antibodies and two of these eight serum samples had neutralizing activity, indicating that the particles produced in VV:ADSP-infected cells were immunogenic. Finally, when lysates from VV:ADSP-infected cells were compared with standard ADV antigens in counterimmunoelectrophoresis assays, a similar pattern of specific reactivity was observed for sera from normal and infected mink.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexandersen S. Acute interstitial pneumonia in mink kits: experimental reproduction of the disease. Vet Pathol. 1986 Sep;23(5):579–588. doi: 10.1177/030098588602300506. [DOI] [PubMed] [Google Scholar]
- Alexandersen S., Bloom M. E., Perryman S. Detailed transcription map of Aleutian mink disease parvovirus. J Virol. 1988 Oct;62(10):3684–3694. doi: 10.1128/jvi.62.10.3684-3694.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexandersen S., Uttenthal-Jensen A., Aasted B. Demonstration of non-degraded Aleutian disease virus (ADV) proteins in lung tissue from experimentally infected mink kits. Brief report. Arch Virol. 1986;87(1-2):127–133. doi: 10.1007/BF01310549. [DOI] [PubMed] [Google Scholar]
- Bablanian R., Goswami S. K., Esteban M., Banerjee A. K., Merrick W. C. Mechanism of selective translation of vaccinia virus mRNAs: differential role of poly(A) and initiation factors in the translation of viral and cellular mRNAs. J Virol. 1991 Aug;65(8):4449–4460. doi: 10.1128/jvi.65.8.4449-4460.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom M. E., Alexandersen S., Garon C. F., Mori S., Wei W., Perryman S., Wolfinbarger J. B. Nucleotide sequence of the 5'-terminal palindrome of Aleutian mink disease parvovirus and construction of an infectious molecular clone. J Virol. 1990 Jul;64(7):3551–3556. doi: 10.1128/jvi.64.7.3551-3556.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom M. E., Alexandersen S., Perryman S., Lechner D., Wolfinbarger J. B. Nucleotide sequence and genomic organization of Aleutian mink disease parvovirus (ADV): sequence comparisons between a nonpathogenic and a pathogenic strain of ADV. J Virol. 1988 Aug;62(8):2903–2915. doi: 10.1128/jvi.62.8.2903-2915.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom M. E., Kaaden O. R., Huggans E., Cohn A., Wolfinbarger J. B. Molecular comparisons of in vivo- and in vitro-derived strains of Aleutian disease of mink parvovirus. J Virol. 1988 Jan;62(1):132–138. doi: 10.1128/jvi.62.1.132-138.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom M. E., Race R. E., Aasted B., Wolfinbarger J. B. Analysis of Aleutian disease virus infection in vitro and in vivo: demonstration of Aleutian disease virus DNA in tissues of infected mink. J Virol. 1985 Sep;55(3):696–703. doi: 10.1128/jvi.55.3.696-703.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom M. E., Race R. E., Hadlow W. J., Chesebro B. Aleutian disease of mink: the antibody response of sapphire and pastel mink to Aleutian disease virus. J Immunol. 1975 Oct;115(4):1034–1037. [PubMed] [Google Scholar]
- Bloom M. E., Race R. E., Wolfinbarger J. B. Characterization of Aleutian disease virus as a parvovirus. J Virol. 1980 Sep;35(3):836–843. doi: 10.1128/jvi.35.3.836-843.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom M. E., Race R. E., Wolfinbarger J. B. Identification of a nonvirion protein of Aleutian disease virus: mink with Aleutian disease have antibody to both virion and nonvirion proteins. J Virol. 1982 Aug;43(2):608–616. doi: 10.1128/jvi.43.2.608-616.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown C. S., Van Lent J. W., Vlak J. M., Spaan W. J. Assembly of empty capsids by using baculovirus recombinants expressing human parvovirus B19 structural proteins. J Virol. 1991 May;65(5):2702–2706. doi: 10.1128/jvi.65.5.2702-2706.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson J., Rushlow K., Maxwell I., Maxwell F., Winston S., Hahn W. Cloning and sequence of DNA encoding structural proteins of the autonomous parvovirus feline panleukopenia virus. J Virol. 1985 Sep;55(3):574–582. doi: 10.1128/jvi.55.3.574-582.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarti S., Brechling K., Moss B. Vaccinia virus expression vector: coexpression of beta-galactosidase provides visual screening of recombinant virus plaques. Mol Cell Biol. 1985 Dec;5(12):3403–3409. doi: 10.1128/mcb.5.12.3403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho H. J., Greenfield J. Eradication of Aleutian disease of mink by eliminating positive counterimmunoelectrophoresis test reactors. J Clin Microbiol. 1978 Jan;7(1):18–22. doi: 10.1128/jcm.7.1.18-22.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens D. L., Carlson J. O. Regulated expression of the feline panleukopenia virus P38 promoter on extrachromosomal FPV/EBV chimeric plasmids. J Virol. 1989 Jun;63(6):2737–2745. doi: 10.1128/jvi.63.6.2737-2745.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clemens K. E., Cerutis D. R., Burger L. R., Yang C. Q., Pintel D. J. Cloning of minute virus of mice cDNAs and preliminary analysis of individual viral proteins expressed in murine cells. J Virol. 1990 Aug;64(8):3967–3973. doi: 10.1128/jvi.64.8.3967-3973.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colledge W. H., Richardson W. D., Edge M. D., Smith A. E. Extensive mutagenesis of the nuclear location signal of simian virus 40 large-T antigen. Mol Cell Biol. 1986 Nov;6(11):4136–4139. doi: 10.1128/mcb.6.11.4136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cotmore S. F., Tattersall P. The autonomously replicating parvoviruses of vertebrates. Adv Virus Res. 1987;33:91–174. doi: 10.1016/s0065-3527(08)60317-6. [DOI] [PubMed] [Google Scholar]
- Eklund C. M., Hadlow W. J., Kennedy R. C., Boyle C. C., Jackson T. A. Aleutian disease of mink: properties of the etiologic agent and the host responses. J Infect Dis. 1968 Dec;118(5):510–526. doi: 10.1093/infdis/118.5.510. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Hadlow W. J., Race R. E., Kennedy R. C. Comparative pathogenicity of four strains of Aleutian disease virus for pastel and sapphire mink. Infect Immun. 1983 Sep;41(3):1016–1023. doi: 10.1128/iai.41.3.1016-1023.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hruby D. E. Vaccinia virus vectors: new strategies for producing recombinant vaccines. Clin Microbiol Rev. 1990 Apr;3(2):153–170. doi: 10.1128/cmr.3.2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jongeneel C. V., Sahli R., McMaster G. K., Hirt B. A precise map of splice junctions in the mRNAs of minute virus of mice, an autonomous parvovirus. J Virol. 1986 Sep;59(3):564–573. doi: 10.1128/jvi.59.3.564-573.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajigaya S., Fujii H., Field A., Anderson S., Rosenfeld S., Anderson L. J., Shimada T., Young N. S. Self-assembled B19 parvovirus capsids, produced in a baculovirus system, are antigenically and immunogenically similar to native virions. Proc Natl Acad Sci U S A. 1991 Jun 1;88(11):4646–4650. doi: 10.1073/pnas.88.11.4646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajigaya S., Shimada T., Fujita S., Young N. S. A genetically engineered cell line that produces empty capsids of B19 (human) parvovirus. Proc Natl Acad Sci U S A. 1989 Oct;86(19):7601–7605. doi: 10.1073/pnas.86.19.7601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalderon D., Roberts B. L., Richardson W. D., Smith A. E. A short amino acid sequence able to specify nuclear location. Cell. 1984 Dec;39(3 Pt 2):499–509. doi: 10.1016/0092-8674(84)90457-4. [DOI] [PubMed] [Google Scholar]
- Kozak M. Compilation and analysis of sequences upstream from the translational start site in eukaryotic mRNAs. Nucleic Acids Res. 1984 Jan 25;12(2):857–872. doi: 10.1093/nar/12.2.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labieniec-Pintel L., Pintel D. The minute virus of mice P39 transcription unit can encode both capsid proteins. J Virol. 1986 Mar;57(3):1163–1167. doi: 10.1128/jvi.57.3.1163-1167.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lanford R. E., Butel J. S. Construction and characterization of an SV40 mutant defective in nuclear transport of T antigen. Cell. 1984 Jul;37(3):801–813. doi: 10.1016/0092-8674(84)90415-x. [DOI] [PubMed] [Google Scholar]
- Mackett M., Smith G. L. Vaccinia virus expression vectors. J Gen Virol. 1986 Oct;67(Pt 10):2067–2082. doi: 10.1099/0022-1317-67-10-2067. [DOI] [PubMed] [Google Scholar]
- Mayer L. W., Aasted B., Garon C. F., Bloom M. E. Molecular cloning of the Aleutian disease virus genome: expression of Aleutian disease virus antigens by a recombinant plasmid. J Virol. 1983 Dec;48(3):573–579. doi: 10.1128/jvi.48.3.573-579.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan W. R., Ward D. C. Three splicing patterns are used to excise the small intron common to all minute virus of mice RNAs. J Virol. 1986 Dec;60(3):1170–1174. doi: 10.1128/jvi.60.3.1170-1174.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori S., Wolfinbarger J. B., Miyazawa M., Bloom M. E. Replication of Aleutian mink disease parvovirus in lymphoid tissues of adult mink: involvement of follicular dendritic cells and macrophages. J Virol. 1991 Feb;65(2):952–956. doi: 10.1128/jvi.65.2.952-956.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss B. Vaccinia virus: a tool for research and vaccine development. Science. 1991 Jun 21;252(5013):1662–1667. doi: 10.1126/science.2047875. [DOI] [PubMed] [Google Scholar]
- Pintel D., Merchlinsky M. J., Ward D. C. Expression of minute virus of mice structural proteins in murine cell lines transformed by bovine papillomavirus-minute virus of mice plasmid chimera. J Virol. 1984 Nov;52(2):320–327. doi: 10.1128/jvi.52.2.320-327.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter D. D., Larsen A. E., Porter H. G. Aleutian disease of mink. Adv Immunol. 1980;29:261–286. doi: 10.1016/s0065-2776(08)60046-2. [DOI] [PubMed] [Google Scholar]
- Porter D. D., Larsen A. E., Porter H. G. The pathogenesis of Aleutian disease of mink. 3. Immune complex arteritis. Am J Pathol. 1973 May;71(2):331–344. [PMC free article] [PubMed] [Google Scholar]
- Porter D. D., Larsen A. E., Porter H. G. The pathogenesis of Aleutian disease of mink. I. In vivo viral replication and the host antibody response to viral antigen. J Exp Med. 1969 Sep 1;130(3):575–593. doi: 10.1084/jem.130.3.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter D. D., Larsen A. E., Porter H. G. The pathogenesis of Aleutian disease of mink. II. Enhancement of tissue lesions following the administration of a killed virus vaccine or passive antibody. J Immunol. 1972 Jul;109(1):1–7. [PubMed] [Google Scholar]
- Race R. E., Chesebro B., Bloom M. E., Aasted B., Wolfinbarger J. Monoclonal antibodies against Aleutian disease virus distinguish virus strains and differentiate sites of virus replication from sites of viral antigen sequestration. J Virol. 1986 Jan;57(1):285–293. doi: 10.1128/jvi.57.1.285-293.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatos N. M., Chakrabarti S., Moss B., Hare J. D. Expression of polyomavirus virion proteins by a vaccinia virus vector: association of VP1 and VP2 with the nuclear framework. J Virol. 1987 Feb;61(2):516–525. doi: 10.1128/jvi.61.2.516-525.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stolze B., Kaaden O. R. Apparent lack of neutralizing antibodies in Aleutian disease is due to masking of antigenic sites by phospholipids. Virology. 1987 May;158(1):174–180. doi: 10.1016/0042-6822(87)90251-0. [DOI] [PubMed] [Google Scholar]
- Tartaglia J., Pincus S., Paoletti E. Poxvirus-based vectors as vaccine candidates. Crit Rev Immunol. 1990;10(1):13–30. [PubMed] [Google Scholar]